Back to Journals » OncoTargets and Therapy » Volume 13
Use of Programmed Death Receptor-1 and/or Programmed Death Ligand 1 Inhibitors for the Treatment of Brain Metastasis of Lung Cancer
Authors Wang S, Hu C , Xie F, Liu Y
Received 23 October 2019
Accepted for publication 24 December 2019
Published 23 January 2020 Volume 2020:13 Pages 667—683
DOI https://doi.org/10.2147/OTT.S235714
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Shiqiang Wang,1,2 Chongling Hu,2 Fei Xie,3 Yanhui Liu1
1Department of Neurosurgery, West China Hospital, Sichuan University, Chengdu 610041, People’s Republic of China; 2Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital, Chongqing 400030, People’s Republic of China; 3Department of Neurosurgery, Ziyang First People’s Hospital, Ziyang 641300, People’s Republic of China
Correspondence: Yanhui Liu
Department of Neurosurgery, West China Hospital, Sichuan University, No. 37, Guoxue Lane, Wuhou Zone, Chengdu 610041, People’s Republic of China
Email [email protected]
Abstract: The central nervous system (CNS) is regarded as an immune privileged environment; however, changes in the neuroimmunology paradigm have led to an increased interest in systematic immunotherapy in lung cancer therapy. The presence of the lymphatic system in the CNS as well as the physiological and biochemical changes in the blood–brain barrier in the tumor microenvironment suggests that immunocytes are fully capable of entering and exiting the CNS. Emerging clinical data suggest that inhibitors of programmed death receptor-1/programmed death ligand 1 (PD-1/PD-L1) can stimulate surrounding T cells and thus have antitumor effects in the CNS. For example, PD-1 antibody (pembrolizumab) monotherapy has displayed a 20– 30% encephalic response rate in patients with brain metastases from malignant melanoma or non-small cell lung cancer. Combined application of nivolumab and ipilimumab anti-PD-1 and anti-cytotoxic T-lymphocyte-associated protein 4 showed an encephalic response rate of 55% in patients with brain metastases of melanoma. Further evidence is required to verify these response rates and identify the mechanisms of curative effects and drug tolerance. While regional treatments such as whole-brain radiosurgery, stereotactic radiosurgery, and brain surgery remain the mainstream, PD-1/PD-L1 inhibitors display potential decreased neurotoxic effects. To date, five drugs have been approved for use in patients with encephalic metastases of lung carcinoma: the anti-PD-1 drugs, pembrolizumab and nivolumab, and the anti-PD-L1 agents, atezolizumab, durvalumab, and avelumab. In recent years, clinical trials of inhibitors in combination with other drugs to treat brain metastasis have also emerged. This review summarizes the biological principles of PD-1/PD-L1 immunotherapy for brain metastasis of lung cancer, as well as ongoing clinical trials to explore unmet needs.
Keywords: immunotherapy, blood–brain barrier, brain metastasis, lung cancer, PD-1/PD-L1 inhibitors
Introduction
The mortality of lung cancer was 46.92/100,000 and the age-standardized mortality rate was 28.59/100,000 in the last ten years. During the same period, the total number of lung cancer deaths in China was 106,300, with 399,300 malignant cancer deaths, accounting for 26.62% of all deaths due to malignant cancer.1 Lung carcinoma remains the leading cause of cancer morbidity and death in China. The national cancer center of China in February 2017 stated that the incidence of lung cancer was 57.70/100,000 and the age-standardized incidence rate was 36.23/100,000.2
Reasons for the high mortality of lung cancer include partial recrudescence and distal metastasis, and the brain is a common site of lung carcinoma recurrence. The rate of brain metastasis in end-stage non-small cell lung cancer (NSCLC) is up to 44%, especially in adenocarcinoma.3 Occurrence of brain metastasis is associated with extremely poor prognosis, with a median survival time (MST) of only 1–2 months in untreated patients.4 Among patients receiving standardized radiotherapy, the MST is only 8.8 months.5
Despite recent progress in oncotherapy, brain metastasis remains a destructive complex disease for a lot of solid apparatus phymas, especially lungcarcinomas. Up to 20% of adult systemic malignancies such as lung cancer develop brain metastases.6 This incidence is increasing, partly due to improvements in magnetic resonance imaging (MRI) testing techniques, as well as improvements in systemic therapies that extend patient survival.7 Localized treatments have become mainstream, but may increase the incidencerate of complex postoperative diseases such as stroke, organ thanatosis, and cognizant confusion.8
Generalized immunotherapy has shown favorable data in the treatment of brain metastases and has changed the conventionalpattern of cerebral immune privilege. The immune system recognizes tumor cell antigens, and antigen presenting cells (APCs) activate T cells and subsequent T cell toxicity via APCs, and play an important role in clearing tumor clones.9,10 However, tumor cells can evade the immune system by expressing multiple checkpoints that confer tolerance, and inhibit T cell proliferation.11,12 At present, the most correlated immune checkpoints in clinical are programmed death receptor-1 (PD-1) and programmed death ligand 1 (PD-L1). PD-L1 is expressed on the surface of cancer cells and interacts with PD-1 on T cells to inhibit apoptosis of regulatory T cells and induce apoptosis of cytotoxic T cells.13
The paradigm of CNS immune privilege provided the impetus for the immune system to enter the intracranial compartment and to enter the tumor. Whether immune system can cross the blood–brain barrier (BBB) has remained controversial for decades. In the 1940s, studies14 indicated that the brain had no lymphatic drainage system, and the BBB was recognized as a limitation of pathways of the CNS,15 including immune cells.16 However, recent studies have refuted the notion of immune isolation in the brain.17 In the 1980s, an antigen exit route was discovered from the brain to the deep cervical lymph nodes.18 In 2015, functional lymphatics found in the meninges provided a direct drainage route for cerebrospinal fluid and immune cells from the brain to cervical lymph nodes.19
However, PD-1/PD-L1 immunotherapy may be less hindered by CNS immune privilege than dogma dictates. Unlike traditional chemotherapy and targeted therapy, the core mechanism of PD-1/PD-L1 inhibitors is to remove inhibition of tumor cells on T cells. Therefore, the key to immunotherapy is not whether a drug can cross the BBB to reach the brain, but whether killer T cells can enter the tumor tissue of the brain parenchyma.
Normal parenchyma and primary brain tumors have fewer lymphocytes in the immunoregulatory environment, while metastatic brain tumors have significant tumor infiltrating lymphocytes (TILs). High density CD3+ TILs was relevant to longer median overall survival (OS).20 Many proof-of-principle studies have demonstrated that high TIL quantity and augmented OS consistency in primary tumors and cerebral metastasis support the utilization of immune checkpoint suppression for the treatment of brain metastases. The cerebrum ceases to be a strictly “immunization prerogative” circumstances and that cerebral metastases can cross the BBB and PD-1/PD-L1 inhibitors have shown promising results.
PD-1/PD-L1 Inhibitors Currently in Clinical Use
The theory that the brain is an immunologically specialized region has recently been re-examined due to findings of the presence of lymph vessels in the endocranium that drain cerebrospinal fluid to the extracranial neck lymphatic gland. These findings have changed our understanding of the anatomy of the CNS.21 However, the inborn and elastic immune responses of the CNS are inferior to those of the peripheral tissues.22 Nevertheless, active cyclic CD4+ T cells have been shown to breach the BBB, and once their isogenous antigens are identified on APCs, they trigger partial T cell excitation, secret various cell factors, and further recruit immunocompetent cells, ultimately changing BBB penetrability characteristics.22 The mechanism by which tumors evade the immune system and promote immune tolerability is the objective of immune checkpoint suppressants. Five drugs have been approved for use in patients with encephalic metastases from lung carcinoma: the anti-PD-1 drugs, pembrolizumab and nivolumab, and the anti-PD-L1 agents, atezolizumab, durvalumab, and avelumab.
Pembrolizumab
NSCLC
Use of pembrolizumab as an anti-PD-1 monoclonal antibody was primarily used to demonstrate unequivocal efficacy against brain metastasis in untreated NSCLC. Many proof-of-principle studies have shown that pembrolizumab has therapeutic effects on brain metastasis of lung cancer. The KEYNOTE-001 clinical trial confirmed the efficacy of pembrolizumab in NSCLC with CNS metastasis for the first time and showed that its efficacy was correlated with PD-L1 expression.23 The US Food and Drug Administration (FDA) authorized use of “Pembrolizumab for second-line PD-L1-positive (≥1%) patients with metastatic NSCLC whose disease progression occurs during or after platinum chemotherapy” in 2015.24 Five-year survival is a milestone for patients with advanced NSCLC, and the long-term total survival data reported in the first KEYNOTE series study is encouraging.
The KEYNOTE-010 clinical trial confirmed the predictive value of PD-L1 expression for pembrolizumab efficacy.25 These clinical results are similar to those reported previously, and patients may still benefit from previous immunotherapy if challenged after resistance has occurred. Data from a previous prospective Phase II study into pembrolizumab treatment for brain metastases26 reported at least one untreated or progression of 5–20 mm of brain metastases, no central symptoms or without glucocorticoid treatment PD-L1 positive in NSCLC, 18 patients with midbrain transfer objective response rate (ORR) was 33%, total ORR was 33%. This study preliminarily confirmed that pembrolizumab was effective for selective NSCLC brain metastatic lesions and showed a consistent remission rate of intracranial lesions with extracranial lesions. There was a high consistency between CNS and systemic response, and CNS response was present in 8/9 (88%) of patients diagnosed with systemic response. At 10.3 months of follow-up, the medium OS in the NSCLC group was 7.7 months. Adverse effects were similar to those of other diseases treated with pembrolizumab, and no treatment-related deaths have been reported.26 This clinical trial also reported a patient with brain metastases who received pembrolizumab for three years without targeted brain radiotherapy. Pembrolizumab treatment resulted in necrosis of intracranial frontal lobe lesions and disappearance of cerebellum lesions, demonstrating significant therapeutic effects of pembrolizumab on brain metastases of lung cancer. The KEYNOTE-024 clinical trial evaluated the first-line efficacy of pembrolizumab treatment for metastatic NSCLC versus chemotherapy27. It included 18 patients with brain metastases treated with pembrolizumab and 10 treated with chemotherapy. Pembrolizumab showed significant improvements in progression-free survival (PFS) and OS in these patients.27 The National Comprehensive Cancer Network guidelines recommend use of pembrolizumab for first-line treatment in patients with PD-L1-positive (≥50%) metastatic NSCLC.28 Recent data show significant results were maintained at HR of 0.56, with a medium follow-up time of 24 months (95% CI, 0.32–0.95, P = 0.0151).29 Similar results were shown in the KEYNOTE-028 study by the American Society of Clinical Oncology (ASCO).30
The latest NSCLC data reported by ASCO in 2018 showed the CNS response of the 34 patients registered was 29.4% (http://abstracts.asco.org/214/AbstView_214_228899.html). The medium OS was 8.9 months and 31% of patients survived >2 years. The CNS response was inconsistent with the systemic response in seven patients. Five additional PD-L1 negative or unevaluable tumors were contained, despite no response in this sub cohort. This study provides important insight into the treatment of metastatic encephaloma of lung carcinoma. Pembrolizumab was also shown to be active in brain metastases in patients with NSCLC, and was considered safe.31 Therefore, systemic immunotherapy may have therapeutic effects in patients with untreated or progressive brain metastasis. My personal recommendation is that there is no hesitation about pembrolizumab or chemo first for eligible patients, and in any case, immunotherapy should be given first.
These clinical trials have shown that chemotherapy may be more effective better after use of immunotherapy.
SCLC
Pembrolizumab is an efficient treatment for metastatic small cell lung cancer (SCLC). KEYNOTE 15832 was a phase II clinical trial study that evaluated the antitumor activity of pembrolizumab. The study enrolled 11 cancer patients, including SCLC patients with brain metastases. Pembrolizumab was administered to patients with advanced SCLC brain metastases who had previous treatment failure, progression, or intolerance to standard therapy, with ORR, duration of response (DOR), and PFS as primary end points and OS as secondary end points. The ORR of 107 SCLC patients was 18.7%, and was 35.7% for PD-L1-positive tumor patients and 6.0% for PD-L1-negative tumor patients. The medium PFS of all patients was 2.0 months, and was 2.1 months for PD-L1-positive patients and 1.9 months for PD-L1-negative patients. The medium OS was 9.1 months, and was 14.6 months for PD-L1-positive patients and 7.7 months for PD-L1-negative patients. This study showed that patients with PD-L1-positive orthotopic tumors benefited from pembrolizumab immunotherapy, but PD-L1 expression of in metastases was not analyzed; therefore, the correlation between PD-L1 expression, and the prognosis of brain metastasis could not be demonstrated.
These findings indicate that use of pembrolizumab may be advantageous for first-line and second-line therapy for brain metastasis of lung cancer, and may provide flexible options for clinical treatment. These findings also support the use immunotherapy followed by sequential chemotherapy.
Short-term treatment with pembrolizumab may have long-term therapeutic effects on the treatment of lung cancer and brain metastasis. In the event of adverse reactions or discomfort, the treatment time, and dose of pembrolizumab can be reduced.
Nivolumab
NSCLC
Nivolumab has similar therapeutic effects to pembrolizumab for lung cancer with brain metastasis. Studies suggest the potential therapeutic use of nivolumab for brain metastasis. The results of the CheckMate-01733 and CheckMate-05734 studies provided a theoretical basis for nivolumab for the treatment of brain metastases. The FDA granted permission for the application of nivolumab for the treatment of metastatic or advanced NSCLC, prompting research into immunotherapy for brain metastasis of lung cancer. Nivolumab has become a second-line drug for NSCLC treatment.
In the Expanded Access Programme of nivolumab, advanced lung squamous cell carcinoma (SCC) (http://abstracts.asco.org/214/AbstView_214_228899.html), and terminal non-squamous cell carcinoma (NSCLC)35 were studied. In the study of pulmonary SCC, 371 patients in stage III/IV were enrolled, including 37 with asymptomatic brain metastases. The illness inhibition proportion of this cohort was 47.3%, including one complete response (CR), six partial responses (PRs), and 11 with a steady condition. Four patients were treated for cancer progression. The median PFS and OS were 5.5 months and 6.5 months, respectively, and only one patient stopped treatment due to adverse reactions. The objective remission rate of CNS patients treated with nivolumab was 19%, disease control rate (DCR) was 49%, 1-year OS ratio was 35%, and 1-year PFS ratio was 31%.36 The study into nivolumab as a treatment for terminal NSCLC enrolled 1588 patients, including 409 with asymptomatic brain metastasis. The study found that the ORR of patients with CNS metastasis was 17%, the DCR was 40%, the medium OS was 8.6 months, and the 1-year OS ratio was 43%.37 These studies suggest that nivolumab is equally effective for extracranial lesions and showed that nivolumab could improve the condition of both lung SCC and non-SCC patients with brain metastasis. Similarly, a study conducted in Israel that enrolled 260 patients with advanced NSCLC, including 55 with CNS metastasis, showed that the median OS of patients with CNS metastasis and patients without CNS metastasis was 7.0 months and 5.2 months, respectively (P = 0.5), suggesting that the survival benefit of patients with brain metastasis receiving nivolumab was similar to that of patients without cerebral metastases.38
A summary analysis of nivolumab treatment for advanced NSCLC39 including 971 patients (544 patients in the treatment nivolumab group, 46 patients with brain metastases, 427 patients in the docetaxel treatment group, 42 patients with brain metastases) showed that subgroup analysis of patients with metastatic encephaloma revealed that nivolumab treatment group and docetaxel treatment group 3 months in CNS lesions were 7% and 12% respectively, the proportion of new CNS lesions observed in 6 months were 13% and 17% respectively, and the medium OS was 8.4 months and 6.2 months respectively. Compared with chemotherapy, nivolumab monotherapy improves OS, and can reduce the occurrence of new intracranial lesions after brain metastases.
A multicenter study reviewed data from patients treated with nivolumab to evaluate the link between of metastatic foci in a real-world setting and the efficacy of nivolumab. Among 201 patients, 51 (25.4%) had cerebrum metastasis. No data were available regarding encephalic progress, prior radiotherapy, symptoms, or corticosteroids used in these patients. Despite the limitations of the study, it is worth noting that 25% of treated patients experienced BMs. Poor PS was correlated with poor prognosis, but encephalon metastases were correlated with poor outcome. In a similar study, poor PS was associated with poor clinical outcome; however, conflicting results demonstrated that CNS metastases were associated with poor prognosis.40 Data on intracranial activity and safety in NSCLC patients with metastatic encephaloma treated with nivolumab were collected in a multicenter retrospective study conducted in France. Forty-three patients with brain metastasis were included, including 37% with abnormal encephalic diseases. Brain tumor activity and extracranial efficacy were similar, with acceptable toxicity.41 To date, studies have shown that nivolumab can provide sustained survival benefits to patients with NSCLC with brain metastases, validating its preferred choice as second-line treatment.42
SCLC
An exploratory analysis of tumor mutational burden (TMB) reported by World Conference on Lung Cancer (WCLC) recruited 211 evaluable SCLC patients with TMB, including 69 with low, 69 with medium, and 73 with high TMB.43 The ORR of high TMB patients treated with nivolumab was 21.3%, the PFS was 1.4 months, the ORR of combined treatment was 42.6%, and the PFS was 7.8 months. In a single drug cohort, the median OS of low, medium, and high TMB was 3.1, 3.9, and 5.4 months, respectively. In the combined group, the median OS of low, medium, and high TMB was 3.4, 3.6, and 22.0 months, respectively. This study evaluated the role of TMB in SCLC and its relationship with prognosis after PD-1 blockade and found that use of nivolumab monotherapy was more effective in patients with high TMB compared medium or low TMB.
Studies have reported an association between high TMBs and increased response to PD-1 pathway blockade in cancer patients. In NSCLC patients treated with pembrolizumab, a higher burden of non-synonymous mutations in tumors was found to be correlated with improved OS, sustained clinical benefit, PFS, and neoantigen-specific CD8+ T cell responses parallel with tumor regression.44 Similarly, whole-exome sequencing analysis showed that high TMB load was associated with increased numbers of TILs and improved survival.45 In December 2017, the FDA approved a laboratory test known as Foundation One for use to assess a patient’s TMB status. This is a new generation of solid tumor fluid biopsy test that has been used in clinical trials to predict the response of anti-PD-1/PD-L1 therapy in various cancer types.46–49
PD-L1 expression of in in situ tumors may not represent expression in intracranial tumors.25 The KEYNOTE-010 study reported 49 patients with brain metastases from lung cancer who remained immunoresponsive after withdrawal. While most patients had a history of smoking and strong expression of PD-L1 in the in situ tumor, 17% were non-smokers, and 39% with brain metastasis of NSCLC with weakly positive expression of PD-L1 received lasting survival benefits. These findings indicate that TMB may be a more suitable predictor of immune treatment response of SCLC than PD-L1 expression.
The CheckMate-032 study was a phase I/II open-label clinical study that evaluated the safety and efficacy of nivolumab monotherapy for advanced or metastatic SCLC. The study showed that 7 of 20 treated patients had partial remission, with an ORR of 35% and a medium remission time of 8.6 weeks.50 While this was an early clinical study, use of nivolumab alone is currently widely accepted as the preferred treatment for brain metastasis of SCLC, based on the National Comprehensive Cancer Network (NCCN) guideline recommendations and the poor efficacy of the second-line drug topotecan. Despite a lack of systematic evaluation comparing immunotherapy with other treatment options, these results are significant since potential effective treatment options other than immunotherapy are still at the clinical trials stage.
These findings show that second-line treatment nivolumab is effective in the treatment of brain metastasis of NSCLC and SCLC. In future, brain metastasis maintenance treatment will be further enriched with nivolumab and treatment may include patients with brain metastasis of lung cancer at all stages.
Atezolizumab
NSCLC
Atezolizumab is a human engineered monoclonal antibody against the IgG1 PD-L1 isotype. Studies have convincingly shown that atezolizumab could increase the life expectancy of patients with brain metastases. The FIR study51 investigated the efficacy of atezolizumab in the treatment of local progression of PD-L1 expression or metastatic NSCLC. This study includes three cohorts, includes one with more than two lines. Thirteen patients with brain metastasis receiving treatment are enrolled, with an ORR of 23%, medium PFS, and OS of 4.3 months and 6.3 months, respectively. These outcomes indicate that patients with asymptomatic intracranial metastatic tumors of NSCLC may benefit from treatment using atezolizumab alone.
The OAK study52 was a Phase III clinical trial comparing atezolizumab versus docetaxel to treat late-stage NSCLC. The atezolizumab group and docetaxel group included 38 and 47 asymptomatic patients with stable metastatic encephaloma after previous local injection, respectively. Compared with docetaxel, atezolizumab reduced disease progress by 39%, and fatalities by 45% in patients with brain metastasis in the POPLAR study.53 In asymptomatic patients with stable brain metastases who had received previous local treatment, atezolizumab showed significant benefits to OS compared with chemotherapy.
Based on these findings,52 the FDA approved atezolizumab for patients with brain metastatic NSCLC who previously underwent unsuccessful chemotherapy or targeted drug treatment due to its good efficacy and safety that it was unaffected by tissue type and PD-L1 expression. In the PD-L1 ≥1% group, median survival was increased by 5.4 months (15.7 months vs 10.3 months). In patients with low or no expression of PD-L1, the medium subsistence rate was extended by 3.7 months (12.6 months vs. 8.9 months). Therefore, atezolizumab may represent a good choice for patients with metastatic lung cancer as its actions do not depend on expression levels of PD-L1, and it can prolong patient survival to a greater extent than chemotherapy.
SCLC
Patients with SCC and NSCLC showed similar survival benefits. The PCD4989g study54 was a Phase I study that investigated atezolizumab treatment for diverse solid tumors containing SCLC. This study included 17 SCLC patients with brain metastasis, 65% of whom had received third-line or above treatment. The median PFS was 1.5 months (95% CI: 1.2–2.7) and a median OS of 5.9 months (95% CI: 4.3–20.1). This study demonstrated that atezolizumab improved ORR and PFS in patients with SCLC brain metastasis and was well tolerated.
Preliminary outcomes highlight good tolerance of atezolizumab in the treatment of metastatic SCLC, and the efficacy of its use as a single drug is encouraging. However, fewer cases were included in this study; therefore, more data are needed to support its use in clinical practice. The IMPower-133 study was a stage III study that assessed the combined use of atezolizumab and chemotherapy (carboplatin plus etoposide) compared with chemotherapy alone for cerebral metastasis in SCLC patients treated without the efficacy and safety. A total of 403 patients were recruited, and the primary endpoints of the study included PFS and OS of the patients. Medium follow-up was 13.9 months, and atezolizumab significantly extended OS, with a median PFS of 5.2 and 4.3 months for the atezolizumab and control groups, respectively. The atezolizumab group maintained control of the disease for longer than the control group, representing a milestone in the use of immunotherapy of SCLC with brain metastases. Impower-133 became the first study for more than 20 years to show that first-line treatment significantly improved OS using immune combination therapy for brain metastases in a stage III SCLC clinical trial. This treatment will likely become a new standard for the treatment of cerebral metastasis in patients with SCLC. Twenty-seven patients with BMs, four lanthanic and untreated and 23 patients in a stable condition who had received brain radiotherapy were analyzed in a combination of four studies using atzolizumab as second-line or above treatment. The morbidity of therapy-correlated neuro-untoward effects was 9% in the non-BMs group and 15% in the BMs group, suggesting atzolizumab was tolerated.55 The IMPower-133 study56 showed that treatment of atezolizumab with carboplatin and etoposide remarkably improved OS and PFS compared with carboplatin and etoposide alone. IMpower133 was the first study in more than 30 years to show significant clinical improvement of OS compared with the current first-line standard treatment regimen. Atezolizumab became the first approved immunological drug for first-line treatment of SCLC, introducing the use of immunotherapy for SCLC. A study conducting subgroup analysis on Japanese patients obtained similar results.57 However, the clinical benefits of atezolizumab for Chinese patients remain unclear.
Atezolizumab is effective in the treatment of brain metastasis of lung cancer. However, the cost of using atezolizumab is quite high. Therefore, it is necessary to further optimize the drug composition and reduce the cost to enable more patients with lung cancer and brain metastasis to receive treatment.
Durvalumab
NSCLC
Durvalumab is a PD-L1 antibody that was approved by the FDA in May 2017 through an accelerated approval process for the therapy of advanced metastatic bladder cancer. The therapeutic effect of durvalumab in the treatment of stage 3 NSCLC was verified by a stochastic steerable clinical study that included 713 patients with stage 3 NSCLC. Some patients had brain metastasis and the disease did not deteriorate temporarily after chemotherapy and radiotherapy. There was no significant tumor growth (median) for 16.8 months after durvalumab compared with 5.6 months for the placebo group. Globally, around 30% of patients with NSCLC have stage 3, and the slow disease progression strategy for stage 3 unrespectable NSCLC is radiation and chemotherapy. In 2017, the European Society of Oncology Conference reported the result of the PACIFIC stage III clinical trial,58 in which the PFS of the experimental group was 11.2 months, and there was no obvious increase in incidence of adverse events. The FDA approved the use of durvalumab for unrespectable NSCLC. Patients who received radiation and chemotherapy after stage 3 NSCLC disease did not progress, indicating that this immune therapy is the first drug to slow the progression of NSCLC. Since 89% of stage 3 NSCLC patients will experience progression of brain metastasis, treatment that slows the disease progression is crucial.
SCLC
In 2018, ASCO published the results of the NCT01693562 study,59 in which durvalumab was used as monotherapy in patients with terminal stage SCLC brain metastasis. A total of 21 patients (20 treated) with extensive stage SCLC with metastatic brain tumors received treatment with durvalumab 10 mg/kg, once every two weeks. The ORR was 9.5%, PR was observed in two cases, and DCR was seen in 14.3%. Among the two effectively treated patients, the curative effect lasted for 14.6 months in one case of initial treatment, and more than 25.5 months in the other case (after platinum resistant third-line chemotherapy). The medium PFS was 1.5 months, the PFS ratio was 14% at 12 months, the medium OS was 4.8 months, and the OS ratio at 12 months was 27.6%. Although this was a small sample study, the results highlight the initial curative effect of durvalumab in patients with SCLC brain metastases.
Durvalumab not only outperforms in survival data, but is also easier to use clinically. The occurrence of immunotherapy-related adverse reactions also increases its difficulty to use in clinical treatment.
Avelumab
Avelumab is a humanized monoclonal antibody against PD-L1. In March 2017, the FDA approved the PD-L1 antibody avelumab to treat a rare form of skin cancer known as Merck cell cancer. No data have been published regarding the use of avelumab in the treatment of brain metastasis from NSCLC or SCLC, as most studies have adopted a combined strategy. The PAVE study (NCT03568097; https://www.clinicaltrials.gov/) used avelumab combination chemotherapy for patients with advanced SCLC as first-line treatment of single arm open tag stage II study, including 55 cases with cerebral metastatic tumors from SCLC.
The result will bring the first-line therapy for brain metastases from SCLC to new ideas. First-line application of avelumab alone may show clinical activity and good tolerance in patients with advanced brain metastasis of lung cancer and indicate that avelumab may be equivalent to current standard treatment. Table 1 summarizes the clinical trials involving PD-1/PD-L1 inhibitor monotherapy for brain metastasis of lung cancer.
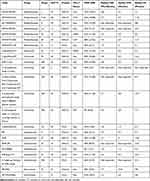 |
Table 1 The Efficacy of PD-1/PD-L1 Inhibitor Monotherapy in Patients with Brain Metastasis of Lung Cancer |
Application of PD-1/PD-L1 Inhibitors Combined with Other Therapeutic Methods in Brain Metastasis of Lung Cancer
PD-1/PD-L1 Inhibitors Combined with Chemotherapy
NSCLC
Treatment using PD-1/PD-L1 inhibitors combined with chemotherapy has a higher clinical response rate than chemotherapy alone. In May 2017, the FDA authorized use of pembrolizumab combined with pemetrexed plus carboplatin for first-line therapy of previously untreated NSCLC patients with intracranial metastases based on findings from the KEYNOTE-021 clinical trial.60
Use of atzolizumab was approved by the FDA as a second-line therapy based on the findings from a phase II clinical trial, and was verified in the OAK III clinical trial. Compared with docetaxel, atzolizumab showed improved efficacy in advanced and metastatic NSCLC.52,61 Subgroup analysis of the OAK trial revealed that 85 patients with asymptomatic and stable BM showed a medium OS improvement of 20.1 months in patients treated with atazolizumab, which was 11.9 months more than that seen in patients treated with docetaxel.62 Atzolizumab improved PFS and OS compared with bevacizumab and carboplatin-taxol chemotherapy, as a first-line therapy for patients with metastatic non-squamous NSCLC without epidermal growth factor receptor (EGFR) or ALK mutations.63 However, this was a large multinational study and only evaluated the efficacy of atzolizumab compared with bevacizumab combined with chemotherapy. A retrospective cohort study comparing use of carboplatin in combination with pemetrexed or without pembrolizumab revealed the potential benefits of the use of pembrolizumab for patients with BMs.64 Since few patients showed significant PD-L1 expression in this study, it was not possible to estimate the impact of PD-L1 expression on PFS and OS. Furthermore, although the study found better outcomes in brain metastases treated with pembrolizumab combined with chemotherapy, the effect was not statistically significant due to the small sample size.
The medium analytic results of the IMPower-131 clinical trial that investigated use of atezolizumab combined with chemotherapy for first-line treatment of advanced SCC-type NSCLC patients with brain metastasis and the results were released at the meeting of the ASCO (http://abstracts.asco.org/214/AbstView_214_214607.html). Combined used of chemotherapy reduced the coefficient of disease progression or mortality rate by 29%, and the 1-year PFS rate doubled (24.7% vs 12.0%). These findings applied also to PD-L1-negative tumors and patients with tumor metastasis. The benefits to patients with superior PD-1 expression increased in a stepwise manner, suggesting that although the combined treatment effect was generally better than single drug chemotherapy, patients with high expression of PD-L1 still benefited.
SCLC
The CheckMate-331 study involving 803 patients compared the head-to-head efficacy of nivolumab with topotecan or amrubicin for SCLC with brain metastases after first-line treatment. Patients were randomly divided into two cohorts and received intravenous treatment with nivolumab, topotecan, or amrubicin. Primary endpoints were OS after 12 months of treatment, and secondary endpoints were PFS and ORR. The trial was declared a failure in 2018. The results showed that nivolumab did not significantly extend the OS and failed to reach the primary efficacy endpoint of the study, compared with relapsing SCLC with second-line standard topotecan or amrubicin chemotherapy. The biosecurity of nivolumab in this study was in accordance with the findings from previous single drug studies of SCLC patients with brain metastases. It remains unclear whether PD-L1 expression in the tumors of the patients participating in the study was an influential factor.
In August 2018, the FDA approved nivolumab for use in brain metastatic SCLC treated with platinum chemotherapy and at least one other therapy. This was granted based on the CheckMate-032 study ORR (12%) and DOR (17.9 months) findings, and nivolumab became a new approved treatment for SCLC with brain metastasis.
PD-1/PD-L1 Inhibitors Combined with Radiotherapy
Localized radiotherapy is the standard treatment for brain metastasis. The KEYNOTE-001 trial evaluated pembrolizumab monotherapy with advanced NSCLC as a phase I study. The study used radiotherapy from the immune therapy single center secondary data analysis and found,65 42 patients underwent radiotherapy, 38 patients with extracranial radiation therapy, four patients underwent cranial radiation therapy, and 55 patients did not receive radiotherapy, radiotherapy in patients with and without radiotherapy in patients with a median PFS were 4.4 and 2.1 months (P = 0.019), the median OS was 10.7 months and 5.3 months, respectively (P = 0.026). Combined with radiotherapy can increase PFS and OS in patients with advanced NSCLC, and immunotherapy combined with radiotherapy plays a synergistic role in NSCLC. Limitations of the study included inability to obtain complete details about radiation dose, grading, and planning for all patients, which limited further analysis, and the PD-L1 status was not available for all patients. Although was a single institution study, this patient group did not appear significantly different from other study groups.
It is unclear whether a combination of immunotherapy and radiotherapy further improves efficacy. Studies have found that radiotherapy promotes the release of various factors in tumors and surrounding tissues, leading to immunogenic death of tumor cells, activating APCs, releasing various cytokines, activating T cells, and promoting the immune system to attack tumor cells.66
A previous retrospective study67 included 17 cases treated with PD-1/PD-L1 inhibitors and stereotactic radiotherapy therapy for NSCLC patients, starting from the radiation of OS 5.6 months, starting from the diagnosis of OS 17.9 months. PD-1/PD-L1 antibodies before or patients with stereotactic radiosurgery (SRS) in the treatment and after treatment for six months in patients with intracranial ORR of SRS were 57% and 0%, respectively. Therefore, PD-1/PD-L1 antibody treatment with adjuvant radiotherapy, especially before treatment or with SRS, may help to control CNS metastasis. This study was the first to report the use of stereotactic radiation and anti-PD-1/PD-L1 in patients with brain metastases of NSCLC; however, this report was ignored due to inherent defects of the retrospective analysis and the heterogeneity of the treatment cohort. Prospective testing of combined use of stereotactic radiation and anti-PD-1/PD-L1 therapy may define safety and may brain metastasis and OS.
A retrospective study68 evaluated the safety of 163 patients with intracranial radiotherapy for brain metastasis of NSCLC, among which 50 patients received PD-1/PD-L1 antibody treatment. In contrast to intracranial radiotherapy, the morbidity of intracranial radiation toxicity was similar, suggesting intracranial radiotherapy combined with PD-1/PD-L1 antibody therapy was safe, and feasible for patients with brain metastasis. Further prospective studies are needed to determine the best timing for the use of this combination therapy and determine its safety and tolerability.
Another study into the effects of immunotherapy combined with radiotherapy on extracranial lesions in patients with lung adenocarcinoma69 suggested that immunotherapy increased the chance of distant effects in patients with low immunogenicity primary tumors with brain metastases after radiation therapy. Since this clinical study only included one case and the evaluation method was relatively unique, further verification of this finding is required.
Radiation “burn” of tumor cells can be viewed as a type of in situ tumor vaccine that stimulates the immune system to recognize tumor cells and promote the immune cells to attack tumor cells. Combining this treatment with PD-1 antibodies may achieve twice the result with half the effort, which is the theoretical basis of radiotherapy combined PD-1 antibodies.
PD-1/PD-L1 Inhibitors in Combination with Other Immune Checkpoint Inhibitors
NSCLC
Several studies have shown that PD-1 inhibitor coupled with CTLA-4 inhibitor has a more effective treatment outcome on brain metastasis of NSCLC. Since the dual immune blocking mode was approved by the FDA for malignant melanoma and renal cell cancer in 2011 and 2017, respectively, studies into therapy of metastatic encephaloma among lung carcinoma patients have been performed. Currently, the main modes of dual immune blocking include PD-1/PD-L1 inhibitor in combination with CTLA-4 inhibitor and PD-1/PD-L1 inhibitor combined with lung cancer vaccine. However, no studies have investigated the use of PD-1/PD-L1 inhibitors combined with CTLA-4 inhibitors.70 Since PD-L1 and CTLA-4 target the activation and effect stages of immune regulation, respectively,71 blocking these two key points simultaneously may lead to unexpected effects. The CheckMate-227 clinical study compared the therapeutic effects of nivolumab combined with ipilimumab and chemotherapy in NSCLC patients. The main data reported at present indicate that combined use of nivolumab and ipilimumab had a relatively high safety, especially for patients with high tumor load. PD-L1 expression levels were not related to high tumor load.72 One of the highlights of this clinical study was the first prospective use of tumor mutation load as a biomarker for exploratory analysis. While data from the CheckMate-227 study are not sufficient to define the role of TMB, they provide an important basis for subsequent clinical studies.
SCLC
The CheckMate-032 clinical study evaluated the therapeutic effect and biosecurity of nivolumab alone or nivolumab combined with ipilimumab to treat metastatic SCLC.73 In this study, 216 SCLC patients with brain metastasis after treatment were enrolled and divided into four groups. The first group received nivolumab, while the other groups received different doses of nivolumab combined with ipilimumab. The primary endpoint of this study was ORR. Secondary endpoints included OS, PFS, and biomarkers. Nivolumab combined with ipilimumab showed favorable efficacy and tolerance in the early treatment group, which was unrelated to the expression of PD-L1 or the sensitivity of patients to platinum. These findings prompted long-term follow-up this of cohort as well as a randomized amplified cohort study in SCLC patients with brain metastases to further evaluate the efficacy of nivolumab combined with ipilimumab.
The NCCN adopted nivolumab ± ipilimumab as a recommended second-line treatment for metastatic SCLC in the first edition guidelines published in 2017.74 Subsequently, ASCO reported the results of the CheckMate-012 study in 2017,75 in which 247 patients were randomly assigned to either nivolumab or nivolumab combined with ipilimumab groups. Nivolumab and nivolumab combined with ipilimumab showed long-lasting effects in previously treated SCLC patients. These findings confirmed that nivolumab combined with ipilimumab may be an alternative treatment for metastatic SCLC. One limitation of this study is that it was not designed to directly compare the safety and efficacy of treatment regimens, had a small sample size, and lacked stratification of relevant baseline characteristics. Another limitation is that since these treatments were not randomized, only indirect comparisons could be made between the nivolumab plus ipilimumab group and the previously reported nivolumab alone or nivolumab plus chemotherapy combination groups.
The results of the NCT02261220 study reported the safety and efficacy of durvalumab combined with tremelimumab (CTLA-4 antibody) in the treatment of metastatic SCLC (abstract number 8517, http://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.8517). Thirty patients with previous systematic treatment were included in the study. The confirmed ORR was 13.3%, the median remission duration was 18.9 months, the medium PFS was 1.8 months, the medium OS was 7.9 months, and the median OS was 41.7% at 12 months. In terms of safety, 20 patients (67%) reported ≥1 case of adverse events or treatment-related adverse events (TRAEs). The most common adverse events were tiredness (n = 7, 23%) and itching (n = 7, 23%), and seven patients (23%) had level 3/4 TRAE. No patients died due to of TRAE withdrawal or treatment-related death. Considering the efficacy and safety, the combination of dual immunity could be a therapy scheme for patients with SCLC brain metastasis.
Therefore, doubling the therapeutic effects led to doubling of the side effects. Grade 3/4 adverse reactions occurred in 55% of patients receiving combined therapy, compared with 27%, and 16.3% for single therapy using durvalumab or tremelimumab, respectively. However, treatment using CTLA-4 with PD-1 inhibitor did not increase immune-related adverse reactions. Therefore, it is suggested that CTLA-4 inhibitor should applied first in clinical practice, followed by PD-1 inhibitor when adverse reactions are weakened.
PD-1/PD-L1 Inhibitors in Combination with Molecular Targeted Drugs
NSCLC
Recent studies have shown conclusively that molecular targeted drugs can significantly improve the inhibitory rate of PD-1/PD-L1 inhibitors on brain metastasis of lung carcinoma. Around 50% of Asian NSCLC patients with brain metastasis with mutations in the EGFR gene.76 This gene mutation can upregulate the expression of PD-L1 in lung carcinoma cells, while PD-1/PD-L1 inhibitor can reduce the expression of PD-L1 in mutant lung cancer cells and reduce their ability to metastasize.77,78 This demonstrates the potential use of PD-1/PD-L1 inhibitor combined with EGFR-tyrosine kinase inhibitor to treat brain metastasis of pulmonary carcinoma.
A clinical study using nivolumab combined with erlotinib in the treatment of patients with late-stage NSCLC metastasis showed the efficacy of NCT01454102.75 It is unclear whether the combination of PD-1/PD-L1 antibody can achieve twice the result with half the effort if PD-L1 expression is downregulated by EGFR-tyrosine kinase inhibitor. The vascular endothelial growth factor pathway not only promotes tumor angiogenesis, but also promotes immune evasion of tumor cells by transmitting inhibitory immune signals. Bevacizumab increases the tumor-killing effect of atezolizumab-activated T cells by reversing the immune suppression mediated by vascular endothelial growth factor. The IMPower-150phase III clinical trial assessed the use of atezolizumab plus carboplatin and paclitaxel in combination with chemotherapy or combined bevacizumab bead sheet resistance (groups B and C) on the scale of initial IV curative effect and safety of NSCLC patients with metastatic encephaloma, also into the group of patients for the treatment of biopsy specimen (http://www.abstractsonline.com/pp8/#!/4562/presentation/11130).
According to data updated by the ASCO, the OS of group B was better than that of group C in control patients. The total survival time of group A (bevacizumab plus chemotherapy) was longer than that of group C, but the diversity was not statistically remarkable. Patients with high expression of PD-L1 and brain metastasis had obvious benefits. In patients with EGFR+/ALK+, PFS was better in group B than in group C, and the medium OS was expected to be extended by 12.5 months. Most clinical studies using combined immunotherapy with first-line therapy exclude EGFR+/ALK+ patients with brain metastasis of lung cancer, whereas the IMpower150 study analyzed the efficacy of EGFR+/ALK+ patients with brain metastasis of lung cancer, which is a major feature. However, further studies are required to investigate whether these patients are suitable for first-line immunotherapy. This study is unique in that it did not exclude patients with a positive driver gene, and explored tumor immunotherapy in this population. The most significant benefit (HR = 0.39) was observed in patients with high expression levels of PD-L1, and the PFS was 12.6 months, which was almost doublecompared to patients with low PD-L1expression (2018 AACR). However, PFS was not significantly prolonged when compared with that seen with pembrolizumab alone in the KEYNOTE-024 study, suggesting that use of pembrolizumab alone may be sufficient when PD-L1 TPS ≥ 50%, although no prospective study has been conducted to answer this question.
SCLC
In 2017, WCLC published the results of the MEDIOLA study (https://library.iaslc.org/search-speaker?search_speaker=49493), which was a basket study of durvalumab combined with PARP inhibitor olaparib in the treatment of multi-tumor species. A total of 38 patients with encephalic metastasis from SCLC were included in the study, including one patient with PR, one patient with CR, and 29% with DCR at 12 weeks. It is suggested that durvalumab plus olaparib in the treatment of brain metastasized SCLC is well tolerated and may have long-term clinical benefits. In conclusion, PD-1/PD-L1 inhibitors combined with targeted drugs have been shown to significantly improve the response rate of patients, and combined used with anti-angiogenic targeted drugs in clinical has shown a positive effect. Table 2 summarizes the clinical trials involving PD-1/PD-L1 inhibitors combined with other therapeutic methods for brain metastasis of lung cancer.
 |
Table 2 The Efficacy of PD-1/PD-L1 Inhibitors Combined with Other Therapies in Patients with Brain Metastasis of Lung Cancer |
Potential Immunotherapy Markers
Use of PD-1/PD-L1 antibodies for the treatment of intracranial metastatic tumors also requires screening of suitable populations to identify ideal markers. Studies have reported that PD-L1 expression is related to the effect of immunotherapy. It is not clear whether PD-L1 expression can predict the effect of immunotherapy on CNS metastasis. A study on pembrolizumab treatment of brain metastasis in NSCLC and melanoma26 aimed to treat NSCLC patients before any parts of the tumor tissue became PD-L1 positive (≥1%). The study found that response rates of intracranial lesions were consistent with the response rates of extracranial lesions; however, it remains unclear whether PD-L1 expression in extracranial lesions can be used as a marker to screen patients with CNS metastasis for immunotherapy. Previous studies also suggest that there are differences in immunophenotypes between primary pulmonary and brain metastases. A retrospective analysis of 11 matched samples of primary and intracranial metastases in NSCLC patients with EGFR mutation negative59 revealed that the expression inconsistency ratio of PD-L1 in matched specimens was 36.3%. It can be seen that extracranial lesions cannot completely replace CNS metastatic foci to screen patients for immunotherapy, but tumor tissues of CNS metastatic foci are difficult to obtain; therefore, it is necessary to find ideal replacement specimens for immune microenvironment of CNS metastatic foci.
Use of PD-1/PD-L1 antibodies has been successful in the treatment of metastatic encephaloma from NSCLC. However, there is some evidence to indicate that only 20% of unselected patients with brain metastasis respond to treatment.79 Therefore, accurate selection of patients who respond to treatment with PD-1/PD-L1 inhibitors is crucial, and is even more important in the development of combined treatment with PD-1/PD-L1 inhibitors. PD-L1 expression and high tumor load are currently relatively certain markers, but are unstable and imperfect predictors.
In addition to PD-1 and PD-L1, immunotherapy markers also include TIM-3, LAG-3, TIGIT, and TILs. It was reported that TIM-3 is highly expressed in human GBM cells and T cells of drug-resistant animals treated with anti-PD-1, which plays an important role in the process of immune tolerance and elimination of apoptotic cells.80 Studies have shown that LAG-3 negatively regulates T cell proliferation and long-lasting memory. Once activated by its ligand, it can promote tumor cells to escape from the immune system and accelerate the occurrence and development of metastatic tumors of the nervous system.81 TIGIT can suppress the role of immune cells in cancer immunotherapy in multiple stages, increasing the probability of brain metastasis.82,83 Drugs enter the body and kills brain metastases by concentrating the superior forces of T cells on a limited number of TILs instead of increasing the total number. Therefore, the number of TILs in brain metastases may not be a marker of immunotherapy. Due to limited relevant studies, it is not clear whether TIM-3, LAG-3, TIGIT, and TILs can be used as markers.
Limitations
Limitations of PD-L1 as a Biomarker
Although expression of PD-L1 protein is the most commonly used predictive marker of immune efficacy at present, it still has limitations.84 Immunohistochemical methods are commonly utilized to detect PD-L1 expression in the clinical studies of the above-mentioned PD-1/PD-L1 inhibitors. Nevertheless, due to commercialization of the platform, there are differences in the detection reagents used.
Detection reagents such as IHC28-8, IHC22C3, SP142, and SP263 differ greatly in staining mode and intensity, and are greatly affected by the fixation method, storage mode, and antigen repair of the detected tissues, which makes it difficult to systematically analyze the divinable value of the efficacy of PD-L1.33,34,52,61,85–87 Meanwhile, the predictive value of PD-L1 expression on different PD-1/PD-L1 suppressants and different NSCLC pathological types may differ.33,34 According to different immune checkpoint inhibitors and different pathological types, individualized determination of PD-L1 positive threshold may represent a solution. However, even in tumor tissues from the same patient, PD-L1 expression is still heterogeneous in time and space.88 During the course of disease development, PD-L1 expression has potential fluctuations and is affected by other factors such as previous chemotherapy or radiotherapy.
Spatially, the expression levels of PD-L1 vary in the primary tumor and metastasis of the same patient.89 A previous study compared the expression of PD-L1 in primary and metastatic NSCLC patients, and showed that the consistency was only 20.8% in patients with PD-L1 expression, ranging from 1% to 50%.89 Even due to the sub clonal polymorphism of the tumor, there may be heterogeneity in the expression of PD-L1 in different parts within the primary or metastatic foci.90 As a dynamic biomarker, the expression levels of PD-L1 limits its reliability and repeatability. Finally, studies11,91 have shown that only PD-L1 expressed in the cell membrane has biological significance, via either dynamic IFN-γ expression or activation of constitutive oncogene. Contrary to oncogene-mediated PD-L1 expression, PD-L1 expression induced by IFN-γ represents a dynamic biomarker, and appears in active inflammatory sites. Therefore, it is more reasonable to analyze the connection between PD-L1 protein on cell membrane and clinical prognosis than intracellular PD-L1 protein or mRNA.
Limitations of Inhibitors in the Treatment of Lung Cancer with BMs
The limitations of PD-1/PD-L1 inhibitors in patients with brain metastasis of lung cancer can be roughly divided into three points. First, the study found that some patients with positive expression of PD-L1 in tumor and positive expression of PD-1 in T cells were not significantly effective after treatment with PD-1/PD-L1 inhibitors, possibly due to a lack of TNF-α mediated inflammation in their brain tumors. PD-1/PD-L1 inhibitors had little effect when TNF-α expression was low in patients with tumors without inflammatory infiltration.92 Second, whether a patient received radiotherapy prior to taking the drug is crucial to the efficacy of the PD-1/PD-L1 inhibitors. A previous clinical study indicated that patients who had not received radiation before treatment with PD-1/PD-L1 inhibitors had a 44% increased risk of disease progression and a 42% increased risk of death compared with those who received radiation.93 Similarly, a follow-up study of the KEYNOTE-001 trial found that patients who continued to be treated with PD-1/PD-L1 inhibitors after radiation therapy had almost double the PFS and OS compared with those who had not been treated directly with the inhibitor.94 Third, a clinical trial showed that PD-1/PD-L1 inhibitors were less effective in patients with NSCLC who had not smoked.95 Patients with a history of smoking had an ORR of 46% (effective in 30 of 65 patients), compared with 27% (effective in 3 out of 11 patients) for patients without a history of smoking. This is because cancer cell mutation rates are higher in smokers than in non-smokers, and cancer cell mutation rates are generally better in smokers. These limitations may provide two suggestions for clinical practice. First, patients should be tested for TNF-α gene and protein levels in brain metastatic tumors prior to receiving PD-1/PD-L1 immunotherapy. Second, patients should undergo radiotherapy prior to receiving PD-1/PD-L1 immunotherapy, not only to improve the cure rate but also to prolong the survival period.
Conclusions
PD-1/PD-L1 immunotherapy is the most concerned method of tumor treatment for patients with advanced brain metastasis. There is increasing evidence to support the use of PD-1/PD-L1 suppressants in the treatment of brain metastasis of pulmonary carcinoma. In general, use of a single PD-1/PD-L1 inhibitor is significantly superior to standard chemotherapy in second-line therapy, and has become the standard treatment for advanced NSCLC brain metastasis after first-line chemotherapy failure. Pembrolizumab has an advantage over other medicines for first-line treatment. In terms of combined therapy, PD-1/PD-L1 suppressants plus chemotherapy have obvious advantages, and combined targeted therapy has great potential. Dual immune blocking mode has the most obvious effect on patients with brain metastasis, and other immune combinations need to be explored in future studies.
Traditionally, patients with metastatic encephaloma have been excluded from clinical trials, which is not conducive to our understanding of the systematic treatment of CNS metastases. A systematic analysis of late-stage NSCLC interventional drug trials formulated on www.ClinicalTrials.gov showed that only 26% of patients received non-targeted treatment for cranial metastases.96 A phase II clinical study (NCT02085070) into use of keytruda in the treatment of melanoma brain metastases reported that keytruda97 was effective for brain metastases, and MRI analysis showed transient pseudo-progression at the beginning, and pathologically confirmed that there were a few tumor cell clusters in the metastases, accompanied by hemorrhage, reactive stellate cells, inflammatory cells, and microglia cells. In 2016, early data from keytruda treatment of patients with melanoma brain metastasis and NSCLC brain metastasis at the beginning of treatment26 showed effective rates of 22% and 33%, respectively, and a lasting effect of immunotherapy. Therefore, systemic immunotherapy is effective for patients with initial treatment or advanced brain metastasis.
In clinical practice, most patients with brain metastases eventually die as a result of systemic disease progression rather than uncontrolled intracranial lesions. Therefore, treatment of metastatic encephaloma of NSCLC patients is coordinated with systemic treatment. It is believed that a better understanding of PD-1 immunotherapy will benefit NSCLC patients with brain metastasis.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Liang H, Song X, Zhang Y, et al. Real-world data on EGFR/ALK gene status and first-line targeted therapy rate in newly diagnosed advanced non-small cell lung cancer patients in Northern China: a prospective observational study. Thorac Cancer. 2019;10(7):1521–1532. doi:10.1111/tca.2019.10.issue-7
2. She C, Wang R, Lu C, et al. Prognostic factors and outcome of surgically treated patients with brain metastases of non-small cell lung cancer. Thorac Cancer. 2019;10(2):137–142. doi:10.1111/tca.2019.10.issue-2
3. Tsakonas G, Petris LD, Ekman S. Management of brain metastasized non-small cell lung cancer (NSCLC) – from local treatment to new systemic therapies. Cancer Treat Rev. 2017;54:122. doi:10.1016/j.ctrv.2017.02.004
4. Hubbs JL, Boyd JA, Donna H, Chino JP, Mert S, Kelsey CR. Factors associated with the development of brain metastases: analysis of 975 patients with early stage nonsmall cell lung cancer. Cancer. 2010;116(21):5038–5046. doi:10.1002/cncr.25254
5. Barlesi F, Gervais R, Lena H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01). Ann Oncol. 2011;22(11):2466–2470. doi:10.1093/annonc/mdr003
6. Johnson JD, Young B. Demographics of brain metastasis. Neurosurg Clin N Am. 1996;7(3):337. doi:10.1016/S1042-3680(18)30365-6
7. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. Cancer Treat Res. 2007;136(1):1.
8. Jindal V, Gupta S. Expected paradigm shift in brain metastases therapy—immune checkpoint inhibitors. Mol Neurobiol. 2018;12:1–7.
9. Hanahan D, Weinberg R. Hallmarks of cancer: the next generation. cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
10. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi:10.1038/nrc3245
11. Herbst RS, Jean-Charles S, Marcin K, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563. doi:10.1038/nature14011
12. Jeffrey W. Immune checkpoint proteins: a new therapeutic paradigm for cancer–preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37(5):430–439. doi:10.1053/j.seminoncol.2010.09.005
13. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi:10.1111/j.1600-065X.2010.00923.x
14. Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29(1):58–69.
15. Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28(1):5–11.
16. Owens T, Bechmann I, Engelhardt B. Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol. 2008;67(12):1113–1121. doi:10.1097/NEN.0b013e31818f9ca8
17. Fecci PE, Heimberger AB, Sampson JH. Immunotherapy for primary brain tumors: no longer a matter of privilege. Clin Cancer Res. 2014;20(22):5620–5629. doi:10.1158/1078-0432.CCR-14-0832
18. Cserr HF, Knopf PM. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol Today. 1992;13(12):507–512. doi:10.1016/0167-5699(92)90027-5
19. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi:10.1038/nature14432
20. Berghoff AS, Fuchs E, Ricken G, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. 2015;5(1):e1057388. doi:10.1080/2162402X.2015.1057388
21. Bradstreet JJ, Ruggiero M, Pacini S. Commentary: structural and functional features of central nervous system lymphatic vessels. Front Neurosci. 2015;9:934. doi:10.3389/fnins.2015.00485
22. Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18(2):123. doi:10.1038/ni.3666
23. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi:10.1056/NEJMoa1501824
24. Kang SP, Gergich K, Lubiniecki GM, et al. Pembrolizumab KEYNOTE-001: an adaptive study leading to accelerated approval for two indications and a companion diagnostic. Ann Oncol. 2017;28(6):1388–1398. doi:10.1093/annonc/mdx076
25. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. doi:10.1016/S0140-6736(15)01281-7
26. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, Phase 2 trial. Lancet Oncol. 2016;17(7):S1470204516300535. doi:10.1016/S1470-2045(16)30053-5
27. Brahmer JR, Rodrã-Guez-Abreu D, Robinson AG, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label Phase 3 trial. Lancet Oncol. 2017. doi:10.1016/S1470-2045(17)30690-3
28. Masters GA, Sarah T, Azzoli CG, et al. Systemic therapy for stage IV non-small-cell lung cancer: american society of clinical oncology clinical practice guideline update. J Oncol Pract. 2017;33(30):832–837.
29. Hossein B, Langer CJ, Shirish G, et al. 24-month overall survival from KEYNOTE-021 cohort G: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for?advanced nonsquamous non–small cell lung?Cancer. J Thorac Oncol. 2019;14(1):124–129.
30. Ott PA, Bang YJ, Piha-Paul SA, et al. T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2019;37(4):318–327. doi:10.1200/JCO.2018.78.2276
31. Di M, Zhang L. Pembrolizumab for non-small cell lung cancer with central nervous system metastases: a two-case report. Thorac Cancer. 2019;10(2):381–385. doi:10.1111/tca.2019.10.issue-2
32. Chung HC, Ros W, Delord JP, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37(17):1470–1478
33. Hossein B, Luis PA, Leora H, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):123–135. doi:10.1056/NEJMoa1504627
34. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi:10.1056/NEJMoa1504627
35. Bidoli P, Chiari R, Catino A, et al. Efficacy and safety data from patients with advanced squamous NSCLC and brain metastases participating in the nivolumab Expanded Access Programme (EAP) in Italy. Ann Oncol. 2016;27(suppl_6). doi:10.1093/annonc/mdw383.28
36. Aaron L, GE B. The Italian nivolumab expanded access program confirms the limitations of single-agent PD-1 inhibition in EGFR -mutant and never-smoking patients with NSCLC. J Thorac Oncol. 2018;13(8):1058–1059. doi:10.1016/j.jtho.2018.06.003
37. Garassino MC, Gelibter AJ, Grossi F, et al. Italian nivolumab expanded access program in nonsquamous non-small-cell lung cancer patients: results in never-smokers and EGFR-mutant patients. J Thorac Oncol. 2018;13(8):1146–1155. doi:10.1016/j.jtho.2018.04.025
38. Dudnik E, Yust-Katz S, Nechushtan H, et al. Intracranial response to nivolumab in NSCLC patients with untreated or progressing CNS metastases. Lung Cancer. 2016;98(4 Suppl):114–117. doi:10.1016/j.lungcan.2016.05.031
39. Goldman JW, Crino L, Vokes EE, et al. P2.36: nivolumab (nivo) in patients (pts) with Advanced (adv) NSCLC and Central Nervous System (CNS) Metastases (mets): track: immunotherapy. J Thorac Oncol. 2016;11(10):S238–S239. doi:10.1016/j.jtho.2016.08.107
40. Manrique MCA, Martínez JM, González JG, et al. Real world data of nivolumab for previously treated non-small cell lung cancer patients: a Galician lung cancer group clinical experience. Transl Lung Cancer Res. 2018;7(3):404–415.
41. Gauvain C, Vauléon E, Chouaid C, et al. Intracerebral efficacy and tolerance of nivolumab in non–small-cell lung cancer patients with brain metastases. Lung Cancer. 2018;116:62. doi:10.1016/j.lungcan.2017.12.008
42. Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol. 2018;44(1):12–21.
43. Hellmann MD, Callahan MK, Awad MM, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018;33(5):853–861.e854. doi:10.1016/j.ccell.2018.04.001
44. Rizvi NA, Hellmann MD, Snyder A, et al; Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi:10.1126/science.aaa1348
45. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi:10.1056/NEJMoa1500596
46. Stadler ZK, Battaglin F, Middha S, et al. Reliable detection of mismatch repair deficiency in colorectal cancers using mutational load in next-generation sequencing panels. J Clin Oncol. 2016;34(18):2141–2147. doi:10.1200/JCO.2015.65.1067
47. Meucci S, Keilholz U, Tinhofer I, Ebner OA. Mutational load and mutational patterns in relation to age in head and neck cancer. Oncotarget. 2016;7(43):69188–69199. doi:10.18632/oncotarget.v7i43
48. de Velasco G, Miao D, Voss MH, et al. Tumor mutational load and immune parameters across metastatic renal cell carcinoma risk groups. Cancer Immunol Res. 2016;4(10):820–822. doi:10.1158/2326-6066.CIR-16-0110
49. Danilova L, Wang H, Sunshine J, et al. Association of PD-1/PD-L axis expression with cytolytic activity, mutational load, and prognosis in melanoma and other solid tumors. Proc Natl Acad Sci U S A. 2016;113(48):E7769–E7777. doi:10.1073/pnas.1607836113
50. Janjigian YY, Bendell J, Calvo E, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;36(28):2836–2844. doi:10.1200/JCO.2017.76.6212
51. Spigel DR, Chaft JE, Gettinger S, et al. FIR: efficacy, safety, and biomarker analysis of a phase II open-label study of atezolizumab in PD-L1-selected patients with NSCLC. J Thorac Oncol. 2018;13(11):1733–1742. doi:10.1016/j.jtho.2018.05.004
52. Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837–1846. doi:10.1016/S0140-6736(16)00587-0
53. Barlesi F, Park K, Ciardiello F, et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. Ann Oncol. 2016;27(suppl_6). doi:10.1093/annonc/mdw435.43
54. Sequist LV, Chiang A, Gilbert J, et al. Clinical activity, safety and predictive biomarkers results from a phase Ia atezolizumab (atezo) trial in extensive-stage small cell lung cancer (ES-SCLC). Ann Oncol. 2016;27:
55. Lukas R, Gandhi M, O’Hear C, Hu S, Lai C, Patel J. P2.03b-014 atezolizumab in advanced NSCLC patients with baseline brain metastases: a pooled cohort safety analysis. J Thorac Oncol. 2017;12(1):S941–S942. doi:10.1016/j.jtho.2016.11.1295
56. Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220–2229. doi:10.1056/NEJMoa1809064
57. Hida T, Kaji R, Satouchi M, et al. Atezolizumab in Japanese patients with previously treated, advanced non–small-cell lung cancer: a subgroup analysis of the phase 3 OAK study. Clin Lung Cancer. 2018;19:e405–e415. doi:10.1016/j.cllc.2018.01.004
58. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919. doi:10.1056/NEJMoa1709937
59. Chih-Hsin Yang J, Shepherd FA, Kim DW, et al. Osimertinib plus durvalumab versus osimertinib monotherapy in EGFR T790M-positive NSCLC following previous EGFR TKI therapy: CAURAL brief report. J Thorac Oncol. 2019;14(5):933–939. doi:10.1016/j.jtho.2019.02.001
60. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497–1508. doi:10.1016/S1470-2045(16)30498-3
61. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi:10.1016/S0140-6736(16)32517-X
62. Gadgeel S, Ciardiello F, Rittmeyer A, et al. PL04a.02: OAK, a randomized Ph III study of atezolizumab vs docetaxel in patients with advanced NSCLC: results from subgroup analyses. J Thorac Oncol. 2017;12(1):S9–S10. doi:10.1016/j.jtho.2016.11.011
63. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):NEJMoa1716948. doi:10.1056/NEJMoa1716948
64. Afzal MZ, Dragnev K, Shirai K. A tertiary care cancer center experience with carboplatin and pemetrexed in combination with pembrolizumab in comparison with carboplatin and pemetrexed alone in non-squamous non-small cell lung cancer. J Thorac Dis. 2018;10(6):3575–3584. doi:10.21037/jtd.2018.06.08
65. Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 Phase 1 trial. Lancet Oncol. 2009;18(7):895. doi:10.1016/S1470-2045(17)30380-7
66. Brooks ED, Schoenhals JE, Tang C, et al. Stereotactic ablative radiation therapy combined with immunotherapy for solid tumors. Cancer J. 2016;22(4):257–266. doi:10.1097/PPO.0000000000000210
67. Ahmed KA, Kim S, Arrington J, et al. Outcomes targeting the PD-1/PD-L1 axis in conjunction with stereotactic radiation for patients with non-small cell lung cancer brain metastases. J Neurooncol. 2017;133(2):1–8. doi:10.1007/s11060-017-2437-5
68. Hubbeling HG, Schapira EF, Horick NK, et al. Safety of combined PD-1 pathway inhibition and intracranial radiation therapy in non-small cell lung cancer. J Thorac Oncol. 2018;13(4):550–558. doi:10.1016/j.jtho.2018.01.012
69. Lin X, Lu T, Xie Z, et al. Extracranial abscopal effect induced by combining immunotherapy with brain radiotherapy in a patient with lung adenocarcinoma: a case report and literature review. Thorac Cancer. 2019;10(5):1272–1275. doi:10.1111/tca.2019.10.issue-5
70. Burg SHVD, Arens R, Ossendorp F, Hall TV, Melief CJM. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer. 2016;16(4):219. doi:10.1038/nrc.2016.16
71. D’Angelo SP, Larkin J, Sosman JA, et al. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35(2):226. doi:10.1200/JCO.2016.67.9258
72. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):NEJMoa1801946. doi:10.1056/NEJMoa1801946
73. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. doi:10.1016/S1470-2045(16)30098-5
74. Ettinger DS, Wood DE, Akerley W, et al. NCCN guidelines insights: non-small cell lung cancer, version 4.2016. J Natl Compr Canc Netw. 2016;14(3):255. doi:10.6004/jnccn.2016.0031
75. Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2016;18(1):31. doi:10.1016/S1470-2045(16)30624-6
76. Shim HS, Lee DH, Park EJ, Kim SH. Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med. 2011;135(10):1329–1334. doi:10.5858/arpa.2010-0493-OA
77. Lin K, Cheng J, Yang T, Li Y, Zhu B. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-κB. Biochem Biophys Res Commun. 2015;463(1–2):95–101. doi:10.1016/j.bbrc.2015.05.030
78. Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3(12):1355–1363. doi:10.1158/2159-8290.CD-13-0310
79. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi:10.1056/NEJMoa1801005
80. Karachi A, Yang C, Dastmalchi F, et al. Modulation of temozolomide dose differentially affects T-cell response to immune checkpoint inhibition. Neuro-Oncology. 2019;21(6):730–741. doi:10.1093/neuonc/noz015
81. Wang J, Sanmamed MF, Datar I, et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell. 2019;176(1–2):334–347.e312. doi:10.1016/j.cell.2018.11.010
82. Gao J, Zheng Q, Xin N, Wang W, Zhao C. CD155, an onco-immunologic molecule in human tumors. Cancer Sci. 2017;108(10):1934–1938. doi:10.1111/cas.2017.108.issue-10
83. Manieri NA, Chiang EY, Grogan JLTIGIT. A key inhibitor of the cancer immunity cycle. Trends Immunol. 2017;38(1):20–28. doi:10.1016/j.it.2016.10.002
84. Festino L, Botti G, Lorigan P, et al. Cancer treatment with anti-PD-1/PD-L1 agents: is PD-L1 expression a biomarker for patient selection? Drugs. 2016;76(9):925–945. doi:10.1007/s40265-016-0588-x
85. Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi:10.1016/S1470-2045(15)70054-9
86. Peters S, Gettinger S, Johnson ML, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol. 2017;35(24):2781–2789. doi:10.1200/JCO.2016.71.9476
87. Planchard D, Yokoi T, McCleod MJ, et al. A phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: rationale and protocol design of the ARCTIC study. Clin Lung Cancer. 2016;17(3):232–236.e231. doi:10.1016/j.cllc.2016.03.003
88. McLaughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2016;2(1):46–54. doi:10.1001/jamaoncol.2015.3638
89. Kim S, Koh J, Kwon D, et al. Comparative analysis of PD-L1 expression between primary and metastatic pulmonary adenocarcinomas. Eur J Cancer. 2017;75:141–149. doi:10.1016/j.ejca.2017.01.004
90. Pinato DJ, Shiner RJ, White SDT, et al. Intra-tumoral heterogeneity in the expression of programmed-death (PD) ligands in isogeneic primary and metastatic lung cancer: implications for immunotherapy. Oncoimmunology. 2016;5(9). doi:10.1080/2162402X.2016.1213934
91. Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–88. doi:10.1038/nm1517
92. Mostofa AG, Punganuru SR, Madala HR, Al-Obaide M, Srivenugopal KS, Process T. Regulatory components of inflammation in brain oncogenesis. Biomolecules. 2017;7(2). doi:10.3390/biom7020034
93. Song P, Zhang J, Shang C, Zhang L. Real-world evidenceand clinical observations of the treatment of advanced non-small cell lung cancer with PD-1/PD-L1 inhibitors. Sci Rep. 2019;9(1):4278. doi:10.1038/s41598-019-40748-7
94. Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895–903. doi:10.1016/S1470-2045(17)30380-7
95. Mark NM, Kargl J, Busch SE, et al. Chronic obstructive pulmonary disease alters immune cell composition and immune checkpoint inhibitor efficacy in non-small cell lung cancer. Am J Respir Crit Care Med. 2018;197(3):325–336. doi:10.1164/rccm.201704-0795OC
96. Mccoach CE, Berge EM, Lu X, Barón AE, Camidge DR. A brief report of the status of central nervous system metastasis enrollment criteria for advanced non-small cell lung cancer clinical trials: a review of the clinicaltrials.gov trial registry. J Thorac Oncol. 2016;11(3):407–413. doi:10.1016/j.jtho.2015.10.024
97. Cohen J, Alomari AK, Vortmeyer AO, et al. Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol Res. 2015;4(3):179. doi:10.1158/2326-6066.CIR-15-0160
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
