Back to Journals » Journal of Blood Medicine » Volume 12
Use of Plerixafor for Stem Cell Mobilization in the Setting of Autologous and Allogeneic Stem Cell Transplantations: An Update
Authors Bilgin YM
Received 27 February 2021
Accepted for publication 7 May 2021
Published 2 June 2021 Volume 2021:12 Pages 403—412
DOI https://doi.org/10.2147/JBM.S307520
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Yavuz M Bilgin
Department of Internal Medicine/Hematology, Admiraal de Ruijter Hospital, Goes, the Netherlands
Correspondence: Yavuz M Bilgin
Department of Internal Medicine/Hematology, Admiraal de Ruijter Hospital, PO Box 15, Goes, 4460 AA, the Netherlands
Tel +31-88-1250000
Email [email protected]
Abstract: Mobilization failure is an important issue in stem cell transplantations. Stem cells are yielded from the peripheral blood via apheresis. Granulocyte colony-stimulating factor (G-CSF) is the most commonly used mobilization agent among patients and donors. G-CSF is administered subcutaneously for multiple days. However, patients with mobilization failure cannot receive autologous stem cell transplantation and, therefore, cannot be treated adequately. The incidence rate of mobilization failure among patients is about 6– 23%. Plerixafor is a molecule that inhibits the binding of chemokine receptor-4 with stromal-cell-derived factor-1, thereby resulting in the release of CD34+ cells in the peripheral blood. Currently, plerixafor is used in patients with mobilization failure with G-CSF and is administered subcutaneously. Several studies conducted on different clinical settings have shown that plerixafor is effective and well tolerated by patients. However, more studies should be conducted to explore the optimal approach for plerixafor in patients with mobilization failure. The incidence of mobilization failure among donors is lower. However, plerixafor is not approved among donors with mobilization failure. Moreover, several clinical studies in donors have shown a beneficial effect of plerixafor. In addition, the adverse events of plerixafor are mild and transient, which can overcome the adverse events due to G-CSF. This review assessed the current role and effects of plerixafor in stem cell mobilization for autologous and allogeneic stem cell transplantations.
Keywords: stem cell mobilization, apheresis, autologous stem cell transplantation, allogeneic stem cell transplantation
Corrigendum for this paper has been published.
Introduction
Stem cell transplantation (SCT) is an established treatment for many hematologic malignancies. The proportion of patients requiring SCT is still increasing. In 2010 >30,000 patients received SCT in Europe. Meanwhile in 2019 >48,000 SCTs were performed; 59% were autologous and 41% allogeneic SCT. Autologous SCT was commonly performed for plasma cell disorders (55%) and lymphoma (36%) and the main indications for allogeneic SCT were acute leukemia (54%) followed by myelodysplastic syndrome (MDS) (12%).1
Hematopoietic stem cells are collected from the bone marrow or the peripheral blood. Hematologic recovery is faster and morbidity is lower if stem cells are collected from the peripheral blood. In addition, promoting patient’s comfort the use of peripheral blood is preferred for a SCT. CD34, a surface marker, is expressed on progenitor stem cells. The number of CD34+ cells in the peripheral blood is used for monitoring the collection time of the peripheral blood stem cells and is a reliable predictor for a successful stem cell mobilization. Peripheral CD34+ cell count is correlated with the number of collected CD34+ cells.2,3 CD34+ cells can be yielded from peripheral blood after mobilization via apheresis. The most commonly used agent for stem cell mobilization is granulocyte colony-stimulating factor (G-CSF); it is injected subcutaneously for several days until sufficient CD34+ cells are measured in the peripheral blood.
Under normal conditions the number of CD34+ cells in the peripheral blood is negligible (only <0.05% of the total leukocyte count). After mobilization with G-CSF this increases up to 5–15 times and the CD34+ cells accounted for up to 6% of the total leukocyte count.4 If the pre-apheresis CD34+ count is <5×109/L, sufficient stem cell collection is not likely. If the CD34+ count is >20×109/L the chance of collecting sufficient stem cells in one apheresis session is >90%.5 A position statement by the European Group for Blood and Marrow Transplantation mentioned that a pre-apheresis CD34+ count >20×109/L is sufficient to start stem cell collection.6 Whereas in patients with a pre-apheresis CD34+ count between 10–20 x 109/L the collection of sufficient stem cells can be frequently achieved with >1 apheresis sessions.6,7
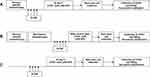
Stem cells can be mobilized either with (chemomobilization) or without chemotherapy. In mobilization without chemotherapy, G-CSF is administered for 4 days after disease-specific chemotherapy and stem cells are collected by apheresis on day 5, if the CD34+ cells in the peripheral blood are > 20×109/L (Figure 1A). In chemomobilization, G-CSF is administered after the mobilization-chemotherapy until there are sufficient CD34+ cells for a successful stem cell collection (Figure 1B). Therefore, with chemomobilization the timing of stem cell collection is unpredictable. In addition, there is a higher risk of bone marrow damage and toxicity resulting in more hospitalization. Nonetheless, chemomobilization results in a higher mobilization yield and has an anticancer effect.8 In patients with non-Hodgkin’s lymphoma (NHL) disease-specific chemotherapy followed by stem cell mobilization is the preferred strategy, which is effective and avoids additional chemomobilization in these heavily treated patients.6 In patients with multiple myeloma (MM) chemomobilization with high-dose (≥3 g/m2) cyclophosphamide is the most commonly used strategy. However, a recent study reported that chemomobilization with low-dose cyclophosphamide (2 g/m2) is a safe mobilization regimen with stem cell collection rates comparable to that of high-dose cyclophosphamide.9 Further, healthy donors are mobilized with G-CSF 1–2 daily subcutaneous injections for 4–5 days (Figure 1C).
A collection of CD34+ cell count of ≥2×106/kg is considered as sufficient for a SCT. Nevertheless, transplantations with a CD34+ cell count of ≥5×106/kg are associated with faster hematopoietic recovery, thereby resulting in a lower incidence of blood transfusions and shorter hospital stay.10 Further, a recent study showed a better 5-year overall survival when the patients are transplanted with higher number of CD34+ cells (>2.65×106/kg).11
Mobilization Failure Among Patients and Donors
Mobilization failure is defined as not able to collect 2×106/kg CD34+ cells.12 Mobilization failure is an important issue and has significant consequences among patients. The incidence of mobilization failure among patients is not fully documented, however the rate varies between 6% and 23%.8,13 These patients cannot receive an autologous SCT and, subsequently, cannot be treated adequately. This has a significant impact on outcome with a 3-year survival rate of 33% in patients with mobilization failure and 71% in those with a successful mobilization.14 A second attempt to remobilize with G-CSF is not effective and has a high failure rate. In a previous study only 23% of patients collected sufficient CD34+ cells with remobilization with G-CSF.15 Mobilization failure is associated with several factors including age, advanced disease, premobilization platelet count <100×109/L, multiple chemotherapy lines, previous radiotherapy, pretreatment with alkylating agents, purine analogs or immune-modulators, diabetes and smoking.4,7
The Italian Group for Stem Cell Transplantation GITMO (Gruppo Italiano Trapianto di Midollo Osseo) developed definitions for patients with mobilization failure.16 Patients with peripheral blood CD34+ cells <20×109/L after adequate mobilization with G-CSF and patients who are not able to collect 2×106/kg after ≤3 apheresis are characterized as proven poor mobilizers. Further, patients are defined as predicted poor mobilizers if they had a previous mobilization failure, they previously received extensive radiotherapy or they met two of the following criteria: advanced disease (≥2 lines of chemotherapy), refractory disease, extensive bone marrow involvement or cellularity <30% at time of mobilization or age ≥65 years.
In contrast, in healthy donors mobilization failure with G-CSF is uncommon, with an estimated incidence rate between 5% and 10%.17 Female gender, older age, low weight and premobilization low leukocyte count were associated with mobilization failure among donors.18 Most donors experienced side effects after administration of G-CSF (>80%); which commonly include bone pain, headache, fatigue and nausea/vomiting.19 Further, transient splenomegaly and even spleen rupture are observed.20
Use of Plerixafor for Autologous SCT
Plerixafor (AMD3100) is a bicyclam molecule, which reversibly blocks chemokine receptor-4 (CXCR-4), thereby inhibiting binding with its ligand stromal-cell-derived factor-1 (SDF-1). This mechanism results in the release of hematopoietic progenitor cells in the blood circulation.21 Randomized (phase III) trials including patients with MM and NHL have shown that addition of plerixafor to upfront mobilization was associated with significantly higher CD34+ cells.22,23 Patients with MM have a higher CD34+ cell count than those with NHL (71% vs 59%). The two trials were followed by compassionate use programs in patients with mobilization failure with G-CSF. These patients received G-CSF in combination with plerixafor in a remobilization attempt; the success rates varied from 60% to 80%.24 Based on these studies the use of plerixafor in patients with mobilization failure with G-CSF was approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Several approaches with the use of plerixafor were investigated in patients with (predicted) mobilization failure: pre-emptive and risk-adapted approaches and in a second attempt to mobilize. The possible approaches are depicted in Figure 1. Plerixafor is added in the pre-emptive approach for patients with predicted mobilization failure based on pre-apheresis CD34+ cell count. Plerixafor is recommended in the mobilization scheme when the targeted CD34+ cell count in the peripheral blood is <10×109/L on days 4–5 of mobilization with G-CSF alone. If the CD34+ cell count is <10×109/L in patients with chemomobilization after 12–14 days, the incidence rate of predicted mobilization failure is high.25 Therefore, in these patients plerixafor is recommended if the CD34+ cell count in the peripheral blood is <10×109/L and the leukocyte count is increasing. In patients with CD34+ cell count between 10–20×109/L a dynamic approach is suggested based on patient’s characteristics and treatment history.6,7 In these patients apheresis can be started and plerixafor is mandatory if insufficient CD34+ is collected (<2×106/kg). In addition, plerixafor is recommended when <25% of the targeted CD34+ cells is collected on the first day of apheresis (Figure 2A).6,7 Several studies showed that this pre-emptive approach resulted in almost 4-fold increase in CD34+ cells in the peripheral blood after administration of plerixafor, with mobilization rates of >90%.26–28 In another approach plerixafor is administered to patients who are at high-risk for mobilization failure (predicted poor mobilizers) based on baseline characteristics (Figure 2B). A low failure rate (~4%) was observed with this approach as well.28 Until now, it is not clear which approach is superior. Both approaches were compared in two nation-wide surveys (in France and Canada). In both studies the success rates of both approaches were comparable (>70%).29,30 However, more studies should be performed to investigate the optimal use of plerixafor in patients with (predicted) mobilization failure.
Some studies investigated the effect of plerixafor in a second remobilization attempt after previous failure with plerixafor (Figure 2C). Also, in these studies high success rates (66–83%) were achieved.31,32 In one analysis pretreatment with fludarabine, low premobilization platelet count (<140×109/L), age >65 years and radiotherapy were significant predictors of mobilization failure with plerixafor.33 Other studies revealed that patients receiving fludarabine- and lenalidomide-based induction regimens required higher number of plerixafor administrations to achieve a successful mobilization.34,35 To date novel drugs are used in induction therapy among patients with hematologic malignancies. Daratumumab, a novel monoclonal CD38 antibody, is increasingly administered to transplant-eligible newly diagnosed myeloma patients. In one study patients who received daratumumab during induction therapy required significant more administrations of plerixafor for a successful mobilization than in those who did not received it (21.7% versus 7.9%).36 Further, the effects of the novel drugs in hematologic diseases should be elucidated in the future.
Patients with a very low pre-apheresis CD34+ cell count (<5×109/L) have a significantly low probability for a successful mobilization. Even in these patients the addition of plerixafor was beneficial. The success rate was >70% in patients with a pre-apheresis CD34+ count of <5×109/L.37 In another analysis, mobilization was successful in about 50% of patients with pre-plerixafor CD34+ cell count of 0–1×109/L and >70% of those with a CD34+ cell count >2×109/L.34 Plerixafor is recommended to be injected subcutaneously 9–11 hours before the planned apheresis. Some studies have shown that the peak of CD34+ cells with plerixafor was observed at an earlier time (3 and 8 hours) in patients with poor mobilization.38,39 Therefore, in these patients the peak of CD34+ cells can be missed and consequently sufficient CD34+ cells cannot be collected. One recent study has shown that the efficacy is higher if apheresis was performed on the same day as the administration of plerixafor.40 However, there are limited studies regarding the effect of plerixafor on the kinetics of stem cell mobilization.
To date plerixafor is widely used in patients with MM and NHL with mobilization failure with G-CSF. Further, plerixafor improved mobilization rates in patients with other diagnoses. In patients with Hodgkin’s lymphoma the success rate was 74%.41 This was even higher in patients with nonhematologic malignancies (85%) and in children with malignant diseases (87%).42,43 In addition, plerixafor was successful for the purpose of gene therapy in sickle cell disease.44 Due to the possible mobilization of leukemic cells plerixafor is not recommended for acute myeloid leukemia (AML). Although a recent small study showed a successful mobilization with plerixafor in 5 (minimal residual disease negative) AML patients with mobilization failure.45
With plerixafor-induced mobilization more immature CD34+/CD38-cells and T- and NK-cells are collected, which can lead to faster hematopoietic recovery after transplantation.46,47 Few studies have shown that the grafts of MM patients had a higher number of NK-cells and CD19+ cells than those of NHL patients. However, no difference was observed in recovery.48–50 Further, the long-term effects of mobilization with plerixafor compared with G-CSF did no differ in progression-free survival or 5-year survival.51 However, one study showed that 5 of 43 patients developed secondary MDS or AML 29 months after an autologous SCT.52
The administration of plerixafor is well tolerated and <2% of patients reported adverse events. Of which the most common were nausea/vomiting, diarrhea, fatigue, and headache. All adverse events resolved immediately and there were no grade 3 or 4 events.53 Plerixafor is costly, this might be an important factor in the use among patients with mobilization failure. However, some studies have shown that patients who received plerixafor required less apheresis sessions, which can equalize the costs of plerixafor.54,55 Moreover, lower cost was mentioned in cost-simulation analyses in patients with upfront stem cell mobilization with plerixafor compared to G-CSF.56,57 More studies are warranted to evaluate the cost-effectiveness of plerixafor.
Plerixafor for Allogeneic SCT
The use of plerixafor is not approved for allogeneic SCT. Since 2011 several case reports mentioned for the first that donors had a successful mobilization using plerixafor after mobilization failure with G-CSF.58,59 In the last years few studies have investigated the role of plerixafor among healthy donors.
In human leukocyte antigen (HLA)-identical and haplo-identical donors plerixafor was administered using several approaches: upfront,60,64,66,67 pre-emptive after failure with G-CSF,61,62,65,68,69 or remobilization after mobilization failure with G-CSF.63 Moreover plerixafor was used successfully for haplo-identical SCT requiring a higher number of CD34+ cells.61,62,65 The characteristics of these studies are depicted in Table 1. In studies with upfront approach the median collected CD34+ after single injection with plerixafor was 2.9 x 106/kg to 4.7×106/kg. Although the failure rate with a single injection with plerixafor was high (33% to 48%) and subsequently a second gift of plerixafor was necessary to achieve a successful stem cell collection. In two studies published in 2021 plerixafor was added pre-emptively to the mobilization with G-CSF.68,69 The peripheral CD34+ cell count showed 2.9-fold and 3.4-fold increases after adding a single injection of plerixafor. In these two studies, the collected CD34+ cells was 1.1×106/kg and 1.6×106/kg with G-CSF alone and it was raised to 2.8×106/kg and 4.9 x 106/kg with the combination G-CSF and plerixafor. Therefore, these studies suggest that if the collected CD34+ cells are not sufficient for an allogeneic SCT, addition of a single injection of plerixafor to the mobilization with G-CSF can be effective. Further, in some studies with healthy donors plerixafor was administered at different doses (0.24 mg/kg or 0.32 mg/kg). In one study plerixafor was administered at a higher dose (0.48 mg/kg) resulting in a higher peak of CD34+ cells, indicating that this might improve harvesting.70 In contrast, another study showed no difference in failure rates in patients who were injected intravenously with higher doses of plerixafor (0.32 mg/kg) compared with those who were subcutaneously injected with plerixafor (0.24 mg/kg).64 More studies should be conducted to investigate the use of plerixafor for an allogeneic SCT.
 |
Table 1 Studies with Plerixafor for Allogeneic Stem Cell Transplantation |
In addition, among donors the use of plerixafor is well tolerated. About 60–70% presented grade 1–2 adverse events and no donor experienced grade 4 events. The most common events were tingling, pain, fatigue, nausea, diarrhea, abdominal bloating and injection site reactions.66,68 All events resolved immediately, in contrast with G-CSF no bone pain was observed after administration of plerixafor.67 This suggests that the adverse events of administration of G-CSF for multiple days can overcome with one or two injections of plerixafor. Further, it has been suggested that viral infections can negatively influence stem cell mobilization with G-CSF. In one case-report sufficient CD34+ cells was collected with single gift of plerixafor in a donor with influenza A and mobilization failure with G-CSF.71 Nevertheless, the possible effects of the COVID-19 pandemic on mobilization failure should be evaluated in the future.
Immunologic studies have shown higher CD3+ and CD4+ cell counts in grafts mobilized with plerixafor, however there was no increase in the incidence of graft-versus-host disease.72 Some studies have shown that in allogeneic SCT engraftment of neutrophils and platelets after stem cell mobilization with plerixafor was faster than that with mobilization failure with G-CSF.67,73
Future Directions
Plerixafor was the first drug that has been approved for patients with mobilization failure. In recent years, early phase or preclinical trials were conducted to assess the efficacy of novel agents. The use of POL6326, a CXCR-4 antagonist, was effective based on a Phase II trial conducted on myeloma patients,74 and another CXCR-4 antagonist, BKT140, was found to be associated with a 78-fold increase in the number of stem cells.75 Moreover, it was even more effective than the combination of G-CSF and plerixafor. Parathormone (PTH), SEW2871, a sphingosine-1 phosphate agonist, and BIO5192, an inhibitor of VLA-4/VCAM, increased the number of stem cells in mobilization in animal studies.76 However, the use of more novel agents with promising results among healthy donors and animals are still investigated.
Conclusions
SCT is a well-established for patients with hematologic malignancies. Mobilization failure is an important issue as it is associated with poor survival. Plerixafor is the first agent approved for patients with mobilization failure with G-CSF. The success rates are high. Therefore, more patients can be treated adequately with autologous SCT. In patients with pre-apheresis very low CD34+ cell count plerixafor is effective. Plerixafor is well tolerated with mild adverse events which resolve immediately. However, there is no consensus on the optimal approach with plerixafor in patients with mobilization failure with G-CSF. The use of plerixafor for allogeneic SCT plerixafor has not been approved yet. Only few studies have shown that plerixafor is efficient among donors. Moreover, the adverse events of G-CSF can be overcome with the use of one or two injections of plerixafor among donors. To assess the optimal use of plerixafor for autologous and allogeneic SCTs more clinical, immunologic and cost-effectiveness studies should be conducted. Further, the effects of the COVID-19 pandemic in stem cell mobilization must be elucidated in the future.
Disclosure
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Passweg JR, Baldomero H, Chabannon C, et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years. Bone Marrow Transplant. 2021. doi:10.1038/s41409-021-01227-8.
2. To LB, Dyson PG, Juttner CA. Cell-dose effect in circulating stem-cell autografting. Lancet. 1986;2(8503):404–405. doi:10.1016/s0140-6736(86)90096-6.
3. Weaver CH, Hazelton B, Birch R, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86(10):3961–3969. doi:10.1182/blood.V86.10.3961.bloodjournal86103961
4. Namdaroglu S, Korkmaz S, Altuntas F. Management of mobilization failure in 2017. Transfus Apher Sci. 2017;56(6):836–844. doi:10.1016/j.transci.2017.11.017.
5. Armitage S, Hargreaves R, Samson D, Brennan M, Kanfer E, Navarrete C. CD34 counts to predict the adequate collection of peripheral blood progenitor cells. Bone Marrow Transplant. 1997;20(7):587–591. doi:10.1038/sj.bmt.1700938.
6. Mohty M, Hubel K, Kroger N, et al. Autologous haematopoietic stem cell mobilisation in multiple myeloma and lymphoma patients: a position statement from the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2014;49:865–872. doi:10.1038/bmt.2014.39.
7. Goker H, Etgul S, Buyukasik Y. Optimizing mobilization strategies in difficult-to-mobilize patients: the role of plerixafor. Transfus Apher Sci. 2015;53(1):23–29. doi:10.1016/j.transci.2015.05.011.
8. Wuchter P, Hubel K. How to find the optimal mobilisation strategy – impact, challenges and solutions. Eur Oncol Haematol. 2016;12(2):87–92. doi:10.17925/EOH.2016.12.02.87.
9. Zannetti BA, Saraceni F, Cellini C, et al. Low-dose cyclophosphamide versus intermediate-high-dose cyclophosphamide versus granulocyte colony-stimulating factor alone for stem cell mobilization in multiple myeloma in the era of novel agents: a multicenter retrospective analysis. Transplant Cell Ther. 2021;27(3):
10. Siena S, Schiavo R, Pedrazzoli P, Carlo-Stella C. Therapeutic relevance of CD34 cell dose in blood cell transplantation for cancer therapy. J Clin Oncol. 2000;18(6):1360–1377. doi:10.1200/JCO.2000.18.6.1360.
11. Partanen A, Turunen A, Valtola J, et al. Mobilization characteristics, blood graft composition, and outcome in diffuse large B-cell lymphoma after autologous stem cell transplantation: results from the prospective multicenter GOA study. Transfusion. 2021;61(2):516–525. doi:10.1111/trf.16198.
12. Ataca Atilla P, Bakanay Ozturk SM, Demirer T. How to manage poor mobilizers for high dose chemotherapy and autologous stem cell transplantation? Transfus Apher Sci. 2017;56(2):190–198. doi:10.1016/j.transci.2016.11.005.
13. Hopman RK, DiPersio JF. Advances in stem cell mobilization. Blood Rev. 2014;28(1):31–40. doi:10.1016/j.blre.2014.01.001.
14. Pavone V, Gaudio F, Console G, et al. Poor mobilization is an independent prognostic factor in patients with malignant lymphomas treated by peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006;37(8):719–724. doi:10.1038/sj.bmt.1705298.
15. Pusic I, Jiang SY, Landua S, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant. 2008;14(9):1045–1056. doi:10.1016/j.bbmt.2008.07.004.
16. Olivieri A, Marchetti M, Lemoli R, et al. Proposed definition of ‘poor mobilizer’ in lymphoma and multiple myeloma: an analytic hierarchy process by ad hoc working group Gruppo Italiano Trapianto di Midollo Osseo. Bone Marrow Transplant. 2012;47(3):342–351. doi:10.1038/bmt.2011.82.
17. Ings SJ, Balsa C, Leverett D, Mackinnon S, Linch DC, Watts MJ. Peripheral blood stem cell yield in 400 normal donors mobilised with granulocyte colony-stimulating factor (G-CSF): impact of age, sex, donor weight and type of G-CSF used. Br J Haematol. 2006;134(5):517–525. doi:10.1111/j.1365-2141.2006.06223.x.
18. Wang TF, Wen SH, Chen RL, et al. Factors associated with peripheral blood stem cell yield in volunteer donors mobilized with granulocyte colony-stimulating factors: the impact of donor characteristics and procedural settings. Biol Blood Marrow Transplant. 2008;14(11):1305–1311. doi:10.1016/j.bbmt.2008.09.002.
19. D’Souza A, Jaiyesimi I, Trainor L, Venuturumili P. Granulocyte colony-stimulating factor administration: adverse events. Transfus Med Rev. 2008;22(4):280–290. doi:10.1016/j.tmrv.2008.05.005.
20. Becker PS, Wagle M, Matous S, et al. Spontaneous splenic rupture following administration of granulocyte colony-stimulating factor (G-CSF): occurrence in an allogeneic donor of peripheral blood stem cells. Biol Blood Marrow Transplant. 1997;3:45–49.
21. Uy G, Rettig M, Cashen AF. Plerixafor, a CXCR4 antagonist for the mobilization of hematopoietic stem cells. Expert Opin Biol Ther. 2008;8(11):1797–1804. doi:10.1517/14712598.8.11.1797.
22. DiPersio JF, Stadtmauer EA, Nademanee A, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–5726. doi:10.1182/blood-2008-08-174946.
23. DiPersio JF, Micallef IN, Stiff PJ, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27(28):4767–4773. doi:10.1200/JCO.2008.20.7209.
24. Hubel K, Fresen MM, Apperley JF, et al. European data on stem cell mobilization with plerixafor in non-Hodgkin’s lymphoma, Hodgkin’s lymphoma and multiple myeloma patients. A subgroup analysis of the European consortium of stem cell mobilization. Bone Marrow Transplant. 2012;47(8):1046–1050. doi:10.1038/bmt.2011.216.
25. Farina L, Spina F, Guidetti A, et al. Peripheral blood CD34+ cell monitoring after cyclophosphamide and granulocyte-colony-stimulating-factor: an algorithm for pre-emptive use of plerixafor. Leuk Lymphoma. 2014;55(2):331–336. doi:10.3109/10428194.2013.802783.
26. Worel N, Fritsch G, Agis H, et al. Plerixafor as preemptive strategy results in high success rates in autologous stem cell mobilization failure. J Clin Apher. 2017;32(4):224–234. doi:10.1002/jca.21496.
27. Bilgin YM, de Greef GE. Plerixafor for stem cell mobilization: the current status. Curr Opin Hematol. 2016;23(1):67–71. doi:10.1097/MOH.0000000000000200.
28. Giralt S, Costa L, Schriber J, et al. Optimizing autologous stem cell mobilization strategies to improve patient outcomes: consensus guidelines and recommendations. Biol Blood Marrow Transplant. 2014;20(3):295–308. doi:10.1016/j.bbmt.2013.10.013.
29. Sheppard D, Bredeson C, Huebsch L, Allan D, Tay J. A plerixafor-based strategy allows adequate hematopoietic stem cell collection in poor mobilizers: results from the Canadian Special Access Program. Bone Marrow Transplant. 2014;49(6):751–7555. doi:10.1038/bmt.2014.33.
30. Chabannon C, Bijou F, Miclea JM, Milpied N, Grouin JM, Mohty M. A nationwide survey of the use of plerixafor in patients with lymphoid malignancies who mobilize poorly demonstrates the predominant use of the “on-demand” scheme of administration at French autologous hematopoietic stem cell transplant programs. Transfusion. 2015;55(9):2149–2157. doi:10.1111/trf.13141.
31. Veeraputhiran M, Jain T, Cronin S, et al. Successful hematopoietic stem cell collection in patients who fail initial plerixafor mobilization for autologous stem cell transplant. J Clin Apher. 2014;29(6):293–298. doi:10.1002/jca.21321.
32. Yuan S, Nademanee A, Krishnan A, Kogut N, Shayani S, Wang S. Second time a charm? Remobilization of peripheral blood stem cells with plerixafor in patients who previously mobilized poorly despite using plerixafor as a salvage agent. Transfusion. 2013;53(12):3244–3250. doi:10.1111/trf.12198.
33. Lanza F, Lemoli RM, Olivieri A, et al. Factors affecting successful mobilization with plerixafor: an Italian prospective survey in 215 patients with multiple myeloma and lymphoma. Transfusion. 2014;54(2):331–339. doi:10.1111/trf.12265.
34. Bilgin YM, Visser O, Beckers EA, et al. Evaluation of Dutch guideline for just-in-time addition of plerixafor to stem cell mobilization in patients who fail with granulocyte-colony-stimulating factor. Transfusion. 2015;55(5):1021–1027. doi:10.1111/trf.12979.
35. Laurent V, Fronteau C, Antier C, et al. Autologous stem-cell collection following VTD or VRD induction therapy in multiple myeloma: a single-center experience. Bone Marrow Transplant. 2021;56(2):395–399. doi:10.1038/s41409-020-01033-8.
36. Hulin C, Offner F, Moreau P, et al. Stem cell yield and transplantation in transplant-eligible newly diagnosed multiple myeloma patients receiving daratumumab+bortezomib/thalidomide/dexamethasone in the Phase 3 CASSIOPEIA study. Haematologica. 2021. doi:10.3324/haematol.2020.261842.
37. Maziarz RT, Nademanee AP, Micallef IN, et al. Plerixafor plus granulocyte colony-stimulating factor improves the mobilization of hematopoietic stem cells in patients with non-Hodgkin lymphoma and low circulating peripheral blood CD34+ cells. Biol Blood Marrow Transplant. 2013;19(4):670–675. doi:10.1016/j.bbmt.2013.01.005.
38. Lefrere F, Mauge L, Réa D, et al. A specific time course for mobilization of peripheral blood CD34+ cells after plerixafor injection in very poor mobilizer patients: impact on the timing of the apheresis procedure. Transfusion. 2013;53(3):564–569. doi:10.1111/j.1537-2995.2012.03744.x.
39. Shi PA, Miller LK, Isola LM. Prospective study of mobilization kinetics up to 18 hours after late afternoon dosing of plerixafor. Transfusion. 2014;54(5):1263–1268. doi:10.1111/trf.12459.
40. Cid J, Castillo C, Marin P, et al. Increased collection efficiency of CD34+ cells after mobilization with preemptive use of plerixafor followed by leukocytapheresis on the same day. Transfusion. 2020;60(4):779–785. doi:10.1111/trf.15711.
41. Yuan S, Nademanee A, Kaniewski M, Palmer J, Shayani S, Wang S. Efficacy of just-in-time plerixafor rescue for Hodgkin’s lymphoma patients with poor peripheral blood stem cell mobilization. Transfusion. 2014;54(3):2015–2021. doi:10.3109/10428194.2012.713480.
42. Worel N, Apperley JF, Basak GW, et al. European data on stem cell mobilization with plerixafor in patients with nonhematologic diseases: an analysis of the European consortium of stem cell mobilization. Transfusion. 2012;52(11):2395–2400. doi:10.1111/j.1537-2995.2012.03603.x.
43. Bhunia N, Abu-Arja R, Stanek JR, et al. A multicenter report on the safety and efficacy of plerixafor based stem cell mobilization in children with malignant disorders. Transfusion. 2021;61(3):894–902. doi:10.1111/trf.16260.
44. Esrick EB, Manis JP, Daley H, et al. Successful hematopoietic stem cell mobilization and apheresis collection using plerixafor alone in sickle cell patients. Blood Rev. 2018;2(19):2505–2512. doi:10.1182/bloodadvances.2018016725.
45. Shumilov E, Novak U, Jeker B, Taleghani BM, Bacher U, Pabst T. Hematopoietic stem cell mobilization with plerixafor is safe and effective in poorly mobilizing acute myeloid leukemia patients. HemaSphere. 2019;3(2):e176. doi:10.1097/HS9.0000000000000176
46. Porrata LF. Autograft immune effector cells and survival in autologous peripheral blood hematopoietic stem cell transplantation. J Clin Apher. 2018;33(3):324–330. doi:10.1002/jca.21611.
47. Saraceni F, Shem-Tov N, Olivieri A, Nagler A. Mobilized peripheral blood grafts include more than hematopoietic stem cells: the immunological perspective. Bone Marrow Transplant. 2015;50(7):886–891. doi:10.1038/bmt.2014.330.
48. Valtola J, Silvennoinen R, Ropponen A, et al. Preemptive plerixafor injection added to pegfilgrastim after chemotherapy in non-Hodgkin lymphoma patients mobilizing poorly. Ann Hematol. 2017;96(11):1897–1906. doi:10.1007/s00277-017-3123-6.
49. Turunen A, Partanen A, Valtola J, et al. CD34+cell mobilization, blood graft composition and posttransplant recovery in myeloma patients compared to non-Hodgkin’s lymphoma patients: results of the prospective multicenter GOA study. Transfusion. 2020;60(7):1519–1528. doi:10.1111/trf.15820.
50. Valtola J, Silvennoinen R, Ropponen A, et al. Blood graft composition and post-transplant recovery in myeloma patients mobilized with plerixafor: a prospective multicenter study. Leuk Lymphoma. 2019;60(2):453–461. doi:10.1080/10428194.2018.1485911.
51. Micallef IN, Stiff PJ, Nademanee AP, et al. Plerixafor plus granulocyte colony-stimulating factor for patients with non-Hodgkin lymphoma and multiple myeloma: long-term follow-up report. Biol Blood Marrow Transplant. 2018;24(6):1187–1195. doi:10.1016/j.bbmt.2018.01.039.
52. Deol A, Abrams J, Masood A, et al. Long-term follow up of patients proceeding to transplant using plerixafor mobilized stem cells and incidence of secondary myelodysplastic syndrome/AML. Bone Marrow Transplant. 2013;48(8):1112–1116. doi:10.1038/bmt.2013.10.
53. Fruehauf S. Current clinical indications for plerixafor. Transfus Med Hemother. 2013;40(4):246–250. doi:10.1159/000354229.
54. Milone G, Martino M, Spadaro A, et al. Plerixafor on-demand combined with chemotherapy and granulocyte colony-stimulating factor: significant improvement in peripheral blood stem cells mobilization and harvest with no increase in costs. Br J Haematol. 2014;164(1):113–123. doi:10.1111/bjh.12606.
55. Hundemer M, Engelhardt M, Bruckner T, et al. Rescue stem cell mobilization with plerixafor economizes leukapheresis in patients with multiple myeloma. J Clin Apher. 2014;29(6):299–304. doi:10.1002/jca.21323.
56. Watts NL, Marques MB, Peavey DB, et al. Mobilization of hematopoietic progenitor cells for autologous transplantation using pegfilgrastim and plerixafor: efficacy and cost implications. Biol Blood Marrow Transplant. 2019;25(2):233–238. doi:10.1016/j.bbmt.2018.09.005.
57. Mohty M, Azar N, Chabannon C, et al. Plerixafor in poor mobilizers with non-Hodgkin’s lymphoma: a multi-center time-motion analysis. Bone Marrow Transplant. 2018;53(3):246–254. doi:10.1038/s41409-017-0033-0.
58. Neumann T, Kruger WH, Busermann C, Kiefer T, Dolken G. Successful mobilization of PBSCs in a healthy volunteer donor by addition of plerixafor after failure of mobilization with G-CSF alone. Bone Marrow Transplant. 2011;46(5):762–763. doi:10.1038/bmt.2010.178.
59. Schriber J, Fauble V, Sproat LO, Briggs A. Plerixafor ‘just in time’ for stem cell mobilization in a normal donor. Bone Marrow Transplant. 2011;46(7):1026–1027. doi:10.1038/bmt.2010.226.
60. Devine SM, Vij R, Rettig M, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112(4):990–998. doi:10.1182/blood-2007-12-130179.
61. Hauge AW, Haastrup EK, Sengeløv H, Minulescu L, Dickmeiss E, Fischer-Nielsen A. Addition of plerixafor for CD34+ cell mobilization in six healthy stem cell donors ensured satisfactory grafts for transplantation. Transfusion. 2014;54(4):1055–1058. doi:10.1111/trf.12383.
62. Gattillo S, Marktel S, Rizzo L, et al. Plerixafor on demand in ten healthy family donors as a rescue strategy to achieve an adequate graft for stem cell transplantation. Transfusion. 2015;55(8):1993–2000. doi:10.1111/trf.13059.
63. Fiala MA, Park S, Slade M, DiPersio JF, Stockerl-Goldstein KE. Remobilization of hematopoietic stem cells in healthy donors for allogeneic transplantation. Transfusion. 2016;56(9):2331–2335. doi:10.1111/trf.13688.
64. Schroeder MA, Rettig MP, Lopez S, et al. Mobilization of allogeneic peripheral blood stem cell donors with intravenous plerixafor mobilizes a unique graft. Blood. 2017;129(19):2680–2692. doi:10.1182/blood-2016-09-739722.
65. Jaiswal SR, Bhakuni P, Joy A, et al. Impact of single-dose plerixafor as an adjunct to granulocyte colony-stimulating factor-based peripheral blood stem cell mobilization on the graft composition and outcome for T cell-replete haploidentical peripheral blood stem cell transplantation with post-transplantation cyclophosphamide: a comparative study. Biol Blood Marrow Transplant. 2018;24(3):542–548. doi:10.1016/j.bbmt.2017.11.014.
66. de Greef GE, Braakman E, van der Holt B, et al. The feasibility and efficacy of subcutaneous plerixafor for mobilization of peripheral blood stem cells in allogeneic HLA-identical sibling donors: results of the HOVON-107 study. Transfusion. 2019;59(1):316–324. doi:10.1111/trf.15037.
67. Chen YB, Le-rademacher J, Brazauskas R, et al. Plerixafor alone for the mobilization and transplantation of HLA-matched sibling donor hematopoietic stem cells. Blood Adv. 2019;3(6):875–883. doi:10.1182/bloodadvances.2018027599.
68. Holig K, Schmidt H, Hutter G, et al. Salvage treatment with plerixafor in poor mobilizing allogeneic stem cell donors: results of a prospective Phase II-trial. Bone Marrow Transplant. 2021;56(3):635–645. doi:10.1038/s41409-020-01053-4.
69. Cid J, Monsalvo S, Castillo C, et al. Addition of plerixafor to G-CSF in poor mobilizing healthy related donors overcame mobilization failure: an observational case series on behalf of the Grupo Espanol de Trasplante Hematopoyetico (GETH). Transfus Apher Sci. 2021;60(2):103052. doi:10.1016/j.transci.2021.103052.
70. Pantin J, Purev E, Tian X, et al. Effect of high-dose plerixafor on CD34+ cell mobilization in healthy stem cell donors: results of a randomized crossover trial. Haematologica. 2017;102(3):600–609. doi:10.3324/haematol.2016.147132.
71. Yeral M, Aytan P, Boga C. Use of plerixafor to mobilize a healthy donor infected with influenza A. Turk J Haematol. 2018;35(2):138–139. doi:10.4274/tjh.2017.0304.
72. Couban S, Wong PC, Schultz KR. The case for plerixafor to replace filgrastim as the optimal agent to mobilize peripheral blood donors for allogeneic hematopoietic cell transplantation. Exp Hematol. 2019;70:1–9. doi:10.1016/j.exphem.2018.11.003.
73. Green MM, Chao N, Chhabra S, et al. Plerixafor (a CXCR4 antagonist) following myeloablative allogeneic hematopoietic stem cell transplantation enhances hematopoietic recovery. J Hematol Oncol. 2016;9(1):71. doi:10.1186/s13045-016-0301-2.
74. Schmitt S, Weinhold N, Dembrowsky K, et al. First results of a phase-II study with the new CXCR4 antagonist POL6326 to mobilize hematopoietic stem cells (HSC) in multiple myeloma (MM). Blood. 2010;116:824. doi:10.1182/blood.V116.21.824.824
75. Abraham M, Biyder K, Begin M, et al. Enhanced unique pattern of hematopoietic cell mobilization induced by the CXCR4 antagonist 4F-benzoyl-TN14003. Stem Cells. 2007;25(9):2158–2166. doi:10.1634/stemcells.2007-0161.
76. Domingues MJ, Nilsson SK, Cao B. New agents in HSC mobilization. Int J Hematol. 2017;105(2):141–152. doi:10.1007/s12185-016-2156-2.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.