Back to Journals » Medical Devices: Evidence and Research » Volume 15
Usability of a Novel Enteral Feeding System: A Summative Study
Authors Mohamed Elfadil O , Keaveney E , Patel A, Abdelmagid MG, Patel I, Patel J, Hurt RT, Mundi MS
Received 19 March 2022
Accepted for publication 8 July 2022
Published 5 August 2022 Volume 2022:15 Pages 253—262
DOI https://doi.org/10.2147/MDER.S367100
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract of "Usability of a Novel Enteral Feeding System" [ID 367100].
Views: 267
Osman Mohamed Elfadil,1 Edel Keaveney,2 Ankitaben Patel,1 Marwa G Abdelmagid,1 Ishani Patel,1 Jalpan Patel,1 Ryan T Hurt,1,3,4 Manpreet S Mundi1
1Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, MN, USA; 2Rockfield Medical Devices, Galway, Ireland; 3Division of Internal Medicine, Mayo Clinic, Rochester, MN, USA; 4Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA
Correspondence: Manpreet S Mundi, Division of Endocrinology, Diabetes, Metabolism, and Nutrition, Mayo Clinic, 200 First St SW, Rochester, MN, 55905, USA, Tel +1 507-284-0106, Fax +1 507-284-5745, Email [email protected]
Background: Utilization of long-term home enteral nutrition (HEN) for nutrition therapy is increasing across the world. However, HEN can be a mobility-limiting experience affecting quality of life (QoL). Improvement of QoL for patients receiving HEN is a universal goal within the nutrition community. This study evaluated usability of Mobility+®, a novel enteral feeding system (EFS).
Methods: A summative study evaluating usability of the novel EFS was conducted with novices (NV), non-novices (NN), and healthcare professionals (HCP). Subjects in NV and NN groups received familiarization training where they were introduced to the novel EFS and walked through steps to fill pouch, simulate feeding, flush (rinse), and wear the system, using the Instructions for Use (IFU) booklet, followed by a testing session where they simulated system use on their own. HCP self-trained using the IFU and instructional videos. A fill from ready-to-hang (RTH) formula bag method was also tested in HCP. Participants’ ability to loosely coil the tubing and sit, stand, and move around wearing a filled feeding pouch inside a crossbody bag was also evaluated.
Results: Forty-five participants completed the study. All participants successfully and safely simulated use of the novel EFS, with 97.8% (44/45) doing so on first attempt. All participants could wear the novel EFS in crossbody bag and move around without any use errors or safety issues.
Conclusion: The examined novel EFS can be safely used in intended use population, with or without previous experience with enteral nutrition, on provision of basic familiarization training and written IFU. Additionally, HCP can successfully self-train on this system with instructional videos.
Keywords: feeding system, enteral feeding, mobility, mobility-enhancing enteral feeding system, novel EFS
Introduction
Enteral nutrition (EN) is a lifesaving tool that can be used to provide nutrition for patients with functional gastrointestinal tract when they are not able to use natural oral route and/or adequately absorb nutrients. Several mechanisms can be used to deliver EN, including use of nasal or percutaneous tubes with gastric or small intestinal access.1 EN is used for inpatient or home nutrition support and can be provided for extended periods of time or permanently.2 Although the history of tube feeding goes back to the 16th century, major milestones were reached in the 20th century including advancements in formula production, and use of pumps for intermittent and continuous tube feeding.3 These advancements have led to increased prevalence of EN in both inpatient nutrition support and home enteral nutrition (HEN) over the last few decades.4,5
While HEN can provide life-sustaining nutrition support for many patients, the question about the burden of this alternative feeding route on patients and caregivers remains valid.6–8 Living with long-term HEN can be very challenging due to constraints on personal freedom, mobility, and partaking in physical and social activities. These challenges require many lifestyle adjustments, which in addition to the burden of underlying medical condition(s) can lead to significant hardships for patients and caregivers.9 If the enteral access is gastric, HEN can be provided via syringe or gravity feedings, with the latter often requiring a pole that limits mobility. Other modalities used include flexible container systems with tubing. If the patient is intolerant to the above methods or is providing feedings into the intestines, then an infusion pump is used to provide continuous feeds, estimated to be used by 40–92% of patients across homecare, acute and long-term care settings.10 However, as described earlier, HEN can be an overwhelming experience for patients and caregivers due to a number of factors.8,11 In fact, regular assessment for HEN burden can help understand QoL of patients and caregivers.7 Mobility enhancement has been described as possibly improving QoL in the elderly and those with limited functional capacity.12–14
In medical nutrition, although ambulatory peristaltic pumps were introduced more than 30 years ago15 allowing portable feeding via use of backpacks and removing the need for users to be tied to an IV pole, little innovation has occurred since then to further advance mobility. Many infusion pump users are mobile16 and innovative solutions which allow users to have active physical and social lives while feeding would likely reduce burdens on both users and caregivers, leading to improvement in QoL.
The current manuscript reviews the usability of a novel EFS that could provide an alternative option to the HEN population, filling a key gap between simple bolus/gravity systems and more complex pumps requiring external power, with the goal of improving mobility, reducing burden of HEN, and improving QoL.
Materials and Methods
The Examined Feeding System
Mobility+® (Novel enteral feeding system (EFS); Rockfield Medical Devices, ATU Innovation Hub, Dublin Road, Galway, Ireland) is a simple lightweight portable enteral feeding system intended to deliver liquid nutrition formula to a feeding tube with an ISO 80369-3 connector (ENFit®).
This novel EFS is designed to allow for enhanced mobility and ease of use, compared to current infusion pumps, and consists of 3 components, a feeding pouch and 2 tubing sets, all contained within a plastic package. The feeding pouch is the reservoir for up to 500mL (16.9oz) of feed, is lightweight (weighing 16g when empty) and consists of a protective sleeve which acts as a barrier to an internal elastomeric pump, made from silicone, an expansile material. Filling-set tubing is used to fill the pouch with feed, and giving-set tubing delivers the feed to the feeding tube or extension set (Figure 1). An ENFit® 60mL syringe is an additional item required to fill the novel EFS.
As the feeding pouch is filled with feed by the user, the elastomeric pump inflates. When the giving-set tubing is inserted into the spout of the pouch, the elastomer begins to contract and the feed is delivered through the giving-set in a controlled manner, silently, without any external power source or need for gravity. The novel EFS package contains one of 4 available giving-sets, all different lengths, which allows for feed flow rates from the system that match the typical flow rate ranges users require, with the giving-set tubing length and diameter determining the flow rate. The progress of feed delivery is indicated by reductions in pouch inflation and weight, and completion of feed delivery is indicated by a cessation in feed flow out of the giving-set. The novel EFS is intended for patients aged 2 years and older in clinical or home care settings; it works with commercial enteral feeds; is for single-patient use over a 24-hour period; and is disposable. Clinicians will consult product labelling to determine which giving-set patients should be guided to use, to provide for their individual flow rate needs. The system can be worn in a bag of choice, or in clothing, that can fit the system without kinking the tubing and without squeezing the pouch. Figure 2 illustrates preparing the system for mobile use. Supplementary Material Figure S1 shows filling method and flushing steps.
ASPEN has given guidance that facility-specific recommendations on hang times should be followed, as these recommendations vary by institution.2 This guidance is expected to be followed to ensure microbial safety.
Study Design
A simulated-use summative study was conducted between November 2020 and February 2021, where the primary objective was to evaluate the usability of the novel EFS, in participants reflective of the intended user population, by providing training consistent with typical real-world scenarios, and evaluating if tasks associated with system set-up, filling, feeding (simulated), flushing, and wearing could be safely executed without unacceptable use error (user action or lack of action that leads to a different result than that intended by the manufacturer or expected by the user17). Participants in novice (NV) and non-novice (NN) groups were trained by a medically trained operator, and the healthcare professional group (HCP) self-trained, in the set-up, filling, simulated feeding, flushing, and wearing of the novel EFS, before ability to use the system was assessed by requiring participants to open the package containing the system, set-up and fill the system with enteral feed to a minimum of 400mL, connect system to an extension set, simulate feeding, flushing, and wearing the system while walking, sitting and standing, without assistance from the operator but with Instructions For Use (IFU) available for guidance.
The IFU provided to participants was a booklet containing an overview of the novel EFS components and step-by-step instructions on how to operate the system, with a filling method for filling from a container of feed (pour fill method), in addition to a filling method for filling from an RTH feed bag (RTH fill method). The pour fill method, typically used in the home, was tested in all groups; however, the RTH fill method was tested in HCP only, as HCP typically use RTH in clinical settings. Instructional videos provided were replications of the content of the IFU and demonstrated the system operation. Devices used in the study were fully functional. The study followed FDA guidance on Human Factors18 and was approved by Mayo Clinic Institutional Board Review (IRB).
Population
Novices (NV) were defined as individuals who have never used or cared for someone who used a standard enteral nutrition system such as bolus, gravity bag, pump, or combination. Non-novices (NN) were either current or past consumers of HEN or caregivers of patients who had received or are currently receiving HEN. Healthcare professionals (HCP) included licensed advanced practice clinicians, nurses, and dietitians with expertise in adult and/or pediatric HEN.
Recruitment of NV and NN aimed to include males and females from three age groups (18–21, 22–55, and 56+ years of age) to ensure feasibility of use across age. Age and sex distribution was not pre-set for HCP, but recruitment aimed to represent expertise as above. The setting was a room in a clinic environment which is reflective of the intended use environment of the novel EFS (hospital, home, work/school and social settings), and typical distractions, such as ambient noise, were present.
Training session: Subjects recruited into NV and NN groups attended a 1:1 (30–60 minutes) familiarization training session with an operator, where they were introduced to the feeding system and walked through steps to set-up system, fill pouch (pour fill method) with decanted liquid from container using filling-set tubing, simulate feeding by delivery of a small volume of liquid back to a container via giving-set tubing, and flush the system, using the IFU booklet for guidance. HCP attended a training session (30–60 minutes) where they self-trained on pour fill and RTH fill methods, using IFU booklet and instructional videos. All participants were shown how pouch weight and degree of pouch inflation reduce as feed delivery into a container continues (over a few minutes); how feed delivery completion is indicated by cessation of feed flow from the giving-set; and how a pouch (prefilled to 500mL) can be worn on the body in a generic crossbody bag. Water with food dye was used as mock feed in training for practical reasons. During training, participants were surveyed to evaluate if they could (A) identify in-progress “feed delivery” by observing liquid coming out of the giving-set tubing over a few minutes, and (B) recognize when “feed delivery” is completed after allowing a pouch containing 400mL to deliver liquid out of the giving-set tubing into a container, until cessation of flow. Furthermore, during training, participants in all groups trialed execution of the steps they would later be tested on.
Testing session: With a decay period of at least 1 hour after the training session, subjects attended a 1:1 (45–60 minutes) testing session to simulate system use, ie, open system package, set-up, fill with feed (pour fill method), simulate feeding, flush, and wear the system, while consulting the IFU as needed, under supervision of the operator, but without assistance. The wearing assessment evaluated ability to coil the giving-set tubing and wear a filled feeding pouch with coiled tubing inside a generic crossbody bag, while sitting, walking around a room, and standing, for short periods. For HCP, RTH fill method was tested in addition to the pour fill method. Where necessary, two attempts to complete filling method steps successfully were offered. Where any use errors occurred, a debriefing interview was conducted following the testing session, to allow participants to provide subjective assessment of why any use error occurred. Figure 3 illustrates study flow.
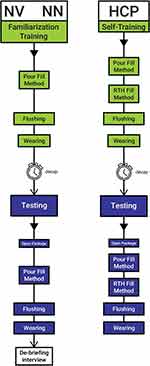 |
Figure 3 Study flow chart. |
Tasks: Tasks involved in getting the device ready to feed, feeding and flushing, detailed in the IFU, were divided into 5 use scenarios (groups of tasks that logically occur in sequence) which are common to both filling methods:
Use scenario 1: Set-up (Steps 1–6)
Use scenario 2: Filling Pouch (Steps 7–11)
Use scenario 3: Preparation to Feed (Steps 12–13)
Use scenario 4: Initiating Feeding (Step 14–15)
Use scenario 5: Flushing
To ensure users will ultimately be able to receive nutrition through the novel EFS and flush it, we checked that participants could successfully complete use scenarios 1–5 inclusive, assigning a pass/fail to each use scenario. User performances which represented success for each task within the 5 use scenarios were defined in advance, including a minimum fill volume of 400mL, chosen as it is considered a reasonable portion a typical user would fill into the novel EFS. Feeding was simulated by obtaining flow of feed out of an extension set connected to the novel EFS, into a container. Successful completion of all tasks deemed critical to the safe and effective use of the novel EFS within each use scenario resulted in a pass score. A risk analysis process conducted prior to the study determined which tasks were critical (based on FDA definition of critical tasks as those which, if performed incorrectly or not performed at all, would or could cause serious harm to the patient or user, where harm is defined to include compromised medical care18). Furthermore, the order in which tasks within a use scenario were completed was deemed inconsequential in terms of potential introduction of use error, and the protocol therefore allowed for participants to complete steps within a use scenario in any order.
Success with opening the novel EFS package, completing the 5 use scenarios, and preparing to wear a filled food pouch was evaluated with check sheets where operators captured observed completion of each step and detail of any use error, and/or participant requirement for operator assistance. Successful preparation for wearing novel EFS was deemed as successfully performing critical tasks of (1) loosely coiling giving-set tubing without kinking it, (2) putting a filled pouch (500mL) with coiled tubing inside a generic crossbody bag, and (3) placing crossbody bag on the body. In addition, operators observed participants being stationary and mobile for short periods, and recorded if participants could sit, stand, and walk while wearing the filled pouch. Given the importance of users identifying when the system is delivering and has finished delivering feed, participants’ ability to identify in-progress and complete feed delivery was evaluated by simple survey questions posed by the operator.
Data Analysis
Descriptive analysis of variables is presented. Data are presented in frequencies and percentages. Given the scope of the study, no contingency analysis was performed.
Results
A total of 47 subjects were recruited by invitation and 2 subjects were withdrawn as they could not attend the testing session. Forty-five participants completed the study in the three groups stratified by 15 participants in each group, with at least 2 participants from each of the 3 age groups present in the NV and NN groups: NV group: (n=15; 66.7% male; 3/15 (18–21 years); 10/15 (22–55 years); 2/15 (56+ years)); NN group: (n=15; 33.3% male; 10/15 HEN recipients; 5/15 caregivers; 2/15 (18–21 years); 3/15 (22–55 years); 10/15 (56+ years)); HCP group: (n=15) (Table 1).
 |
Table 1 Study Population (Novices vs Non-Novices) |
All 45 participants (100%) safely filled the system to 400mL and obtained feed flow out of novel EFS, thereby successfully simulating use; 44/45 (97.8%) of participants successfully and safely simulated use in their first attempt at executing use scenarios 1–5 inclusive. One NV, 1/45 (2.2%) failed to do so on first attempt due to filling the feeding pouch to only 100mL; however, they were successful in filling to 400mL on their second attempt. This participant commented in the debriefing interview that they failed to recall what the target fill volume was, in their first attempt. Operator assistance was required in only 1 instance throughout the study, where 1 HCP required minor assistance to resolve a slight kink in the filling-set tubing.
All 45 participants (100%) were able to open package without difficulty, remove protective caps from system components, fill the pouch with 400mL feed, disconnect filling-set tubing from the pouch, connect system to extension set, simulate feeding, flush system, loosely coil the giving-set tubing and sit, stand and move around the room while wearing a filled feeding pouch and coiled tubing within a generic crossbody bag, safely, without kinking the tubing (see Table 2). Furthermore, within the maximum allowed attempts, no participant made any unanticipated use errors or use errors previously identified in risk management files associated with the novel EFS system. Also, participants all successfully identified in-progress feed delivery and feed delivery completion.
 |
Table 2 Achievement of Usability Elements Tested Across the Groups |
Discussion
Our results indicate that the examined novel EFS is usable across the intended use population, irrespective of previous experience with enteral feeding. All participants were able to simulate use of the feeding system after completing a short familiarization training session and through consulting the IFU booklet as needed. Usability was evidenced by the fact that all participants were able to successfully complete steps to set-up, fill, simulate feeding, and flush the feeding system on the first attempt, with only one participant needing a second attempt. The packaging was adequate in terms of ease of use, and the instructions were shown to effectively guide the users on safely simulating use of the novel EFS. All participants were able to wear the filled feeding system within a crossbody bag and to sit, stand, and move around without kinking the tubing or experiencing any unanticipated or previously identified use-related errors or safety issues. Insights into the usability of the novel EFS from the study help to inform the path to clinical use, as the results validate the system design.
The proven usability of the examined novel EFS could be quite beneficial in improving mobility during feedings, leading to a significant improvement in QoL. Understanding how EN can affect QoL involves an interplay of many factors, including assessment methods, complexity of pathophysiological mechanisms that can lead to long-term HEN and setting realistic goals. An important consideration in this context is that QoL at initiation of HEN is generally poor due to a number of factors, including malnutrition and its related psychological morbidity, along with a fear of unknown complications and patients losing independence in being able to care for themselves.8,19,20 Furthermore, in patients receiving HEN, existence of an underlying acute or chronic disease such as cancer, neurological deficits, eating disorders, or motility disorders along with concurrent treatment for those diseases further exacerbates the decline in QoL.21
Currently, HEN can be delivered intermittently through gravity- or pump-assisted feeding systems for gastric feeding and generally continuously through pump-assisted feedings when provided directly into the small intestine. Gravity systems typically use a pole to raise the feedings well above the head of the patient requiring them to stay in proximity to the system while receiving EN numerous times per day and for potentially extended durations. Infusion pumps can be placed in a backpack; however, the weight of the feeds, infusion pump, and batteries to power the pump can limit mobility in many patients who are underweight or have a decline in strength due to malnutrition or their underlying disease. Due to this, most currently available systems can significantly limit personal freedom and result in difficulty moving around, leaving home, and being socially and physically active, all of which can negatively affect QoL for both consumers and caregivers.11,22
Loss of physical mobility and ability to perform tasks of daily living can place vulnerable patient populations at increased risk of falls, fractures, and institutionalization, which can lead to decline in QoL and increased morbidity, mortality, and health care utilization and cost.23,24 In a study evaluating predictors of mortality in severely disabled children, decline in mobility and use of tube feedings led to a significant increase in risk of mortality (odds ratio 4.02; 95% CI [3.12, 5.19]).25 Similarly, in over 5800 adults age 65 years or older, a decline in mobility was associated with higher annual health care costs ($3919; 95% CI [$1948-5890]), higher number of hospitalizations (22 additional hospitalizations; 95% CI [14–30]), and increased mortality (odds ratio 2.73; 95% CI [1.79–4.15]).26 A recent meta-analysis reviewed the impact of home parenteral nutrition (HPN) on QoL and noted that QoL was worse in HPN patients compared to healthy populations with the impairment in QoL being associated with decrease in physical, psychological, and social functioning.27 Similarly, an assessment of burden in caregivers for patients on home artificial nutrition noted that functional limitations resulted in higher care burden.7 It is important to recognize that these studies are referring to HPN populations. While the concept of the potential positive effect of enhanced mobility on QoL may be generalized with limitations, HEN-specific studies are needed to prove this.
Fortunately, increasing mobility can lead to improvement in many clinical outcomes, including QoL and mortality.24 In a recent Cochrane review of patients with hip fractures, the use of multi-disciplinary rehabilitation led to improvement in mobility at 6 to 12 months as well as decreased requirement of institutional care, hospitalization, and mortality28. Unfortunately, there remains a paucity of data regarding the direct impact of improved mobility on clinical outcomes in the HEN population. We do have some supporting data on the HPN population, as Saqui et al prospectively studied change in QoL with transition from stationary to portable infusion pumps in a HPN recipients cohort from Canada.15 The study noted higher patient satisfaction with use of portable pumps compared to pole-mounted pumps, which was attributed to considerable improvements in convenience with ease of use, ease of movement between rooms, travel, entertaining, noise and sleep quality.
Our study does have a number of limitations as it was designed as a simulated use study to evaluate the usability of a novel EFS, including adequacy of instruction materials. Although every effort was made to enroll a broad spectrum of participants from different age groups, two-thirds of novices were from 22–56 years age group, while two-thirds of non-novices were from 56+ years age group. This may reflect the majority demographics of our non-novice population. Future trials targeting a larger cohort will strive to ensure balanced representation across all age groups. Additionally, our current study focused on training and usability and could not evaluate clinical use of the novel EFS or impact of use of the novel EFS on mobility, QoL and other clinical outcomes. Future trials will be needed to evaluate these variables in the HEN population.
Conclusions
The results indicate that the novel EFS can be safely and effectively used in intended use population, irrespective of experience with EN. Provision of basic familiarization training and written IFU was adequate to successfully train novice and non-novice users and caregivers, and provision of videos alongside a written IFU allowed for successful self-training of healthcare professionals on use of the feeding system. Given the innovative design and lightweight nature of this novel EFS, including how it operates without any external power source or need for gravity, the evaluated system has potential to increase mobility and thus improve psychological and social well-being, QoL, as well as clinical outcomes, which could be evaluated in future prospective studies.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. While the study was sponsor-initiated with involvement with protocol development, investigators at the host institute executed the study and analyzed data independently.
Funding
This work was funded by Rockfield Medical Devices that received grant funding from the Disruptive Technologies Innovation Fund, managed by the Government of Ireland Department of Enterprise, Trade and Employment and administered by Enterprise Ireland.
Disclosure
Edel Keaveney is Senior Research Manager at Rockfield Medical Devices. Rockfield Medical Devices has the following patents granted: EP3554456B1, a portable enteral feeding apparatus; and EP3416609B1, docking station for an enteral feeding device. In addition, Rockfield Medical Devices has a number of other patents pending. Ryan T. Hurt is a consultant for Nestlé outside the submitted work. Manpreet S. Mundi reports research grants from Rockfield Medical Devices during the conduct of the study; and grants from Realfood blends, Nestlé, VectivBio, and Fresenius Kabi, and is on advisory boards for Baxter and NorthSea, outside the submitted work. The aforementioned authors report no other potential conflicts of interest in relation to this work. Osman Mohamed Elfadil, Ankitaben Patel, Marwa G. Abdelmagid, Ishani Patel, and Jalpan Patel have no relevant conflicts of interest in relation to this work to report.
References
1. Kwon RS, Banerjee S, Desilets D, et al.; ASGE Technology Committee. Enteral nutrition access devices. Gastrointest Endosc. 2010;72(2):236–248. doi:10.1016/j.gie.2010.02.008
2. Boullata JI, Carrera AL, Harvey L, et al. ASPEN safe practices for enteral nutrition therapy. J Parenter Enter Nutr. 2017;41(1):0148607116673053. doi:10.1177/0148607116673053
3. Harkness L. The history of enteral nutrition therapy: from raw eggs and nasal tubes to purified amino acids and early postoperative jejunal delivery. J Am Diet Assoc. 2002;102(3):399–404. doi:10.1016/S0002-8223(02)90092-1
4. Kim H, Stotts NA, Froelicher ES, Engler MM, Porter C. Why patients in critical care do not receive adequate enteral nutrition? A review of the literature. J Crit Care. 2012;27(6):702–713. doi:10.1016/j.jcrc.2012.07.019
5. Mohamed Elfadil O, Ewy M, Patel J, Patel I, Mundi MS. Growing use of home enteral nutrition: a great tool in nutrition practice toolbox. Curr Opin Clin Nutr Metab Care. 2021;24(5):446–452. doi:10.1097/MCO.0000000000000777
6. Barrett D, Li V, Merrick S, Murugananthan A, Steed H. The hidden burden of community enteral feeding on the emergency department. JPEN J Parenter Enteral Nutr. 2020;45(6):1347–1351. doi:10.1002/jpen.2021
7. Villar-Taibo R, Martínez-Olmos MA, Bellido-Guerrero D, et al. Burden assessment in caregivers of patients with home artificial nutrition: a need and a challenge. Eur J Clin Nutr. 2017;71(2):192–197. doi:10.1038/ejcn.2016.239
8. Asiedu GB, Carroll K, Griffin JM, Hurt RT, Mundi M. Home enteral nutrition: use of photo-elicitation to capture patient and caregiver experiences. Health Sci Rep. 2018;1(8):e56. doi:10.1002/hsr2.56
9. Thomas A, Sowerbutts AM, Burden ST. The impact of living with home enteral feeding: perspectives of people who have had a diagnosis of head and neck cancer. J Hum Nutr Diet off J Br Diet Assoc. 2019;32(5):676–683. doi:10.1111/jhn.12691
10. ASPEN enteral nutrition by the numbers: EN data across the healthcare continuum; 2017. Available from: http://www.nutritioncare.org/ENMarketReport/.
11. Ojo O, Keaveney E, Wang XH, Feng P. The effect of enteral tube feeding on patients’ health-related quality of life: a systematic review. Nutrients. 2019;11(5):1046. doi:10.3390/nu11051046
12. La Grow S, Yeung P, Towers A, Alpass F, Stephens C. The impact of mobility on quality of life among older persons. J Aging Health. 2013;25(5):723–736. doi:10.1177/0898264313490198
13. Pinto C, Salazar AP, Marchese RR, Stein C, Pagnussat AS. The effects of hydrotherapy on balance, functional mobility, motor status, and quality of life in patients with Parkinson disease: a systematic review and meta-analysis. PM R. 2019;11(3):278–291. doi:10.1016/j.pmrj.2018.09.031
14. Groessl EJ, Kaplan RM, Rejeski WJ, et al. Physical activity and performance impact long-term quality of life in older adults at risk for major mobility disability. Am J Prev Med. 2019;56(1):141–146. doi:10.1016/j.amepre.2018.09.006
15. Saqui O, Fernandes G, Allard JP. Quality of life analysis during transition from stationary to portable infusion pump in home parenteral nutrition patients: a Canadian experience. Nutr Clin Pract off Publ Am Soc Parenter Enter Nutr. 2014;29(1):131–141. doi:10.1177/0884533613516129
16. Stratton R, Evill R, Smith T, Report BANS. Home Enteral Tube Feeding (HETF) in adults (2010–2015); 2018. Available from: https://www.bapen.org.uk/pdfs/reports/bans/bans-report-2018.pdf.
17. ISO. ISO 14971:2019. Available from: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/27/72704.html.
18. Applying human factors and usability engineering to medical devices. U.S. Food and Drug Administration; 2016. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/applying-human-factors-and-usability-engineering-medical-devices.
19. Alberda C, Gramlich L, Jones N, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009;35(10):1728–1737. doi:10.1007/s00134-009-1567-4
20. Sánchez-Torralvo FJ, Contreras-Bolívar V, Ruiz-Vico M, et al. Relationship between malnutrition and the presence of symptoms of anxiety and depression in hospitalized cancer patients. Support Care Cancer. 2021;30(2):1607–1613. doi:10.1007/s00520-021-06532-y
21. Howard L, Ament M, Fleming CR, Shike M, Steiger E. Current use and clinical outcome of home parenteral and enteral nutrition therapies in the United States. Gastroenterology. 1995;109(2):355–365. doi:10.1016/0016-5085(95)90321-6
22. Day T. Home enteral feeding and its impact on quality of life. Br J Community Nurs. 2017;22(Sup7):S14–S16. doi:10.12968/bjcn.2017.22.Sup7.S14
23. Mundi MS, Patel J, McClave SA, Hurt RT. Current perspective for tube feeding in the elderly: from identifying malnutrition to providing of enteral nutrition. Clin Interv Aging. 2018;13:1353–1364. doi:10.2147/CIA.S134919
24. Shafrin J, Sullivan J, Goldman DP, Gill TM. The association between observed mobility and quality of life in the near elderly. PLoS One. 2017;12(8):e0182920. doi:10.1371/journal.pone.0182920
25. Strauss D, Eyman RK, Grossman HJ. Predictors of mortality in children with severe mental retardation: the effect of placement. Am J Public Health. 1996;86(10):1422–1429. doi:10.2105/AJPH.86.10.1422
26. Hardy SE, Kang Y, Studenski SA, Degenholtz HB. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J Gen Intern Med. 2011;26(2):130–135. doi:10.1007/s11606-010-1543-2
27. Winkler MF. Quality of life in adult home parenteral nutrition patients. J Parenter Enter Nutr. 2005;29(3):162–170. doi:10.1177/0148607105029003162
28. Handoll HH, Cameron ID, Mak JC, Panagoda CE, Finnegan TP. Multidisciplinary rehabilitation for older people with Hip fractures. Cochrane Database Syst Rev. 2021;11:CD007125. doi:10.1002/14651858.CD007125.pub3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.