Back to Journals » OncoTargets and Therapy » Volume 9
URG4 expression is a novel prognostic factor for the progression of nasopharyngeal carcinoma and overall survival of patient
Authors Yu G, Meng Q, Zhang T, Zeng C, He B, Zhang S
Received 31 August 2015
Accepted for publication 3 February 2016
Published 23 May 2016 Volume 2016:9 Pages 3059—3065
DOI https://doi.org/10.2147/OTT.S95476
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Daniele Santini
Guodong Yu,1,* Qingxiang Meng,2,* Tian Zhang,1 Chen Zeng,1 Benfu He,3 Shanshan Zhang1
1ENT and HN Surgery Department, Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, 2Department of Otorhinolaryngology – Head and Neck Surgery, Guangzhou First People’s Hospital, Guangzhou Medical University, Guangdong, 3Oncology Department, PLA421 Hospital, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Abstract: URG4, a novel oncogene, is involved in the development and progression of various tumors. This study investigated the clinicopathological significance of URG4 in nasopharyngeal carcinoma (NPC). We used five NPC tissues and adjacent normal nasopharyngeal tissues to determine URG4 expression and found that URG4 was upregulated in NPC tissues. Immunohistochemistry analysis found URG4 was expressed positively in 97.1% (99/102) of NPC samples and highly expressed in 41.2% (42/102) of NPC samples. Its level was positively correlated with advancing clinical stage. Kaplan–Meier analysis with the log-rank test found that patients with high URG4 expression had poor outcome and patients with low URG4 expression had better survival. Statistical analysis showed that there was a significant correlation between URG4 expression and clinical stage, larger tumor size, and lymph node involvement. Cox-regression analysis showed that URG4 expression could serve as a prognostic factor for NPC patients. In summary, this study showed that URG4 was upregulated in NPC tissues, patients with high URG4 expression had poor outcome, and URG4 was found to be a valuable biomarker for NPC progression.
Keywords: URG4, nasopharyngeal carcinoma, prognostic factor
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common malignant neoplasms in southern China – the incidence in Guangdong Province is about 100-fold higher compared to Europe and North America. Some susceptibility loci and oncogenes have been demonstrated to promote or suppress NPC progression, and in the past few decades, they have been found to serve as prognostic factors and therapy targets; for example, TNFRSF19, CDKN2A-CDKN2B, and MDS1-EVI1 are some newly identified susceptibility loci.1 BMI1 is a new oncogene for NPC and promotes epithelial–mesenchymal transition (EMT) Mechanistic analyses have found that it binds to tumor suppressor PTEN directly and inhibits its activity to activate PI3K/Akt/GSK-3β signaling pathway. This signaling can increase the stability of Snail that facilitates EMT. Snail also suppresses PTEN expression, PTEN, and PI3K/Akt/GSK-3β and forms a positive feedback loop.2 But the regulatory mechanism of the development and progression of NPC has not been understood well, and hence the survival of patients with NPC is still poor. However, early diagnosis is critical for NPC treatment and identification of useful biomarkers is essential for NPC prognosis.
URG4, a novel oncogene, promotes the progression of various tumors. For example, it is a natural product of hepatitis B × antigen (HB×Ag), which participates in hepatocarcinogenesis.3 URG4 is upregulated in hepatocellular carcinoma (HCC) tissues. Statistical analyses suggest that its expression is significantly correlated with clinical stages and poor survival of HCC patients. Functional analyses have found that URG4 not only activates Akt signaling pathway, and suppresses cell cycle inhibitors p27 and p21 to promote cell proliferation,4,5 but also activates NF-κB pathway to facilitate angiogenesis.6 URG4 expression is higher in ovarian cancer tissues and hence can serve as a new prognostic marker for ovarian cancer.7,8 Apart from HCC and ovarian cancer, URG4 also plays critical roles in gastric cancer,9 bladder cancer,10 glioblastoma multiforme,11 non-small-lung cancer,12 medullary thyroid cancer,13 neuroblastoma,14 prostate cancer,15 and leukemia.16 But the role of URG4 in NPC has not been reported yet.
In this study, we determined URG4 expression in NPC tissues and adjacent normal nasopharyngeal tissues and analyzed the relationship between URG4 expression and various clinicopathologic characteristics. Finally we used Cox-regression analysis to evaluate whether URG4 can serve as a prognostic marker for NPC patients.
Materials and methods
Patients and tissue specimens
A total of 102 paraffin-embedded NPC samples were histopathologically diagnosed in 1998–2003 at the Guangzhou First People’s Hospital, Guangzhou Medical University, Guangdong, People’s Republic of China. These clinical materials were used for research purposes after obtaining patient’s written consent. This study was approved by the Institutional Research Ethics Committee of the Guangzhou First People’s Hospital. Clinicopathological information of these samples is shown in Table 1. The clinical stages of all patients were defined according to the Chinese 1992 staging system.17
  | Table 1 Clinicopathological characteristics of NPC patient samples |
Five NPC specimens (T) and adjacent normal nasopharyngeal specimens were also obtained from the Guangzhou First People’s Hospital, Guangzhou Medical University. These samples were embedded using paraffin and were stored at 4°C until use.
Immunohistochemistry
Immunohistochemistry (IHC) was performed according to the previously described method by Cao et al.18 Briefly, all paraffin-embedded specimens were cut into 4 μm sections and deparaffinized with xylenes. They were then rehydrated with graded ethanol to distilled water. Antigen retrieval was performed by submerging sections into ethylene diamine tetraacetic acid buffer (pH 8.0) and heating in a microwave oven. A total of 0.3% H2O2 was used to treat sections for 15 minutes to block endogenous peroxidase after being heated, and then the sections were incubated with 1% bovine serum albumin to block nonspecific binding and then incubated with anti-URG4 antibody (1:150, HPA020134, Sigma-Aldrich Co., St Louis, MO, USA) overnight at 4°C. After washing with phosphate-buffered saline, the sections were incubated with biotinylated secondary antibody, followed by further incubation with streptavidin–horseradish peroxidase at 37°C for 30 minutes. The sections were immersed in 3,3′-diaminobenzidine for 10 minutes and counterstained with 10% Mayer’s hematoxylin, dehydrated, and mounted in crystal mount. For negative controls, the primary antibody was replaced by normal goat serum.
The results of staining were scored independently by three pathologists blinded to clinical outcome according to the proportion of positively stained tumor cells and the intensity of staining. The proportion of positively stained tumor cells was scored as follows: 0 (no positive tumor cells), 1 (<20% positive tumor cells), 2 (20%–50% positive tumor cells), 3 (50%–80% positive tumor cells), and 4 (>80% positive tumor cells). The intensity of staining was graded as follows: 0 (no staining), 1 (weak staining, light yellow), 2 (moderate staining, yellow brown), and 3 (strong staining, brown). The staining index (SI) was calculated as the product of the staining intensity score and the proportion of positive tumor cells score, resulting in scores of 0, 1, 2, 3, 4, 6, 8, 9, and 12. Cutoff values for high and low expression of URG4 were chosen according to the measurement of heterogeneity using the log-rank test with respect to overall survival. The optimal cutoff for high expression of URG4 was identified as an SI score ≥4 and the cutoff for low expression of URG4 was identified as an SI score ≤3.
Statistical analysis
All statistical analyses were performed using the SPSS 19.0 statistical software package. Chi-square and Fisher’s exact tests were used to analyze the relationship between URG4 expression and clinicopathologic parameters. Bivariate correlations between variables were calculated by Spearman’s rank correlation coefficients. Survival curve was plotted using Kaplan–Meier method and compared by the log-rank test. Univariate and multivariate Cox-regression analyses were used to analyze the significance of various variables for survival. A value of P<0.05 (two tailed) was considered significant in all cases.
Results
URG4 is upregulated in NPC tissues
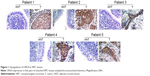
To investigate the role of URG4 in NPC, we determined the expression of URG4 in the NPC tissues and adjacent normal nasopharyngeal tissues of five patients by IHC. We found that URG4 was expressed only in primary NPC tissues and no specific URG4 staining was observed in adjacent normal nasopharyngeal tissues (Figure 1). These suggested that URG4 was upregulated in NPC tissues.
URG4 expression is positively correlated with clinical aggressiveness of NPC
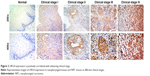
We determined URG4 expression in 102 paraffin-embedded archival NPC tissues using IHC. These samples included four stage I tumors, 27 stage II tumors, 50 stage III tumors, and 21 stage IV tumors. URG4 was detected in 99 of 102 (97.1%) cases. Its expression was high in 42 (41.2%) cases and low in 60 (58.8%) cases (Table 2). We also found that URG4 was mainly located in cytomembrane (Figure 2). We further investigated URG4 expression in different clinical stages and the detailed data are as follows: 0% for stage I (0/4), 6.9% for stage II (2/27), 46% for stage III (23/50), and 81% for stage IV (17/21) (Table 3); this suggests that URG4 expression was positively correlated with advancing clinical stage (Figure 2).
  | Table 2 The expression of URG4 in NPC tissues |
  | Table 3 The correlation between URG4 expression and clinicopathologic characteristics of NPC |
We analyzed the association between URG4 expression and the patient’s clinicopathologic characteristics. As shown in Table 3, we found that URG4 expression was significantly correlated with clinical stage, larger tumor size (T classification), and lymph node involvement (N classification). But whether URG4 expression was significantly correlated with distant metastasis (M classification) is not clear because different P-values were calculated according to different statistical methods (P=0.036 using chi-square test and P=0.067 using Fisher’s exact test). However, there was no significant correlation between URG4 expression, sex, and age. We further analyzed the correlation between URG4 expression and clinical stage, larger tumor size, lymph node involvement, and distant metastasis using Spearman’s correlation analysis and found that URG4 expression was positively correlated with these clinicopathologic characteristics (Table 4).
  | Table 4 Spearman correlation analysis between URG4 and clinical pathologic factors |
High URG4 expression is associated with an unfavorable prognosis of NPC
We investigated the correlation between URG4 expression and patient’s survival using Kaplan–Meier analysis with the log-rank test. We found that URG4 expression was significantly negatively correlated with the overall survival of NPC patients, patients with low expression of URG4 had better outcome, and patients with high expression of URG4 had poor survival (Figure 3).
  | Figure 3 Kaplan–Meier curves with log rank test. |
We used univariate Cox-regression analysis to determine the clinical significance of various prognostic factors that might influence the survival and tumor progression in NPC patients. The results showed that clinical stage, T classification, and URG4 expression were significant poor prognostic factors. Multivariate Cox-regression analysis revealed that clinical stage, T classification, and URG4 expression were independent prognostic factors for patients with NPC (Table 5). Thus, these results suggested that URG4 expression has a significant correlation with the prognosis of NPC and could serve as a biomarker for the prognosis of NPC patients.
  | Table 5 Univariate and multivariate analyses of various prognotic parameters in patients with NPC Cox-regression analysis |
Discussion
In this study, we found that URG4 was overexpressed in NPC tissues and its expression was positively correlated with advancing clinical stage. Statistical analysis revealed that URG4 expression significantly correlated with NPC progression and patients with high URG4 expression had poor clinical outcome. Therefore, it can function as an unfavorable prognostic factor.
New valuable prognostic factors are important to develop novel therapeutic strategies. Recently, many prognostic factors, such as centromere protein H (CENP-H)17 and MMP9,19 have been found. To estimate cancer progression and the outcomes of NPC patients, more biomarkers have to be discovered. We used a cohort of 102 patients’ samples to determine the relationship between URG4 expression and patient survival. We found that patients with high URG4 expression had poor survival. To demonstrate whether URG4 could serve as an indicator of NPC progression, we analyzed the correlation between URG4 expression and clinicopathologic parameters; we found that with the progression of NPC, the URG4 expression was upregulated gradually. This suggested that URG4 expression was significantly positively correlated with advancing clinical stage. We also found that there was a significant correlation between clinical stage, larger tumor size, and lymph node involvement. These suggested that URG4 was a biomarker of NPC progression. Univariate and multivariate Cox-regression analyses showed that patients with high URG4 expression had poor prognosis. These results emphasize that URG4 is a novel biomarker for NPC and it can be combined with other indentified biomarkers to choose appropriate therapy method, such as adjuvant chemotherapy, radiotherapy, or chemotherapy, and to predict the clinical stage and survival rate.
URG4 localization is different in different kinds of tumors. In ovarian cancer and HCC, URG4 mainly is expressed in cytoplasm,4,8 whereas in cervical cancer, URG4 mainly is found in plasma membrane.7 We found that presence of URG4 in cytomembrane in NPC conditions facilitated clinical diagnosis.
Previous reports have shown that URG4 activates Akt and NF-κB pathway in HCC.4,6 Akt pathway7 plays critical role in survival, glucose uptake, angiogenesis, proliferation, and metabolism. It inhibits Wee1, Myt1, p27, p21, and cyclin D1 and suppresses the progression of cell cycle. It also inhibits FOXO1, FOXO3a, and Bad, and meanwhile activates MDM2 to regulate apoptosis.20 Akt has been demonstrated to regulate EMT in NPC.2 NF-κB pathway regulates tumor cellular proliferation, apoptosis, angiogenesis, and metastasis.21 Akt2 and NF-κB pathway22 regulate proliferation, metastasis, and apoptosis of NPC. There was a significant positive correlation between URG4 expression and clinical stage, larger tumor size, lymph node involvement, and distant metastasis, which suggested that URG4 might regulate proliferation and metastasis of NPC. We speculated that URG4 might regulate proliferation and metastasis through Akt pathway or NF-κB pathway. These findings suggested that URG4 might be a novel target for NPC therapy. But the role of URG4 in NPC should be studied in depth.
Conclusion
In conclusion, our study suggested that URG4 was upregulated in NPC tissues and its level showed a significant correlation with clinical stage, larger tumor size (T classification), and lymph node involvement (N classification). Patients with high URG4 level had poor outcome and URG4 was found to be an independent prognosis factor for NPC patients.
Acknowledgments
This study was supported by the Foundation of Guizhou Science and Technology Department (D2011-16), the Foundation of Governor of Guizhou Province (S2010-8), the Foundation of Guizhou Provincial Education Department (F2010-8), and the Science and Technology Planning Project of Guangdong Province, People’s Republic of China (2012B031800480).
Disclosure
The authors report no conflict of interest in this work.
References
Bei JX, Li Y, Jia WH, et al. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nature Genet. 2010;42(7):599–603. | ||
Song LB, Li J, Liao WT, et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest. 2009;119(12):3626–3636. | ||
Tufan NL, Lian Z, Liu J, et al. Hepatitis Bx antigen stimulates expression of a novel cellular gene, URG4, that promotes hepatocellular growth and survival. Neoplasia. 2002;4(4):355–368. | ||
Xie C, Song LB, Wu JH, et al. Upregulator of cell proliferation predicts poor prognosis in hepatocellular carcinoma and contributes to hepatocarcinogenesis by downregulating FOXO3a. PLoS One. 2012;7(7):e40607. | ||
Satiroglu-Tufan NL, Dodurga Y, Gok D, Cetinkaya A, Feitelson MA. RNA interference-mediated URG4 gene silencing diminishes cyclin D1 mRNA expression in HepG2 cells. Genet Mol Res: GMR. 2010;9(3):1557–1567. | ||
Xing S, Zhang B, Hua R, et al. URG4/URGCP enhances the angiogenic capacity of human hepatocellular carcinoma cells in vitro via activation of the NF-kappaB signaling pathway. BMC Cancer. 2015;15(1):368. | ||
Zhang L, Huang H, Zhang L, et al. URG4 overexpression is correlated with cervical cancer progression and poor prognosis in patients with early-stage cervical cancer. BMC Cancer. 2014;14:885. | ||
Li W, Zhou N. URG4 upregulation is associated with tumor growth and poor survival in epithelial ovarian cancer. Arch Gynecol Obstet. 2012;286(1):209–215. | ||
Song J, Xie H, Lian Z, et al. Enhanced cell survival of gastric cancer cells by a novel gene URG4. Neoplasia. 2006;8(12):995–1002. | ||
Wu M, Chen J, Wang Y, et al. URGCP/URG4 promotes apoptotic resistance in bladder cancer cells by activating NF-kappaB signaling. Oncotarget. 2015;6(31):30887–30901. | ||
Chen LC, Zhang HY, Qin ZY, et al. Serological identification of URGCP as a potential biomarker for glioma. CNS Neurosci Ther. 2014;20(4):301–307. | ||
Cai J, Li R, Xu X, et al. URGCP promotes non-small cell lung cancer invasiveness by activating the NF-kappaB-MMP-9 pathway. Oncotarget. 2015;6(34):36489–36504. | ||
Dodurga Y, Eroglu C, Secme M, Elmas L, Avci CB, Satiroglu-Tufan NL. Anti-proliferative and anti-invasive effects of ferulic acid in TT medullary thyroid cancer cells interacting with URG4/URGCP. Tumour Biol. Epub 2015 Sep 3. | ||
Dodurga Y, Gundogdu G, Koc T, Yonguc GN, Kucukatay V, Satiroglu-Tufan NL. Expression of URG4/URGCP, Cyclin D1, Bcl-2, and Bax genes in retinoic acid treated SH-SY5Y human neuroblastoma cells. Contemp Oncol. 2013;17(4):346–349. | ||
Dodurga Y, Avci CB, Susluer SY, Satiroglu Tufan NL, Gunduz C. The expression of URGCP gene in prostate cancer cell lines: correlation with rapamycin. Mol Biol Rep. 2012;39(12):10173–10177. | ||
Dodurga Y, Oymak Y, Gunduz C, et al. Leukemogenesis as a new approach to investigate the correlation between up regulated gene 4/upregulator of cell proliferation (URG4/URGCP) and signal transduction genes in leukemia. Mol Biol Rep. 2013;40(4):3043–3048. | ||
Liao WT, Song LB, Zhang HZ, et al. Centromere protein H is a novel prognostic marker for nasopharyngeal carcinoma progression and overall patient survival. Clin Cancer Res. 2007;13(2 Pt 1):508–514. | ||
Cao JY, Liu L, Chen SP, et al. Prognostic significance and therapeutic implications of centromere protein F expression in human nasopharyngeal carcinoma. Mol Cancer. 2010;9:237. | ||
Liu Z, Li L, Yang Z, et al. Increased expression of MMP9 is correlated with poor prognosis of nasopharyngeal carcinoma. BMC Cancer. 2010;10:270. | ||
Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. | ||
Perkins ND. The diverse and complex roles of NF-kappaB subunits in cancer. Nat Rev Cancer. 2012;12(2):121–132. | ||
Huang W, Liu J, Feng X, et al. DLC-1 induces mitochondrial apoptosis and epithelial mesenchymal transition arrest in nasopharyngeal carcinoma by targeting EGFR/Akt/NF-kappaB pathway. Med Oncol. 2015;32(4):115. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.