Back to Journals » Risk Management and Healthcare Policy » Volume 16
Uptake, Safety and Attitudes Towards COVID-19 Vaccination: A Cross-Sectional Study on First and Second Doses Among the General Public
Authors Mahmoud MA , Ibrahim A , Alharbi F, Alalawi AM, Alnezary F, Aldafiri A, Alahmadi Y , Alolayan SO, Althaqfan SS, Alsultan MM , Omer S, Alsahly MB
Received 8 May 2023
Accepted for publication 26 July 2023
Published 18 August 2023 Volume 2023:16 Pages 1633—1643
DOI https://doi.org/10.2147/RMHP.S418300
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Mansour A Mahmoud,1 Alnada Ibrahim,2 Fawaz Alharbi,3 Ali Mohammed Alalawi,4 Faris Alnezary,1 Ahmed Aldafiri,3 Yaser Alahmadi,1 Sultan Othman Alolayan,1 Sultan S Althaqfan,1 Mohammed M Alsultan,5 Safaa Omer,6 Musaad B Alsahly7
1Department of Clinical and Hospital Pharmacy, College of Pharmacy, Taibah University, Al-Madinah Al-Munawarah, Saudi Arabia; 2Department of Pharmacy Practice, College of Pharmacy, Princess Nourah bint Abdulrahman University, P.O.Box 84428, Riyadh, 11671, Saudi Arabia; 3AlHaram Hospital, Ministry of Health, Al-Madinah Al-Munawarah, Saudi Arabia; 4Department of Pharmacology and Toxicology, College of Pharmacy, Taibah University, Al-Madinah Al-Munawarah, Saudi Arabia; 5Department of Pharmacy Practice, College of Clinical Pharmacy, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 6Department of Clinical Biochemistry, College of Medicine, King Khalid University, Abha, Saudi Arabia; 7College of Medicine, Department of Physiology, King Saud University, Riyadh, Saudi Arabia
Correspondence: Alnada Ibrahim, Department of Pharmacy Practice, College of Pharmacy, Princess Nourah bint Abdulrahman University, P.O. Box 84428, Riyadh, 11671, Saudi Arabia, Email [email protected] Mansour A Mahmoud, Department of Clinical and Hospital Pharmacy, College of Pharmacy, Taibah University, AL-Madinah Al-Munawara, Saudi Arabia, Email [email protected]
Objective: To investigate public uptake, attitudes and the safety of the first and second doses of COVID-19 vaccination.
Methods: This was a cross-sectional web-based survey study. A self-administered questionnaire was prepared from a literature search and information about COVID-19 available at various resources. The developed questionnaire was validated for readability by experts and refined in light of the feedback received from the experts and the final version was prepared. The reliability of the questionnaire was 0.7 which shows an acceptable level of scale internal consistency. The data analysis was performed using IBM SPSS software (version 25).
Results: A total of 513 participants completed the survey, including 311 (60.6%) women and 202 (39.4%) men. The mean age was (31.5± 12.8) years. It was found that 493 (96.1%) took the first and second doses of COVID-19 and 376 (73.3%) suffered from side effects, of these 14% (56/376) reported the side effects to the health authorities. The most common side effects were fatigue (51.5%), fever (42.3%), headache (39.5%), and injection site pain (37.6%). Half of the participants (50.5%) had a positive attitude towards COVID-19 preventive measures. Females had higher odds of experiencing side effects than males OR (95% CI); 2.002 (1.312– 3.056). Individuals living in urban areas had lower odds of experiencing side effects than those living in rural areas OR (95% CI); 0.364 (0.142– 0.933).
Conclusion: Vaccine uptake was massive and side effects due to the COVID-19 vaccine were common but minor. The majority of the participants had positive attitudes towards recommended COVID-19 preventive measures. Being female and living in rural areas were associated with experiencing side effects.
Keywords: COVID-19, safety, uptake
Introduction
According to the John Hopkins University’s Coronavirus Resource Center, there is a global total of 676,609,95 COVID-19 cases, with 6.881 million deaths and 13,338,833,19 vaccine doses administered.1 Saudi Arabia had 841,469 confirmed cases of COVID-19, with 9,646 deaths and a total of 68,534,631 vaccine doses administered.2 In mid-December 2020, the BNT162b2 mRNA vaccine developed by Pfizer-BioNTech became the first vaccine to receive approval for use in the Kingdom of Saudi Arabia.3 (KSA) By July 2021, other three COVID-19 vaccines were approved for use in Saudi Arabia including the Oxford/AstraZeneca vaccine (AZD1222), Johnson & Johnson (Ad26.COV2.S), and Moderna (mRNA-1273).4
In December 2019, the onset of SARS-CoV-2 presented a formidable challenge to the world, both in terms of public health and economics. The virus spread rapidly across the globe, leading the World Health Organization (WHO) to declare it a pandemic and public health emergency of international concern on January 30, 2020.5 COVID-19 can manifest in a variety of clinical symptoms, ranging from no symptoms at all to acute respiratory distress syndrome (ARDS) and multiple organ failure. Common symptoms include cough, fever, sore throat, muscle aches, headache, and shortness of breath. In some cases, the disease can rapidly progress from pneumonia and respiratory failure to death within the first week, likely due to a cytokine storm.6 The primary mode of transmission is through human-to-human contact, primarily through respiratory droplets.5
The impact of COVID-19 on daily life has been significant, placing a tremendous burden on healthcare workers and governments worldwide. Coordination between manufacturers, government agencies, and healthcare professionals is crucial, along with addressing vaccine hesitancy and providing transparent information to the public for the success of COVID-19 vaccination programs.7 To combat the spread of the disease, measures such as investigations, quarantine implementation, and emergency hospital response have been put in place. In Saudi Arabia, effective strategies such as curfews, travel restrictions, and limitations on social and religious gatherings, along with mask mandates and digital technology, have been implemented to swiftly restore normal living.8 The development of new vaccines has been a game-changer in the fight against the disease, offering a critical intervention in managing its spread.9 The efficacy of the COVID-19 vaccine can be influenced by several factors such as comorbidities, behavioral factors like social distancing, and genetic variations.10 In addition, vaccine hesitancy and reluctance have posed a significant hindrance in achieving vaccination goals worldwide.11,12 In response, Saudi Arabia has implemented regulations such as the issuance of health passports to individuals who have received two vaccine doses and restricting non-immunized travelers from entering the kingdom.13
Early studies on the COVID-19 vaccine have indicated limited efficacy (33–52%) in reducing coronavirus cases after receiving only one dose.14,15 However, multiple studies from different countries have demonstrated that a two-dose vaccination series is necessary to achieve ≥ 80% protection against SARS-CoV-2 infection.16–18 In Saudi Arabia, various studies have been conducted to assess the public’s willingness to receive the vaccine,19–22 with acceptance rates ranging from 50% to 78%. Concerns regarding safety and efficacy were identified as the primary barriers to not receiving the vaccine.
Prior studies in Saudi Arabia mainly focused on COVID-19 vaccine acceptance and hesitancy before the government approved the third vaccine dose. Therefore, our study aims to explore the prevalence of first and second COVID-19 vaccine doses uptake among the general public, as well as the side effects, and attitudes toward the Ministry of Health’s (MOH) recommended preventive measures at a time when COVID-19 cases have decreased.
Methods
Study Design and Setting
This was a national cross-sectional web-based survey study conducted in the period from 30/12/2021 to 26/1/2022.
Survey Development
A self-administered Arabic language survey was prepared from a literature search and information about COVID-19 available at various resources such as the WHO website and the Saudi Arabia Ministry of Health website. The survey consisted of four sections. The first section consisted of demographic characteristics such as age, gender, marital status, region, and educational level. The second section explored public experiences with the COVID-19 vaccine including previously diagnosed with PCR-positive COVID-19, uptake of the first and second COVID-19 doses, diagnosis with PCR-positive COVID-19 after taking the first and second doses, side effects related to COVID-19 vaccine. The third section explored attitudes towards COVID-19 prevention strategies which were assessed using four questions and the responses were graded using 5 points Likert scale (Strongly disagree, Disagree, neither agree nor disagree, Agree, strongly agree). The preventive measures included; visiting crowded areas, wearing a mask, using hand sanitizer, and washing hands regularly. The item “I often go to crowded areas” has a reversed scoring, meaning that a higher score indicates less adherence to the preventive measure. A total attitude score of 80–100% (positive attitude), a score of 60–79% (neutral), and a score of less than 60% (negative attitude).23 The fourth section explored different issues related to COVID-19 such as reasons for not taking the first and second COVID-19 doses, and types of vaccines received.
Validity and Reliability of the Survey
The developed questionnaire was circulated to four experts from different geographic regions to carefully assess the content of the questionnaire (using a scale of 1—poor to 5—excellent) and were asked to critically appraise the survey tool. The readability of the questionnaire was assessed by randomly selected faculty members. Finally, the questionnaire was refined in light of the feedback received from the experts and the final survey was prepared. The reliability of the questionnaire was measured by calculating Cronbach’s alpha and it was found to be 0.7 which shows an acceptable level of scale internal consistency.24
Sample Size
The sample size was calculated using the RAOSOFT sample size calculator based on population size with a 5% margin of error and a 95% confidence interval for a target population of 36 million.25 The minimum required sample size was 385. An extra 20% was added to manage any dropout and the minimum required sample size was estimated at 462 participants.
Ethical Consideration
Participants were informed about the study objective, and that their participation in the study was completely voluntary. Electronic informed consent was provided on the cover page of the questionnaire. Confidentiality of the information was guaranteed throughout the study period by making participants’ information anonymous and asking them to provide honest answers. The study was conducted in accordance with the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) guidelines.26 Ethical approval was obtained from the Institutional Review Board (IRB), General Directorate of Health Affairs, Ministry of Health (25/8/2021/IRB/-1548). One of the authors of this manuscript is affiliated with the Ministry of Health. This study complies with the Declaration of Helsinki.
Data Analysis
The data was initially recorded in an Excel spreadsheet and underwent a thorough cleaning process to eliminate any incomplete or outlier values. Descriptive statistics were employed to present the data, and categorical variables were reported as frequency and percentages. To assess the overall attitude level across subgroups such as age, gender, education level, marital status, geographical region, occupation, and residence, the Pearson Chi-Square and Kruskal–Wallis Test were applied as appropriate. To determine the factors affecting COVID-19 incidence and potential side effects after the first and second COVID-19 vaccine, a univariate logistic regression was conducted. Variables identified as significant in the univariate regression analysis were included in a multivariate logistic regression model. Crude odds ratio (OR), adjusted OR with their 95% confidence interval (CI), and p-value for each determinant were presented. The adjusted OR takes into account the effects of other variables in the model, whereas the crude OR does not. The significance level was set at p-value < 0.05. The statistical analysis was performed using IBM SPSS software version 25.
Results
Demographic Characteristics of the Participants
A total of 513 participants completed the survey, including 311 (60.6%) women and 202 (39.4%) men. Most participants were ≤31 years 273 (53.2%). The mean age was (31.5±12.8) years. Most participants had university education level 367 (71.5%) and the majority were single 278 (54.2%). More than half of the participants were residing in the western region 279 (54.4%) followed by the central region 98 (19.1%). Table 1 summarized the demographic characteristics of the participants (N=513).
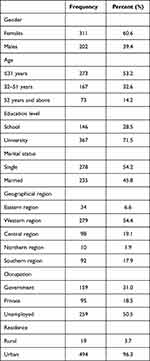 |
Table 1 Demographic Characteristics of the Participants (N=513) |
Public Experience with the COVID-19 Vaccine
It was found that 193 (37.9%) were previously diagnosed with COVID-19, 493 (96.1%) took the first and second dose of COVID-19, 100 (19.5%) were diagnosed with COVID-19 after taking the first and second shots of the vaccines, and 376 (73.3%) suffered from side effects, of these 14% (56/376) reported side effects to the health authorities. Table 2 summarized the public experience with COVID-19 vaccines.
 |
Table 2 Public Experience with the COVID-19 Vaccines |
Table 3 presents the determinants of the incidence of COVID-19 after the first and second COVID-19 vaccine. The results are obtained using logistic regression analysis, and the crude odds ratios (OR) and corresponding 95% confidence intervals (CI) and p-values are presented for each determinant. Individuals with higher education levels were 1.95 times more likely to develop COVID-19 after the first and second COVID-19 vaccine than individuals with lower education levels. 95% CI (1.23–3.09) (p-value = 0.004). However, there was no significant association between gender, age, or marital status and the incidence of COVID-19 after vaccination.
 |
Table 3 Determinants of Incidence of COVID-19 After the First and Second COVID-19 Vaccine |
Table 4 presents the results of a logistic regression analysis examining the determinants of side effects after the first and second doses of the COVID-19 vaccine. The table shows the crude odds ratio (OR), adjusted OR with their 95% confidence interval (CI), and p-value for each determinant. The results show that gender is significantly associated with side effects after vaccination, with females having higher odds of experiencing side effects than males OR (95% CI); 2.060 (1.3533–3.135). This association remains significant even after adjusting for other variables in the model OR (95% CI); 2.002 (1.312–3.056). The residence was significantly associated with side effects, with individuals living in urban areas having lower odds of experiencing side effects than those living in rural areas OR (95% CI); 0.328 (0.130–0.827). This association also remains significant after adjusting for other variables in the model OR (95% CI); 0.364 (0.142–0.933). Age, education level, marital status, and occupation do not show a significant association with side effects after vaccination.
 |
Table 4 Determinants of Side Effects After the First and Second COVID-19 Vaccine Doses |
Reasons for Not Taking COVID-19 Vaccines
Reasons for not taking the first and second doses of the vaccines were side effects (42.6%), fear of getting infection after vaccination (23.1%), hesitance (23.1%), and feeling that vaccines are ineffective against COVID-19 (Figure 1).
 |
Figure 1 Reasons for not taking the first and second COVID-19 doses. |
Side Effects
Participants reported several side effects related to COVID-19 vaccines shots. The most common side effect was fatigue (51.5%), fever (42.3%), headache (39.5%), and injection site pain (37.6%). However, sleeping disturbances, dizziness, swelling at the injection site, nausea, and stress were uncommon and were presented in 13.8% to 10.5% of the participants (Figure 2).
 |
Figure 2 Side effects after taking the first and second shots of COVID-19. |
Attitudes Toward COVID-19 Preventive Measures
The highest mean attitude items score was for the question about “regularly wearing a mask” and the lowest mean score was for the question ‘I often go to crowded areas’ (Table 5).
 |
Table 5 Public Attitudes Towards COVID-19 Preventive Measures |
Half of the participants had positive attitudes, 40.7% had neutral attitudes and 8.8% had negative attitudes about COVID-19 preventive measures (Table 6).
 |
Table 6 Overall Attitude Level Across Subgroups |
Discussion
We conducted a study to determine the prevalence of the first and second COVID-19 vaccine dose uptake among the public, as well as their experiences and attitudes towards COVID-19 prevention strategies. Our findings revealed a high prevalence rate of COVID-19 vaccination uptake (96%). In contrast, during the first quarter of 2021, a study among 1935 adult participants from the general population in Saudi Arabia found that the rate of the vaccine uptake was 22.4%.27 This finding may indicates a growing understanding of the importance of vaccination to control the COVID-19 disease. In the current study although approximately three-quarters of participants experienced side effects, there were mostly minor. Additionally, half of the participants had positive attitudes towards COVID-19 prevention strategies. Being female and living in rural areas were associated with experiencing side effects.
The side effects of COVID-19 vaccination have become well-known, as evidenced by several studies.28–31 With over 200 vaccines currently undergoing preclinical trials,32 it is important to conduct active pharmacovigilance studies and maintain an active reporting system for any side effects that may occur. Among the reported side effects, pain at the injection site is the most commonly observed.28,30 Our study found that body pain, fever, headache, and injection site pain were the most frequently reported side effects, with a higher incidence among women compared to men, which is consistent with previous research.28,33 Hormonal and psychological factors may contribute to this gender discrepancy.33 Intranasal COVID-19 vaccines present a potential alternative to reduce the discomfort typically associated with injection site pain.34
Several studies have documented public reluctance toward receiving COVID-19 vaccines.19,35 However, in the present study, there was a high prevalence of first and second COVID-19 vaccine uptake. This could potentially be attributed to the rules, regulations, and strategies implemented by healthcare authorities in Saudi Arabia. For instance, health authorities in Saudi Arabia mandated the uptake of both the first and second doses of the COVID-19 vaccine. To aid in tracking vaccination status, a mobile application called Tawakkalna® was developed and widely utilized by the public. Individuals who declined vaccination were denied entry to numerous government and private establishments throughout Saudi Arabia, as their vaccination status was checked through the Tawakkalna® mobile application. This has increased the number of vaccinated individuals in Saudi Arabia.
In the current study, side effects were the most common reason for individuals not taking the first and second doses of the COVID-19 vaccine. Hesitancy was the third most common reason for not taking the vaccine, and it seems that the rate of vaccination uptake decreased with the number of doses taken. However, earlier studies showed that people had significant concerns about the safety of the vaccine.19,21,22 Our study found that participants had a positive attitude toward wearing masks and regularly washing their hands, which is consistent with findings from previous studies.36–38 However, participants had a more neutral attitude toward visiting crowded places, which is a high-risk behavior for COVID-19 transmission. This highlights the need for continued public education and communication on the importance of avoiding crowded places to reduce the spread of the virus. Interestingly, a recent study conducted on the Saudi population found positive attitudes towards COVID-19 preventive measures were associated with increasing age.36
The findings of our study suggest that the public’s fear of COVID-19 vaccination has decreased compared to when the vaccines were first introduced.19,21,22 However, it is important to continue awareness strategies and remain vigilant about public attitudes towards prevention strategies. Our study also found that attitudes toward COVID-19 prevention measures, such as wearing masks and washing hands regularly, have improved and are becoming more habitual even as COVID-19 cases decline.
Strengths and Limitations
This study explored the reasons behind COVID-19 vaccine refusal. However, the majority of participants were from the western region, which prevented further analysis stratified by region. Additionally, a relatively small number of participants lived in rural areas compared to those living in urban areas. Lastly, the study was self-administered, which could be subject to recall bias.
Conclusion and Recommendations
The vaccine uptake in Saudi Arabia was massive, and 20% of participants were diagnosed with COVID-19 after taking the first and second doses of the COVID-19 vaccine. Most participants reported side effects, including fatigue, fever, headache, and injection site pain. However, a relatively low percentage of participants reported these side effects to the health authorities. The majority of participants had a positive to neutral attitude towards COVID-19 preventive measures. However, the uptake of the first and second doses of the COVID-19 vaccine was not significantly associated with a positive attitude toward prevention measures. Overall, the results suggest that while many people are adhering to recommended preventive measures such as mask-wearing and hand hygiene, there is still room for improvement in terms of avoiding crowded places and increasing awareness of the risks associated with COVID-19. The survey results provide valuable insights into the general population’s attitudes and experiences with the COVID-19 vaccine. However, further research may be needed to understand the underlying factors influencing these attitudes and behaviors.
Acknowledgments
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R144), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. We would like to acknowledge the students Abdallah Altory and Abdelrahman Alahmadi for their great help and participation in the data collection.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. WHO coronavirus (COVID-19) dashboard: overview; 2023. Available from: https://covid19.who.int/?adgroupsurvey=%7Badgroupsurvey%7D&gclid=Cj0KCQiAw8OeBhCeARIsAGxWtUxR9wuVfKmK8awaDLSRrSm65bKiEJOcvWX34XpP2LX4eD_sR9ZfUqAaArNPEALw_wcB.
2. World Health Organization. Saudi Arabia Situation on COVID-19; 2022. Available from: https://covid19.who.int/region/emro/country/sa.
3. Assiri A, Al-Tawfiq JA, Alkhalifa M, et al. Launching COVID-19 vaccination in Saudi Arabia: lessons learned, and the way forward. Travel Med Infect Dis. 2021;43:102119. doi:10.1016/j.tmaid.2021.102119
4. COVID-19 vaccine tracker. Saudi Arabia; 2022. Available from https://covid19.trackvaccines.org/country/saudi-arabia/.
5. Wang C, Wang Z, Wang G, Lau JY-N, Zhang K, Li W. COVID-19 in early 2021: current status and looking forward. Signal Transduct Target Ther. 2021;6(1):114. doi:10.1038/s41392-021-00527-1
6. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
7. Priyanka COP, Singh I, Singh I. Making sound public health decisions for the roll-out of COVID-19 vaccines. J Travel Med. 2021;28(4). doi:10.1093/jtm/taab031
8. Salam AA, Al-Khraif RM, Elsegaey I. COVID-19 in Saudi Arabia: an overview. Front Public Health. 2022;9:2258. doi:10.3389/fpubh.2021.736942
9. Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: a comprehensive review of Phase 3 candidates. Npj Vaccines. 2021;6(1):28. doi:10.1038/s41541-021-00292-w
10. Choudhary OP. Vaccine efficacy against COVID-19: a foresight on the host-associated factors. J Formos Med Assoc. 2021;120(6):1405–1407. doi:10.1016/j.jfma.2020.11.021
11. El-Elimat T, AbuAlSamen MM, Almomani BA, Al-Sawalha NA, Alali FQ. Acceptance and attitudes toward COVID-19 vaccines: a cross-sectional study from Jordan. PLoS One. 2021;16(4):e0250555. doi:10.1371/journal.pone.0250555
12. Neergaard L, Fingerhut H. AP-NORC poll: half of Americans would get a COVID-19 vaccine. AP News; 2020.
13. Alshahrani SM, Dehom S, Almutairi D, et al. Acceptability of COVID-19 vaccination in Saudi Arabia: a cross-sectional study using a web-based survey. Hum Vaccin Immunother. 2021;17(10):3338–3347. doi:10.1080/21645515.2021.1936869
14. Mahase E. Covid-19: pfizer vaccine efficacy was 52% after first dose and 95% after second dose, paper shows. Br Med J. 2020;371:m4826. doi:10.1136/bmj.m4826
15. Mahase E. Covid-19: reports from Israel suggest one dose of Pfizer vaccine could be less effective than expected. Br Med J. 2021;372:n217. doi:10.1136/bmj.n217
16. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B. 1.617. 2 (Delta) variant. N Engl J Med. 2021;385(7):585–594. doi:10.1056/NEJMoa2108891
17. Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397(10293):2461–2462. doi:10.1016/S0140-6736(21)01358-1
18. Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi:10.1016/S0140-6736(21)02183-8
19. Al-Mohaithef M, Padhi BK. Determinants of COVID-19 vaccine acceptance in Saudi Arabia: a web-based national survey. J Multidiscip Healthc. 2020;13:1657–1663. doi:10.2147/JMDH.S276771
20. Alhasan K, Aljamaan F, Temsah M-H, et al. COVID-19 delta variant: perceptions, worries, and vaccine-booster acceptability among healthcare workers. Healthcare. 2021;9(11):1566. doi:10.3390/healthcare9111566
21. Almaghaslah D, Alsayari A, Kandasamy G, Vasudevan R. COVID-19 vaccine hesitancy among young adults in Saudi Arabia: a cross-sectional web-based study. Vaccines. 2021;9(4):330. doi:10.3390/vaccines9040330
22. Yahia AIO, Alshahrani AM, Alsulmi WGH, et al. Determinants of COVID-19 vaccine acceptance and hesitancy: a cross-sectional study in Saudi Arabia. Hum Vaccin Immunother. 2021;17(11):4015–4020. doi:10.1080/21645515.2021.1950506
23. Seid MA, Hussen MS. Knowledge and attitude towards antimicrobial resistance among final year undergraduate paramedical students at University of Gondar, Ethiopia. BMC Infect Dis. 2018;18:1–8. doi:10.1186/s12879-018-3199-1
24. Boateng GO, Neilands TB, Frongillo EA, Melgar-Quiñonez HR, Young SL. Best practices for developing and validating scales for health, social, and behavioral research: a primer. Front Public Health. 2018;6:149. doi:10.3389/fpubh.2018.00149
25. Raosoft, Inc. Raosoft Sample Size Calculator. Seattle; 2004. Available from: http://www.raosoft.com/samplesize.html.
26. Eysenbach G. Improving the quality of web Surveys: the Checklist For Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res. 2004;6(3):e34. doi:10.2196/jmir.6.3.e34
27. Al-Mansour K, Alyahya S, AbuGazalah F, Alabdulkareem K. Factors affecting COVID-19 vaccination among the general population in Saudi Arabia. MDPI. 2021;2021:1218.
28. Alzarea AI, Khan YH, Alatawi AD, et al. Surveillance of post-vaccination side effects of COVID-19 vaccines among Saudi population: a real-world estimation of safety profile. Vaccines. 2022;10(6):924. doi:10.3390/vaccines10060924
29. Andrzejczak-Grzadko S, Czudy Z, Donderska M. Side effects after COVID-19 vaccinations among residents of Poland. Eur Rev Med Pharmacol Sci. 2021;25(12):4418–4421. doi:10.26355/eurrev_202106_26153
30. El-Shitany NA, Harakeh S, Badr-Eldin SM, et al. Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among Saudi residents: a retrospective cross-sectional study. Int J Gen Med. 2021;14:1389–1401. doi:10.2147/IJGM.S310497
31. Lane S, Shakir S. Assessing case fatality on cases of thrombosis with concurrent thrombocytopenia following COVID-19 vaccine AstraZeneca (Vaxzevria) in the United Kingdom: a review of spontaneously reported data. Drug Saf. 2022;45(9):1003–1008. doi:10.1007/s40264-022-01217-9
32. Medeiros KS, Costa APF, Sarmento ACA, Freitas CL, Gonçalves AK. Side effects of COVID-19 vaccines: a systematic review and meta-analysis protocol of randomised trials. BMJ open. 2022;12(2):e050278. doi:10.1136/bmjopen-2021-050278
33. Al-Qazaz HK, Al-Obaidy LM, Attash HM. COVID-19 vaccination, do women suffer from more side effects than men? A retrospective cross-sectional study. Pharm Pract. 2022;20(2):1–6. doi:10.18549/PharmPract.2022.2.2678
34. Choudhary OP, Mohammed TA, Singh I, Singh I. Intranasal COVID-19 vaccines: is it a boon or bane? Int J Surg. 2021;94:106119. doi:10.1016/j.ijsu.2021.106119
35. Fadhel FH. Vaccine hesitancy and acceptance: an examination of predictive factors in COVID-19 vaccination in Saudi Arabia. Health Promot Int. 2021. doi:10.1093/heapro/daab209
36. Al-Hanawi MK, Angawi K, Alshareef N, et al. Knowledge, attitude and practice toward COVID-19 among the public in the Kingdom of Saudi Arabia: a cross-sectional study. Front Public Health. 2020;8:217. doi:10.3389/fpubh.2020.00217
37. Mahmoud MA, Ibrahim AAM, Alolayan SO, Bhagavathula AS. A study of public knowledge and pharmacists perceptions of their roles in prevention of COVID-19. Lat Am J Pharm. 2021;40(4):871–878.
38. Wajid S, Samreen S, Sales I, Bawazeer G, Mahmoud MA, Aljohani MA. What has changed in the behaviors of the public after the COVID-19 pandemic? A cross-sectional study from the Saudi community perspective. Front Public Health. 2022;10. doi:10.3389/fpubh.2022.723229
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
