Back to Journals » Patient Preference and Adherence » Volume 17
Uptake and Persistence on HIV Pre-Exposure Prophylaxis Among Female Sex Workers and Men Having Sex with Men in Kigali, Rwanda: A Retrospective Cross-Sectional Study Design
Authors Rugira E, Biracyaza E , Umubyeyi A
Received 14 July 2023
Accepted for publication 15 September 2023
Published 25 September 2023 Volume 2023:17 Pages 2353—2364
DOI https://doi.org/10.2147/PPA.S427021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Eugene Rugira,1 Emmanuel Biracyaza,2,3 Aline Umubyeyi1
1Department of Epidemiologuy and Biostatistics, School of Public Health, University of Rwanda, Kigali, Rwanda; 2Centre for Interdisciplinary Research in Rehabilitation of Greater Montreal, Institut Universitaire sur la Réadaptation en Déficience Physique de Montréal (IURDPM), Montreal, Canada; 3School of Rehabilitation, Faculty of Medicine, Université de Montréal, Montreal, QC, Canada
Correspondence: Eugene Rugira, Tel +250788873545, Email [email protected]
Background: Although HIV pre-exposure prophylaxis (PrEP) is known for its effectiveness in preventing HIV transmission; there is a global rise in HIV infection rates, particularly prominent in sub-Saharan Africa. This health concern is mostly evident among high-risk groups, namely Female Sex Workers (FSWs) and Men who have Sex with Men (MSMs), both of whom are more susceptible to sexually transmissible infections. This research examined the persistence, uptake, and associated predictors of PrEP utilization within the FSW and MSM populations.
Methods: A cross-sectional study design was conducted involving 4872 individuals from the FSW and MSM groups who were enrolled in a PrEP program across 10 health centers participating in a pilot initiative. The study population was subject to a year-long follow-up period commencing on March 1st, 2019. To evaluate the determinants of PrEP utilization within FSW and MSM groups, bivariate logistic analyses and multivariate logistic regression models were employed.
Results: The findings revealed that the occurrence of PrEP uptake was 45.55% (n=2219) among FSWs and 35.42% (n=17 participants) among MSM. Regarding PrEP persistence, MSM (88.24%, n=15 participants) presented higher PrEP proportion than FSWs (78.5%, n=1742 women). Our findings disclosed that individuals aged 25– 34 years (aOR=0.82; 95% CI=0.72– 0.93, p=0.002), 35– 44 years (aOR=0.83; 95% CI=0.71– 0.97, p=0.017), and 55 years and older (OR=0.14; 95% CI=0.04– 0.48, p=0.002) exhibited lower likelihoods of having low PrEP uptake than those aged 15– 19 years. Moreover, individuals residing with their families (aOR=0.71; 95% CI=0.58– 0.87, p< 0.001), living with roommates (aOR=0.7; 95% CI=0.5– 0.97, p=0.032) displayed lower odds for experiencing low PrEP uptake than their counterparts living alone.
Conclusion: This study highlighted the low uptake of PrEP among participants when compared to previous studies. These results revealed significant influences of age and living conditions on PrEP usage.
Keywords: pre-exposure prophylaxis, female sex workers, men having sex with men, uptake, persistence
Background
Pre-exposure prophylaxis (PrEP) has been known to hold enormous potential to substantially reduce transmission of Human Immunodeficiency Virus (HIV) not only among the general population but also among key populations globally.1 Using PrEP as a prescribed intervention significantly reduces the risk of being infected with HIV by sex (99%) or by injection drug use (74%).2–4 The global HIV statistics are concerning, with an estimated 38.4 million (33.9–43.8 million) people living with HIV in 2022, 1.5 million (1.1–2.0 million) new infections in the same year and 650,000 (510,000–860,000) AIDS-related deaths in 2021. Further, Sub-Saharan Africa (SSA) bears the highest burden of the HIV epidemic, accounting for over two-thirds of the cases.5–7 Additionally, South Africa, Mozambique, India, and Nigeria are among the countries with the highest numbers of people living with HIV (PLWH) witha higher prevalence of HIV infection in adults compared to young people.8,9
Although efforts have been made in SSA to scale up PrEP through national health strategies and to increase the effectiveness of antiretroviral therapy (ART) and PrEP programs,10,11 the epidemic persists, particularly among key populations. Female sex workers (FSWs) play a significant role in HIV transmission, accounting for a substantial percentage of cases by engaging in unprotected sexual behaviors.12,13 Men who have sex with men (MSM) also face a higher risk of HIV infection compared to the general population and PrEP has shown the potential to reduce HIV incidence among MSM by 20–25% when universally used.14 Furthermore, PrEP has been proven effective in preventing sexually transmitted infections among individuals at high risk.2,15
The occurrence of HIV among MSM varies across regions, ranging from 3% to 25% in the Middle East and North Africa, with Kenya reporting an incidence of 20% among MSM.6,16 Sex workers globally have an estimated HIV occurrence of approximately 12%. —in some countries with high HIV occurrence among sex workers in the general population— the HIV-positive rate reaches 30.7%.17 Indeed, FSWs in SSA have a significantly higher HIV occurrence than other women of reproductive age. Key populations engaging in multiple risk behaviors, such as injecting drugs combined with sex work or MSM who inject drugs, have even higher HIV occurrence rates.18
New HIV infections continue to pose a significant health threat, particularly among key populations, such as MSM and FSWs, who often experience inconsistent condom use.19 In addition to existing HIV control strategies, PrEP is an effective preventive approach, as demonstrated in countries such as South Africa, Uganda, Kenya, and Botswana, where it has been implemented successfully.20,21 Although this novel strategy has been effective in reducing the spread of HIV, access to oral PrEP has been a public health concern in many high-income countries and five other SSA countries, namely Kenya, Nigeria, South Africa, Uganda, and Zambia.22 However, the availability and awareness of PrEP services in Rwanda remains limited. Moreover, many PrEP users discontinued treatment within the first year of initiation, highlighting the importance of ensuring the persistence of PrEP.23 Hence, low awareness, stigma, financial constraints, challenges in accessing PrEP, doubts about its effectiveness, concerns about its side-effects, and partner-related factors are among the barriers to PrEP uptake.23–25 Previous studies have reported a low level of PrEP awareness, uptake, and its effective use among key populations.26,27 Major factors of low uptake were fear of side-effects, family influence, individual and community stigma, individual financial constraints, low financial resources in the health system to meet the demands of PrEP, being pregnant, challenges with accessing PrEP, doubts about effectiveness, concerns with taking pills or the ability to adhere, having faithful sexual partners, and partners stopping users taking PrEP.20,21 Sociodemographic characteristics, including age, employment, education level, and socioeconomic status, have been reported as important factors that also play a role in PrEP uptake and persistence.28
In Rwanda, the uptake and retention of healthcare services for key populations under PrEP medication is important for continued access to therapy, reinforcing adherence, and testing for HIV and other Sexually Transmitted Infections (STIs), which are sometimes a big challenge in achieving sustainable development goals.10 The problem with the uptake and retention of PrEP is that some users of PrEP discontinue using PrEP programs due to diverse factors, such as fear of side-effects, lack of health insurance coverage, self-payment, healthcare providers, and awareness. Thus, these barriers compel PrEP knowledge to be strengthened to promote the quality of health and prevent the risk of HIV among the key population.29–31 Ensuring the uptake and persistence of PrEP among key populations is crucial for its effective implementation; however, challenges persist in terms of information, dissemination, setting appropriate targets for the uptake, effective use, and coverage, ultimately affecting HIV prevention efforts.32,33 Efforts to scale up PrEP in SSA countries through national health systems require the tracking of uptake, adherence, and retention in PrEP programs. Additionally, understanding how and why patients take up PrEP can contribute to cost-effective PrEP interventions and enhance HIV prevention in the country. Therefore, this study aimed to determine PrEP uptake and persistence as well as to identify factors associated with uptake among FSW and MSM in Rwanda to fill the gap in scientific evidence regarding this intervention.
Methods
Study Design
We conducted a retrospective cross-sectional analysis of a key population registered for PrEP at 10 pilot healthcare facilities. The participants were tracked over a span of one year, starting from March 2019 to March 2020, with the objective of exploring the occurrence of PrEP uptake and its associated factors in Kigali City, Rwanda.
Study Settings and Population
The study setting was Kigali City, which is the capital city of Rwanda. Rwanda is a small country sharing borders with Uganda to the north, Burundi to the south, Tanzania to the east, and the Democratic Republic of the Congo (DRC) to the west. The study area has experienced an unprecedented population growth (rate of 4% per year) over the past two decades. Among more than 13.46 million Rwandan population, the majority are from Kigali, with an approximate population of 1.248 million by 2022.34,35 The rise in population within this capital city can be attributed to the expansion of urban infrastructure. Prior to 2005, urban development was minimal due to the effects of 1994 genocide against the Tutsi that left diverse effects including destructions of infrastructures, low access to healthcare services, and extreme poverty. However, since Kigali gained administrative city status in 2005, its territorial boundaries have extended to encompass rural and agricultural zones that previously lacked urban infrastructure and amenities. Notably, informal settlements now accommodate 79% of the urban population, covering 66% of the developed area.36 The city continues to struggle to provide affordable housing units to accommodate an increasing number of urban residents. As a result, urban expansion has now extended to wetland areas and steep slopes, which have previously been considered unsuitable for human habitation. Interestingly, it is estimated that approximately 19% of Kigali’s built environment is on land which is non-conducive for development.37 Regarding health, HIV occurrence in the city of Kigali is more than twice the national average (6.7%), and the majority of the population at a higher risk (MSM and FWS) are found in this area when compared to other settings in the country.38,39 Thus, of the total of 45.8% for the occurrence of FSWs estimated with HIV and 4% for MSM, the majority were in Kigali.32 In collaboration with the Ministry of Health through the Rwanda Biomedical Center (RBC), this study was conducted in Kigali in 10 health centers sampled to pilot the PrEP program to prevent new HIV infections.38,40 These health centers were Remera Health Center (HC), Kagugu HC, Gihogwe HC, Kicukiro HC, Gatenga HC, Gikondo HC, Gahanga HC, Rugarama HC, Kacyiru HC, and Kabusunzu HC.
Study Population and Sample Size
The individuals participating in this trial initiative consisted of specific populations such as MSM and FSWs. The recruitment of participants for the PrEP program was carried out through self-referral or through the involvement of friends, partners, and healthcare providers. The criteria for determining eligibility were as follows: individuals who were HIV-negative or uninfected and belonged to the male or female gender, and were at risk of acquiring HIV within these specific categories; MSM engaging in unprotected anal intercourse; FSWs involved in unprotected sexual intercourse and having sexual partners whose HIV status was unknown to them; and FSWs and MSM diagnosed with a sexually transmitted infection (STI).
The total number of the eligible participants was 5319 participants, comprising 5267 females and 52 males. After removing the incomplete registries of the participants, the final sample size of this study was 4872 that includes 4824 FSWs and 48 MSM.
Study Variables
In this study, the primary outcome variable under investigation was the uptake of PrEP. The PrEP uptake variable was constructed by distinguishing individuals who engaged in PrEP (specifically Truvada) from those who did not. The independent variables encompassed various socio-demographic factors, including sex or group of participants, age, marital status, living situation, education, living situation, and employment status. Another crucial variable of interest was the persistence of PrEP, which was determined based on the HIV status of participants at the conclusion of the follow-up period. Participants who remained on PrEP and still tested HIV-negative were considered to have persisted with the PrEP program, while those who did not maintain their HIV-negative status were not retained in the PrEP program.
Data Collection and Management
The present study used secondary data from registers of the PrEP Program, and these data were obtained from the Health Management Information System (HMIS) collected from 10 health centers sampled for the pre-exposure prophylaxis pilot program. All data were collected from registries of the participants. The information to be collected included socio-demographic information and clinical data. Screening for eligibility information included HIV test results, STI symptoms, previous history of STI symptoms since the last visit, and sexual intercourse without a condom since the last visit. PrEP uptake and acceptability information included self-report of high risk of HIV infection, awareness of daily ARV to reduce the risk of getting HIV, willingness to take daily ART to lower the risk of getting HIV, eligibility for PrEP, and provision of Truvada. Persistence information included HIV testing at the end of the follow-up period. The HMIS database was kept confidential for the privacy of the participants on a locked computer with a personal key. The registries of the patients were kept in their usual safe and locked locations after extracting the required data. All extracted data in the Excel spreadsheet were secured with password data manipulation to protect the patients’ medical information. Participants with missing data for any of the aforementioned study variables were excluded from the study.
Data Analysis
Descriptive statistics were used to summarize the research data, including graphs, frequencies, percentages, and graphs to describe the study variables. Bivariate logistic regression analyses were performed to determine the level of association of each independent variable with the HIV PrEP uptake and crude odds ratios (cOR), 95% for the confidence intervals (CI), and a significance level of p<0.05. All significant variables at p<0.25 in the bivariate analysis were added to the multivariate logistic models to assess factors associated with HIV PrEP uptake. Further, the adjusted odds ratios (aOR), 95% for the CI, and statistical significance levels of p<0.05 were computed. The 95% confidence intervals were used, and all statistical tests allowed a type error of maximum 5%. In this study, all variables in the multivariate logistic regression models were assessed for collinearity, which was measured to indicate whether the study variables possess a variance inflation factor (VIF) more than 3. All statistical analyses were performed using STATA version 13, statistical software used mostly in epidemiological studies.
Ethics
All methods of this study were carried out under the regulations and principles of the Helsinki Declaration and in accordance with the Centers for Disease Control and Prevention (CDC) human research protection procedures and were determined to be research.41 Ethical approval for this study was obtained from the Institutional Review Board of the College of Medicine and Health Sciences at the University of Rwanda (approval No: 220/CMHS IRB/2021). This study used medical information from patients from their electronic medical records. As data sharing and managing privacy and confidentiality are important for protecting participants, confidentiality and privacy were strictly and entirely ensured in this study. Therefore, participants consented that their medical data could be used anonymously for the research purpose.
Results
Socio-Demographic Characteristics of the Participants
The study primarily comprised female participants (99.1%; 4824 women). Furthermore, a substantial proportion fell within the 25–34 age range (46.5%; 2265 individuals), were single (60.3%; 2936 individuals), resided alone (34.6%; 4071 individuals), possessed primary education (71%; 3458 individuals), and lacked employment (73.5%; 3586 individuals) (Table 1).
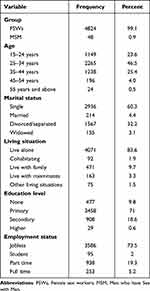 |
Table 1 Socio-Demographic Characteristics of Study Participants (N=4872) |
HIV-PrEP Uptake Among FSW and MSM
Our results suggested that 45.55% (n=2219) of FSWs opted for PrEP uptake, indicating that a majority of FSWs (n=2653, 54.45%) did not initiate PrEP. Turning to the MSM population, our findings determined that 35.42% (n=17) initiated PrEP, leaving a significant portion of respondents (n=31, 64.58%) who chose not to start PrEP (Figure 1).
 |
Figure 1 Uptake level among study participants. Abbreviations: FSW, Female Sex worker; MSM, Male sex with male; PrEP, pre-exposure prophylaxis. |
HIV-PrEP Persistence Level Among FSW and MSM in Kigali
Regarding the continued use of PrEP, our research findings revealed that MSM presented a greater persistence rate, with 88.24% (15 individuals) remaining on PrEP. In contrast, among FSWs, the proportion was slightly lower at 78.5% (1742 women). This implies that 11.76% (11 individuals) of MSM and 21.5% (477 women) of FSWs had ceased their participation in PrEP interventions (Figure 2).
 |
Figure 2 PrEP persistence among FSW and MSM. Abbreviations: FSW, Female Sex worker; MSM, Male sex with male; PrEP, pre-exposure prophylaxis. |
Associated Predictors of Uptake of PrEP Among FSW and MSM in Rwanda
The bivariate analysis examining the relationship between PrEP uptake for HIV prevention and the administration of Truvada medication was predicated on certain factors. As illustrated in Table 2, individuals aged 55 years or older, as opposed to those aged 15–24 years, displayed a lower likelihood of adopting PrEP (cOR=0.20, 95% CI=0.07–0.59). Moreover, living situations had a role to play: cohabitating with roommates versus living alone (cOR=0.66, 95% CI=0.48–0.91), as well as residing with family versus living alone (cOR=0.67, 95% CI=0.56–0.80), were both associated with a low PrEP uptake. Educational attainment also emerged as a factor: individuals with primary education (cOR=0.87, 95% CI=0.81–0.93), secondary education (cOR=0.77, 95% CI=0.67–0.88), and higher education (cOR=0.45, 95% CI=0.20–0.99) were significantly associated with low PrEP uptake when compared to those with no education (Table 2).
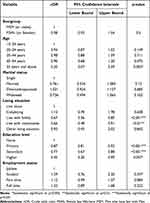 |
Table 2 Bivariate Analysis for the Associated Factors of PrEP Uptake Among Participants |
Multivariate Logistic Regression Models of the Factors Associated with the Uptake of PrEP
The results from multiple logistic regression models revealed participants aged 25–34 years (aOR=0.82, 95% CI=0.72–0.93), 35–44 years (aOR=0.83, 95% CI=0.71–0.97), and 55 years or older (aOR=0.14, 95% CI=0.04–0.48) showed lower likelihoods of accepting PrEP compared to those aged 15–24 years. Moreover, family status emerged as a significant factor affecting PrEP initiation. Individuals living with their families (aOR=0.71, 95% CI=0.58–0.87) and those cohabitating with roommates (aOR=0.70, 95% CI=0.50–0.97) were both less likely to initiate PrEP when compared to those who lived alone (Table 3).
 |
Table 3 Multivariable Logistic Regression Models for the Factors of PrEP Uptake Among FSW and MSM (N=5319) |
Discussion
This study delved into the update and persistence of PrEP, along with its influencing factors, among FSWs and MSMs in Kigali, Rwanda. The findings indicated that 45.55% of FSWs initiated PrEP for the prevention of HIV infection. This uptake percentage falls within the range identified in studies conducted with FSWs and MSMs in South Africa and Kenya.42,43 These results also align with earlier research, demonstrating varied PrEP uptake levels among FSWs, ranging from 2% to 49%.21 However, our results amount PrEP update is lower than the similar study reported in in Senegal with the prevalence of 82%. While our results of PrEP uptake are lower than those from Senegal,44 they surpass the uptake reported in urban settings among MSMs in South Africa, where rates ranged from 10% to 26%.42,45 The PrEP uptake among MSMs in Rwanda, at 35.42%, was relatively higher compared to studies conducted in Atlanta, USA, which reported 34% among young black MSM.46 Similarly, a study in the Netherlands found an uptake of 45.6% among MSMs, surpassing the findings of our study.25 Furthermore, a study in China reported a much lower uptake of 4% among MSMs, highlighting the contrast with our results.47
Among the FSWs who initiated PrEP in our study, a notable 78.50% continued to use the PrEP intervention in Rwanda. This persistence rate proves a higher level of persistence compared to a study conducted among FSWs in other SSA countries such as Senegal and Benin, which reported a persistence rate of 73.4% and 54.6% after follow-up respectively.12,44 Our study also presented a higher rate than an observational study in South Africa that stated a persistence level of 22% among FSWs.48 For MSM, our findings revealed a persistence proportion of 88.24% in Rwanda. This proportion is significantly higher compared to a study conducted in three cities in the US, which reported a persistence rate varying between 30% and 57% among MSM.49 It is also higher than the persistence level of 57.7% documented in some SSA countries like Kenya.50 Overall, our study demonstrated relatively high persistence rates for both FSWs and MSMs in Rwanda compared to various international studies, suggesting favorable adherence to PrEP interventions in the Rwandan context.51
Consistent with previous research,52 our study established that residing with roommates or family, as opposed to living alone, was associated with a decrease in likelihood of PrEP adoption. Likewise, our findings concurred with earlier studies29,52 in revealing that being widowed was associated with a decreased likelihood of PrEP uptake. Furthermore, our results aligned with studies conducted in Kenya and Uganda, which demonstrated that being married to one or more women significantly associated with a higher probability of initiating PrEP.27 Interestingly, cohabitation did not emerge as a significant factor for PrEP uptake in our study, unlike a contradictory finding from a study conducted in the United States53 that identified a positive association between being single and PrEP initiation. Additionally, our study revealed that the age of the participants was a significant factor in PrEP initiation, where young people were more likely to experience a low uptake of PrEP than adults. Consistent with prior studies conducted in South Africa among a key population,42 our results revealed that people aged 25–34 years, 35–44 years, and 55 years and above were less likely to experience PrEP uptake compared to those aged 15–24 years. It is noteworthy that conflicting results have been reported in other studies, where they found a positive correlation between age and PrEP uptake.54,55 Finally, this study uncovered a high level of PrEP persistence among both FSWs and MSMs. It was also noted that living situation (such as residing with family or roommates) and age played a role in the likelihood of PrEP initiation. These results collaborated with prior studies on the factors influencing uptake, continuation, and discontinuation of oral PrEP among FSWs and MSM facilities.21,56
Our study faced some limitations in certain aspects. Firstly, the authors lacked adequate statistical power to observe similar distinctions among participants across all age groups. The absence of data on refusal of screening hindered their ability to gauge the extent to which the failure to account for screening refusals might have led to an overestimation of PrEP uptake at the population level. Secondly, the list of determinants they analyzed, associated with the acceptance and persistence of PrEP, was constrained to those acquired through program instruments. Consequently, the possibility of residual confounding cannot be dismissed. Moreover, their research concentrated on interventions at individual and structural levels to support effective PrEP use. They did not encompass larger systemic predictors of PrEP uptake, such as psychosocial factors, stigma, community stigma, medical mistrust, and economic constraints, which can hinder access to public health services and affect comfort levels with receiving PrEP services among populations at higher risk of HIV.23,57 Thirdly, substantial variables such as knowledge of partner status, past HIV testing history, changes in sexual partnerships over time, and perceived risk of HIV were not recorded, which could have resulted in overestimation or underestimation of the findings. Fourthly, this study was conducted among FSW and MSM sites and employed secondary data from the HMIS in Kigali city; therefore, it may not be generalizable to all PrEP implementation sites nationwide. Fifthly, our study was limited by a study design that was not capable of determining causality between HIV PrEP uptake and independent variables. Lastly, our study was limited to a relatively small sample size and the unequal number of participants between the MSM and FSWs groups. This could potentially impact the generalizability and robustness of our findings. Finally, this study was limited to a study design that could not determine the causality of the factors, but rather the association.
Conclusion
In order to improve the quality of life for those who are at a higher risk of HIV, there is a public health emergency that needs to be addressed with the low uptake of PrEP among study participants from Kigali. Besides, PrEP persistence was high in both FSWs and MSMs. The main causes of the limited uptake of PrEP were the age and housing circumstances of participants of this research. Our results are used to support certain recommendations for future research and policymakers. To promote uptake, policymakers should first increase the sensitization of risk groups to the benefits of PrEP. Second, it is critical to incorporate PrEP education into other programs, like family planning, in order to raise awareness and uptake. Further studies on risk factors of PrEP persistence are important, since this would serve as a tool to maximize PrEP persistence among key populations. There is a need to develop, implement, and evaluate PrEP delivery models and expand their use and persistence in Rwanda to promote the quality of life of people who are at risk of HIV infection. Further, it is essential to conduct additional study using a longitudinal study design to assess the causality of PrEP uptake. Understanding who is at risk of discontinuation and the reasons for discontinuation in a larger sample size is an important contribution to better designing planning to promote PrEP persistence. In addition, It is also critical to undertake mixed-methods research to investigate how sociocultural factors affect PrEP use and how the relevant demographic views the use of PrEP.
Data Sharing Statement
All relevant data and information can be obtained from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
Ethical approval for this study was obtained from the Institutional Review Board of the College of Medicine and Health Sciences at the University of Rwanda. Confidence and anonymity were also ensured. This study was conducted in accordance with the standards and principles of the Helsinki Declaration.
Acknowledgments
The authors express gratitude to the Rwanda Ministry of Health and the Rwanda Biomedical Center for generously sharing the datasets. The participants are also acknowledged for their willingness to contribute data to the Rwanda Biomedical Center and for granting permission for their data to be employed in this research endeavor. Special appreciation is extended to the Ethics Committee of the College of Medicine and Health Sciences at the University of Rwanda for granting ethical approval for this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare no competing interests in this work.
References
1. Bekker LG, Giovenco D, Baral S, et al. Oral pre-exposure prophylaxis uptake, adherence, and adverse events among South African men who have sex with men and transgender women. South Afr J HIV Med. 2022;23(1):1–9. doi:10.4102/SAJHIVMED.V23I1.1405
2. Kagaayi J, Batte J, Nakawooya H, et al. Uptake and retention on HIV pre-exposure prophylaxis among key and priority populations in South-Central Uganda. J Int AIDS Soc. 2020;23(8):1–6. doi:10.1002/jia2.25588
3. Fonner VA, Dalglish SL, Kennedy CE, et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. Aids. 2016;30(12):1973–1983. doi:10.1097/QAD.0000000000001145
4. Pre-Exposure Prophylaxis (PrEP). Centers for disease control and prevention; 2023. Available from: https://www.cdc.gov/hiv/risk/prep/index.html#:~:text=Pre-exposureprophylaxis.
5. Saing CH, Prem K, Uk P, et al. Risk factors associated with HIV and hepatitis C virus co-infection among people who inject drugs in Cambodia. Int J Drug Policy. 2020;86:1–11. doi:10.1016/j.drugpo.2020.102974
6. World Health Organization. HIV/AIDS; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/hiv-aids.
7. Bbosa N, Kaleebu P, Ssemwanga D. HIV subtype diversity worldwide. Curr Opin HIV AIDS. 2019;14(3):153–160. doi:10.1097/COH.0000000000000534
8. Shao Y, Williamson C. The HIV-1 epidemic: low- to middle-income countries. Cold Spring Harb Perspect Med. 2012;2(3):a007187. doi:10.1101/cshperspect.a007187
9. Gengiah TN, Moosa A, Naidoo A, Mansoor LE. Adherence challenges with drugs for pre-exposure prophylaxis to prevent HIV infection. Int J Clin Pharm. 2014;36(1):0–85. doi:10.1007/s11096-013-9861-1
10. Stalter R, Chen M, Uwizeye G, et al. Association of sexual risk behaviour with previous HIV testing among VCT clients in Kigali, Rwanda. Int J STD AIDS. 2015;27(14):1317–1325. doi:10.1177/0956462415617590
11. Uganda Ministry of Health. Consolidated Guidelines for Prevention and Treatment of HIV. Uganda Ministry of Health; 2016.
12. Mboup A, Béhanzin L, Guédou FA, et al. Early antiretroviral therapy and daily pre-exposure prophylaxis for HIV prevention among female sex workers in Cotonou, Benin: a prospective observational demonstration study. J Int AIDS Soc. 2018;21(11):1–11. doi:10.1002/jia2.25208
13. Jourdain H, de Gage SB, Desplas D, Dray-Spira R. Real-world effectiveness of pre-exposure prophylaxis in men at high risk of HIV infection in France: a nested case-control study. Lancet Public Heal. 2022;7(6):e529–e536. doi:10.1016/S2468-2667(22)00106-2
14. World Health Organization. Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations – 2016 Update. World Health Organization; 2016:155.
15. Chou R, Evans C, Hoverman A, et al. Preexposure prophylaxis for the prevention of HIV infection: evidence report and systematic review for the US preventive services task force. JAMA. 2019;321(22):2214–2230. doi:10.1001/jama.2019.2591
16. Havens JP, Scarsi KK, Sayles H, Klepser DG, Swindells S, Bares SH. Acceptability and feasibility of a pharmacist-led human immunodeficiency virus pre-exposure prophylaxis program in the Midwestern United States. Open Forum Infect Dis. 2019;6(10). doi:10.1093/ofid/ofz365
17. Gombe MM, Cakouros BE, Ncube G, et al. Key barriers and enablers associated with uptake and continuation of oral pre-exposure prophylaxis (PrEP) in the public sector in Zimbabwe: qualitative perspectives of general population clients at high risk for HIV. PLoS One. 2020;15(1):1–18. doi:10.1371/journal.pone.0227632
18. Shannon K, Crago AL, Baral SD, et al. The global response and unmet actions for HIV and sex workers. Lancet. 2018;392(10148):698–710. doi:10.1016/S0140-6736(18)31439-9
19. Greenwald ZR, Maheu-Giroux M, Szabo J, et al. Cohort profile: L’Actuel Pre-Exposure Prophylaxis (PrEP) Cohort study in Montreal, Canada. BMJ Open. 2019;9(6):1–10. doi:10.1136/bmjopen-2018-028768
20. Jackson-gibson M, Ezema AU, Orero W, et al. Facilitators and barriers to HIV pre- exposure prophylaxis (PrEP) uptake through a community-based intervention strategy among adolescent girls and young women in Seme Sub-County, Kisumu, Kenya. BMC Public Health. 2021;21:1–13. doi:10.1186/s12889-021-11335-1
21. Pillay D, Stankevitz K, Lanham ML, et al. Factors influencing uptake, continuation, and discontinuation of oral PrEP among clients at sex worker and MSM facilities in South Africa. PLoS One. 2020;15(4):e0228620. doi:10.1371/journal.pone.0228620
22. UNAIDS. Countries as diverse as Italy, Lesotho, Viet Nam and Zimbabwe cut new HIV infections by more than 45% between 2015 and 2021: UNAIDS GLOBAL AIDS UPDATE; 2022. Available from: https://www.unaids.org/sites/default/files/media_asset/2022-global-aids-update-summary_en.pdf.
23. Koppe U, Marcus U, Albrecht S, et al. Barriers to using HIV pre-exposure prophylaxis (PrEP) and sexual behaviour after stopping PrEP: a cross-sectional study in Germany. BMC Public Health. 2021;21(159):1–10. doi:10.1186/s12889-021-10174-4
24. Hojilla JC, Koester K, Cohen S, et al. Sexual behavior, risk compensation, and HIV prevention strategies among participants in the San Francisco PrEP demonstration project: a qualitative analysis of counseling notes. AIDS Behav. 2016;20(7):1461–1469. doi:10.1007/s10461-015-1055-5
25. van Dijk M, de Wit JBF, Guadamuz TE, Martinez JE, Jonas KJ. Slow Uptake of PrEP: behavioral predictors and the influence of price on prep uptake among MSM with a high interest in PrEP. AIDS Behav. 2021;25(8):2382–2390. doi:10.1007/s10461-021-03200-4
26. Nunn AS, Brinkley-Rubinstein L, Oldenburg CE, et al. Defining the HIV pre-exposure prophylaxis care continuum. Aids. 2017;31(5):731–734. doi:10.1097/QAD.0000000000001385
27. Koss CA, Charlebois ED, Ayieko J, et al. Uptake, engagement, and adherence to pre-exposure prophylaxis offered after population HIV testing in rural Kenya and Uganda: 72-week interim analysis of observational data from the SEARCH study. Lancet HIV. 2020;7(4):e249–e261. doi:10.1016/S2352-3018(19)30433-3
28. Ahouada C, Diabaté S, Mondor M, et al. Acceptability of pre-exposure prophylaxis for HIV prevention: facilitators, barriers and impact on sexual risk behaviors among men who have sex with men in Benin. BMC Public Health. 2020;20(1):1–17. doi:10.1186/s12889-020-09363-4
29. Muhumuza R, Ssemata AS, Kakande A, et al. Exploring perceived barriers and facilitators of PrEP uptake among young people in Uganda, Zimbabwe, and South Africa. Arch Sex Behav. 2021;50(4):1729–1742. doi:10.1007/s10508-020-01880-y
30. Beesham I, Milford C, Smit J, et al. Post-trial access to and use of pre-exposure prophylaxis in Durban, South Africa. BMC Public Health. 2023;23(1):1–11. doi:10.1186/s12889-023-16139-z
31. Skovdal M, Magoge-Mandizvidza P, Dzamatira F, et al. Improving access to pre-exposure prophylaxis for adolescent girls and young women: recommendations from healthcare providers in eastern Zimbabwe. BMC Infect Dis. 2022;22(1):1–10. doi:10.1186/s12879-022-07376-5
32. Gueler A, Vanobberghen F, Rice B, Egger M, Mugglin C. The HIV Care Cascade from HIV diagnosis to viral suppression in sub-Saharan Africa: a systematic review and meta-regression analysis protocol. Syst Rev. 2017;6(1):1–6. doi:10.1186/s13643-017-0562-z
33. Spriggs M, Charles T. Should HIV discordant couples have access to assisted reproductive technologies? J Med Ethics. 2003;29(6):325–329. doi:10.1136/jme.29.6.325
34. Ba G, Malonza J, Manirakiza V. Understanding the Concept of Neighbourhood in Kigali City, Rwanda. Sustainability. 2020;12(1555):1–22. doi:10.3390/su12041555
35. Rwanda Biomedical Center. Prevalence and factors associated with preterm birth at Kenyatta National Hospital. Enferm Glob. 2018;18(1):1–15. doi:10.6018/eglobal.16.3.255721
36. Twarabamenye E, Mukashema A. Long-run trend of cities’ informal housing: a solution for the majority of Kigali Urban Dwellers and a Challenge to Urban Development in Rwanda. J Environ Manag. 2012;3:35–56.
37. Nduwayezu G, Sliuzas R, Kuffer M. Modeling urban growth in Kigali city Rwanda. Rwanda J. 2017;1(2). doi:10.4314/rj.v1i2S.7D
38. FHAPCO. Rwanda country operational plan (COP/ROP) 2018 strategic direction summary; 2018. Available from: https://www.state.gov/wp-content/uploads/2019/08/Rwanda-2.pdf.
39. National Institute of statistics of Rwanda (NISR) [Rwanda], Ministry of Health (MoH) [Rwnda], ICF. Rwanda demographic and health survey 2019–20 key indicators report. Natioanal Institute of Statistics of Rwanda; 2020. Available from: https://www.statistics.gov.rw/datasource/demographic-and-health-survey-201920.
40. Nsanzimana S, Rwibasira GN, Malamba SS, et al. HIV incidence and prevalence among adults aged 15–64 years in Rwanda: results from the Rwanda Population-based HIV Impact Assessment (RPHIA) and District-level Modeling, 2019. Int J Infect Dis. 2022;116:245–254. doi:10.1016/j.ijid.2022.01.032
41. Shrestha B, Dunn L. The declaration of Helsinki on medical research involving human subjects: a review of seventh revision. J Nepal Heal Res Counc. 2020;17(4):548–552. doi:10.33314/jnhrc.v17i4.1042
42. Rao A, Mhlophe H, Comins C, et al. Persistence on oral pre-exposure prophylaxis (PrEP) among female sex workers in eThekwini, South Africa, 2016–2020. PLoS One. 2022;17(3):e0265434. doi:10.1371/journal.pone.0265434
43. Irungu EM, Mugwanya KK, Mugo NR, et al. Integration of pre-exposure prophylaxis services into public HIV care clinics in Kenya: a pragmatic stepped-wedge randomised trial. Lancet Glob Heal. 2021;9(12):e1730–e1739. doi:10.1016/S2214-109X(21)00391-0
44. Sarr M, Gueye D, Mboup A, et al. Uptake, retention, and outcomes in a demonstration project of pre-exposure prophylaxis among female sex workers in public health centers in Senegal. Int J STD AIDS. 2020;31(11):1063–1072. doi:10.1177/0956462420943704
45. Donnell D, Beesham I, Welch JD, et al. Incorporating oral PrEP into standard prevention services for South African women: a nested interrupted time-series study. Lancet HIV. 2021;8(8):e495–e501. doi:10.1016/S2352-3018(21)00048-5
46. Rolle CP, Rosenberg ES, Siegler AJ, et al. Challenges in translating PrEP interest into uptake in an observational study of young black MSM. J Acquir Immune Defic Syndr. 2017;76(3):250. doi:10.1097/QAI.0000000000001497
47. Wang Z, Mo PKH, Ip M, Fang Y, Lau JTF. Uptake and willingness to use PrEP among Chinese gay, bisexual and other men who have sex with men with experience of sexualized drug use in the past year. BMC Infect Dis. 2020;20:1–13.
48. Eakle R, Gomez GB, Naicker N, et al. HIV pre-exposure prophylaxis and early antiretroviral treatment among female sex workers in South Africa: results from a prospective observational demonstration project. PLoS Med. 2017;14(11):e1002444. doi:10.1371/journal.pmed.1002444
49. Chan PA, Mena L, Patel R, et al. Retention in care outcomes for HIV pre‐exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc. 2016;19(1):20903. doi:10.7448/IAS.19.1.20903
50. Wahome EW, Graham SM, Thiong’o AN, et al. PrEP uptake and adherence in relation to HIV-1 incidence among Kenyan men who have sex with men. EClinicalMedicine. 2020;26:100541. doi:10.1016/j.eclinm.2020.100541
51. Chan PA, Patel RR, Mena L, et al. Long-term retention in pre-exposure prophylaxis care among men who have sex with men and transgender women in the United States. J Int AIDS Soc. 2019;22(8):1–9. doi:10.1002/jia2.25385
52. Wanga V, Omollo V, Bukusi EA, et al. Uptake and impact of facility-based HIV self-testing on PrEP delivery: a pilot study among young women in Kisumu, Kenya. J Int AIDS Soc. 2020;23(8):14–18. doi:10.1002/jia2.25561
53. Ojikutu BO, Bogart LM, Higgins-Biddle M, et al. Facilitators and Barriers to Pre-Exposure Prophylaxis (PrEP) use among black individuals in the United States: results from the national survey on HIV in the black community (NSHBC). AIDS Behav. 2018;22(11):3576–3587. doi:10.1007/s10461-018-2067-8
54. Morgan E, Moran K, Ryan DT, Mustanski B, Newcomb ME. Threefold increase in PrEP uptake over time with high adherence among young men who have sex with men in Chicago. AIDS Behav. 2018;22(11):3637–3644. doi:10.1007/s10461-018-2122-5
55. Rubtsova AM, Wingood G, Dunkle K, Camp CJ, DiClemente R. Young adult women and correlates of potential adoption of pre-exposure prophylaxis (PrEP): results of a national survey. Curr HIV Res. 2013;11(7):543–548. doi:10.2174/1570162X12666140129104952
56. Chapin-Bardales J, Haaland R, Martin A, et al. HIV pre-exposure prophylaxis persistence and adherence among men who have sex with men in four US Cities. JAIDS J Acquir Immune Defic Syndr. 2013;91(3):34–41. doi:10.1097/QAI.0000000000003160
57. Cahill S, Taylor S, Elsesser S, Mena L, Hickson D, Mayer K. Stigma, medical mistrust, and perceived racism may affect PrEP awareness and uptake in black compared to white gay and bisexual men in Jackson, Mississippi and Boston, Massachusetts. AIDS Care. 2021;29(11):1351–1358. doi:10.1080/09540121.2017.1300633
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
