Back to Journals » Drug Design, Development and Therapy » Volume 9
Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer
Authors Qing Y, Li Q, Ren T, Xia W, Peng Y, Liu GL, Luo H, Yang Y, Dai XY, Zhou S, Wang D
Received 30 September 2014
Accepted for publication 17 November 2014
Published 16 February 2015 Volume 2015:9 Pages 901—909
DOI https://doi.org/10.2147/DDDT.S75152
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Min Li
Yi Qing,1,* Qing Li,1,* Tao Ren,1 Wei Xia,1 Yu Peng,1 Gao-Lei Liu,2 Hao Luo,1 Yu-Xin Yang,1 Xiao-Yan Dai,1 Shu-Feng Zhou,3 Dong Wang1
1Cancer Center, 2Urological Surgery, Daping Hospital and Research Institute of Surgery, Third Military Medical University, Chongqing, People’s Republic of China; 3Department of pharmaceutical Sciences, College of Pharmacy, University of South Florida, Tampa, FL, USA
*These authors contributed equally to this work
Introduction: Gastric cancer is a fatal malignancy with a rising incidence rate. Effective methods for early diagnosis, monitoring metastasis, and prognosis are currently unavailable for gastric cancer. In this study, we examined the association of programmed death ligand-1 (PD-L1) and apurinic/apyrimidinic endonuclease 1 (APE1) expression with the prognosis of gastric cancer.
Methods: The expressions of PD-L1 and APE1 were detected by immunohistochemistry in 107 cases of human gastric carcinoma. The correlation of PD-L1 and APE1 expression with the clinicopathologic features of gastric carcinoma was analyzed by SPSS version 19.0.
Results: The positive expression rates of PD-L1 and APE1 in gastric cancer tissues were 50.5% (54/107) and 86.9% (93/107), respectively. PD-L1 and APE1 positive expressions were significantly associated with depth of invasion, lymph node metastasis, pathological type, overall survival, and higher T stage. Furthermore, the expression of PD-L1 in highly differentiated gastric cancers was higher than that in poorly differentiated cancers (P=0.008). Moreover, the expression of APE1 and PD-L1 in gastric cancers was positively correlated (r=0.336, P<0.01). Multivariate analysis showed that the depth of invasion was a significant prognostic factor (risk ratio 19.91; P=0.000), but there was no significant relationship with PD-L1, APE1, prognosis, and other characteristics.
Conclusion: The deregulation of PD-L1 and APE1 might contribute to the development and the poor prognosis of gastric cancer. Our findings suggest that high expression of PD-L1 and APE1 is a risk factor of gastric cancer and a new biomarker to predict the prognosis of gastric cancer. Furthermore, our findings suggest that targeting the PD-L1 and APE1 signaling pathways may be a new strategy for cancer immune therapy and targeted therapy for gastric cancer, especially in patients with deep invasion and lymph node metastasis.
Keywords: gastric carcinoma, immunohistochemistry, prognostic, potential targets
Introduction
Gastric cancer is one of the most common fatal cancers, and patients are particularly prone to lymph node metastasis.1 The incidence for gastric carcinoma has had a recent increase, likely due to both environmental and social factors.1 Effective methods for early diagnosis, monitoring metastasis, and prognosis are currently unavailable for gastric cancer. Currently, molecular targeted therapy has shown to be effective in HER-2-positive patients with gastric cancer,2,3 but its clinical application is limited due to the rare occurrence of HER-2 mutation. Immunotherapy has shown bright promise in the treatment of gastric cancer.4,5 Unfortunately, it was reported that gastric cancer cells can escape immune surveillance by a variety of mechanisms which prevent the immune system from producing effective antitumor immune responses.6 Moreover, the body’s antitumor immunity is mainly mediated by T lymphocytes, but the majority of tumor antigen-specific T-cells are often in a state of immune tolerance. Therefore, elucidating the relationship between the tumor immune escape mechanism and prognosis of gastric cancer will be of high importance for both cancer biology and translational cancer research.
Programmed death ligand-1 (PD-L1) is a member of the B7 superfamily of costimulating molecules expressed by antigen-presenting cells that functions as a T lymphocyte inhibitory molecule. PD-L1 has been shown to induce T lymphocyte anergy and/or apoptosis through binding to its receptor, PD-1.7–11 PD-L1 increases apoptosis of antigen-specific human T-cell clones and inhibits the activation of CD4 and CD8 T-cells in vitro.7,12 PD-L1 expression has been described in several malignancies, including colon cancer, ovary cancer, melanoma, lung carcinoma, breast cancer, non-small-cell lung carcinoma, gliomas, squamous cell carcinoma of head and neck, renal cell carcinoma, and esophageal carcinoma.9,12–17 High expression of PD-L1 has been demonstrated to be closely associated with the clinicopathological status of patients with non-small-cell lung cancer, renal cell carcinoma, or human esophageal cancer.13,16,17 Several studies have shown that PD-1 or PD-L1 antibodies could relieve the inhibitory effects of PD-L1 on cytotoxic T-cells and hence accelerate the removal of tumor cells by cytotoxic T-cells.18,19 PD-L1 inhibitors have also been shown to be effective in the immunotherapy of melanoma.20 Most recently, Ansen et al have reported that PD-L1 expression was associated with distinct genotypes of EGFR, KRAS, and STK11 in the two most common histological non-small-cell lung cancer subtypes (adenocarcinoma and squamous cell carcinoma) in the 2014 ASCO Annual Meeting.21 This novel finding will facilitate the identification of patients most suitable for anti-PD-1 and anti-PD-L1 therapies. In addition, PD-L1 was found to promote cell proliferation in breast cancer.12 Taken together, these data demonstrate that the PD-1/PD-L1 pathway plays an important role in tumor immunity and tumor cell proliferation and suggest that interrupting the PD1/PD-L1 signal pathway is a promising therapeutic strategy for cancer.
Apurinic/apyrimidinic endonuclease 1 (APE1) plays an important role in multiple biological processes. It can repair DNA damage and suppress tumorigenesis through maintaining genome stability. APE1, as a redox-active protein, also activates many transcription factors including NF-κB and AP-1 to stimulate their DNA binding activities through keeping the cysteine residues of the highly conserved DNA binding domains of these transcription factors in the reduction state.22 Overexpression and distribution in subcellular localization of APE1 have been found in a number of cancers and are correlated with invasion, metastasis, and chemo- or radio-resistance.23,24 However, deregulation of APE1 is not a consistent feature in all tumors, therefore the role of APE1 in tumorigenesis is currently under debate. One previous study suggested that inhibiting the redox function of APE1 could hinder prostate cancer cell proliferation by reducing the NF-κB and DNA binding activity.25 In contrast, another study found that inhibiting the expression of APE1 and VEGF proteins decreased cell proliferation and angiogenesis in osteosarcoma.26 Similarly, it was proposed that APE1 overexpression enhanced the tumorigenesis of gastric cancer.27 But the relationship between APE1 and the tumorigenesis in gastric cancer is still unclear.
A number of studies have shown that the expressions of PD-L1 and APE1 are related to tumor cell proliferation, indicating that both PD-L1 and APE1 can potentially play an important role in the pathogenesis of gastric cancer.28,29 However, the relationship of APE1 and PD-L1 coexpression in tissue type, metastasis, and prognosis of gastric cancers has not yet been reported. In this study, we examined the association between PD-L1 and APE1, especially PD-L1, and tumor immunity in gastric cancer. We analyzed PD-L1 and APE1 gene expression in 107 gastric cancer samples and their relationship with clinical pathological characteristics.
Materials and methods
Study subjects
The study group consisted of 107 gastric cancer patients (71 male and 36 female, aged between 30 and 82 years old, mean age 60.5 years). All subjects were from the Chinese Han population. Patients were consecutively enrolled from January 2009 to December 2013 in Daping Hospital, Third Military Medical University (Chongqing, People’s Republic of China) without restriction of age, gender, histology, or stage. All patients were newly diagnosed with gastric carcinoma based on pathological examination and underwent surgical treatment in Daping Hospital. No patient received chemotherapy or radiotherapy before surgery. At recruitment, informed consent was obtained from each subject, and each participant was then interviewed to solicit detailed information on demographic characteristics. The study was approved by the ethics committee of Daping Hospital. All patients were followed up for 42 months.
Immunohistochemistry
Polyclonal anti-human PD-L1/CD274 antibody was purchased from GeneTex (San Diego, CA, USA); mouse monoclonal antibody against APE1 (1:3,000) was purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Immunohistochemistry was performed using the Dako ElivisionTM Plus two-step system (Dako Denmark A/S, Glostrup, Denmark) according to the manufacturer’s instructions. In brief, tissues were fixed with formalin, processed and embedded in paraffin wax, and cut into 3 mm-thick sections by microtome. The sections were dewaxed and incubated with 3% H2O2 for 10 minutes to block endogenous peroxidase activity, followed by washing twice with phosphate-buffered saline (pH 7.4). Then, the sections were incubated with anti-human PD-L1 (1:100) or anti-human APE1 (1:3,000) antibody at 4°C overnight. After washing twice with phosphate-buffered saline, the sections were further processed using Immuno-Bridge + immunohistochemical staining system (GBI, Bothell, WA, USA) according to the instructions provided by the manufacturer. Reactivity was detected using DAB reagent sets (Sino-American Biotechnology, Beijing, People’s Republic of China), and the cells were counterstained with hematoxylin. Positive staining was detected as a brown color of the cells. Random count 10 HPF of 1,000 tumor cells were counted and graded as follows: negative (−): positive cells rate <10%; positive (+): positive cells rate ≥10%; positive (++): positive cells rate ≥30%; positive (+++): positive cells rate ≥60%.
Statistical analysis
Statistical analysis was performed using SPSS version 19.0 software. Differences between groups were evaluated using a χ2 test, and the prognosis between PD-L1, APE1 immunolabeling and various factors was analyzed by multivariable logistic regression. Overall survival (OS) was calculated using the Kaplan–Meier method. OS was defined as the time from start of treatment to death. The differences between the survival curves were tested by log-rank test. The Cox proportional hazards regression model was used to determine the joint effects of several variables on survival. Two-tailed tests were used and a P-value <0.05 was considered significant.
Results
PD-L1 expression is upregulated in gastric cancer tissues and associated with poor prognosis of patients with gastric cancer
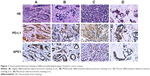
The immunohistochemical staining with the anti-PD-L1 antibody showed pale yellow or brown staining in the cell membrane and cytoplasm of the gastric cancer tissues, while the nucleus was not stained (Figure 1). Weak or no PD-L1 staining was observed in the normal tissue adjacent to the carcinoma. The percentage of PD-L1-positive cells in gastric carcinoma was 50.5% (54/107). There was no correlation between PD-L1 expression and age, sex, or tumor size of patients; however, PD-L1 staining was significantly correlated to the depth of invasion (odds ratio [OR] =3.37; P=0.005), lymph node metastasis (OR =2.68; P=0.020), tumor differentiation (OR =3.19; P=0.008), pathological type (χ2=8.676; P=0.013), and survival time (OR =3.39; P=0.003) (Table 1). These data indicate that PD-L1 expression is a risk factor for all the above clinical characteristics. Moreover, the positive rate of PD-L1 expression is much higher in high differentiation, lymph node metastasis, and higher T stage than that in others. In addition, Kaplan–Meier analysis demonstrated that PD-L1-positive gastric cancers were significantly associated with a poor prognosis (Figure 2) (P<0.05). Thus, PD-L1 expression is upregulated in gastric cancer and is associated with poor prognosis.
  | Table 1 Clinicopathologic characteristics and PD-L1 and APE1 expression in gastric cancer patients |
APE1 expression is upregulated in gastric cancer tissues and is a biomarker of poor prognosis in patients with gastric cancer
There was weak or no apparent expression of APE1 in normal tissues adjacent to the carcinoma. However, expression of APE1 could be observed in the nucleus and cytoplasm of gastric cancer cells (Figure 1). About 86.9% (93/107) of gastric carcinoma tissues showed positive APE1 immunostaining. Moreover, APE1 expression in gastric cancer cells was significantly correlated to lymph node metastasis (OR =3.26; P=0.037), depth of invasion (OR =7.61; P=0.001), and survival time (OR =10.91; P=0.040) (P<0.05). There was no significant association of APE1 expression with age, sex, or tumor size of patients (Table 1) (P>0.05). Kaplan–Meier analysis demonstrated that APE1 positive expression was a marker of poor prognosis in gastric cancer patients (P=0.035) (Figure 3). These results show that APE1 expression is upregulated in gastric cancer and is a marker of poor prognosis in patients with gastric cancer.
Gastric cancers have upregulated expression of both APE1 and PD-L1, and APE1 in combination with PD-L1 is a biomarker of patients with gastric cancer
We next analyzed the relationship between coexpression of APE1 and PD-L1 and the clinical features and prognosis in gastric cancer patients. We found that 49.5% (53/107) of gastric cancer tissues demonstrated both APE1 and PD-L1 positive expression. Spearman’s rank correlation analysis showed that APE1 and PD-L1 expression in gastric cancer were positively correlated (r=0.336, P<0.01) (Table 2). Most importantly, we found that coexpression of APE1 and PD-L1 was significantly correlated to depth of tumor invasion of gastric cancers (P<0.05). In addition, Kaplan–Meier analysis demonstrated that PD-L1 and APE1 co-positivity was significantly related to a poor prognosis (P=0.003) (Figure 4). These results demonstrate that upregulated expression of APE1 in combination with PD-L1 is a biomarker of gastric cancer.
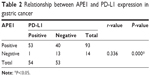  | Table 2 Relationship between APE1 and PD-L1 expression in gastric cancer |
Correlation analysis of clinical data characteristics and OS time
We further analyzed the relationships between clinical characteristics and OS using the Kaplan–Meier method (Table 3). Tumor location (P=0.008), depth of invasion (P=0.000), lymph node metastasis (P=0.000), tumor differentiation (P=0.013), and pathological type (P=0.002) were significantly correlated to OS (P<0.05). However, there was no correlation between OS and either age or sex of the patients. With regards to tumor location and OS, the median survival time of patients with cancers of the cardia was 22±2.42 months, which was shorter than that of gastric body cancers (45±3.86 months, P=0.003). The median survival time of tubular adenocarcinoma was 41.0 months, while the median survival time of poorly differentiated carcinoma was 20±2.24 months (P=0.004). Finally, multivariate analysis using the Cox regression model showed that the depth of invasion was a significant prognostic factor (risk ratio 19.91; 95% confidence interval: 4.83–82.03; P=0.000), but there was no significant relationship between prognostic risk and PD-L1, APE1, or other characteristics.
  | Table 3 Correlation analysis of clinical data characteristics and overall survival time |
Discussion
In this study, we demonstrated that both PD-L1 and APE1 were upregulated in gastric cancer. PD-L1 and APE1 positive expressions were significantly correlated with invasion, lymph node metastasis, pathological type, OS, and higher T stage. The expression of APE1 and PD-L1 in gastric cancer was positively correlated.
Gastric carcinoma occurs early in most patients, and local lymph node metastasis predicts a poor prognosis, but the underlying molecular mechanisms remain to be determined. PD-L1 is a type I transmembrane glycoprotein, encoding 290 amino acids, and containing IgV- and IgC-like regions.30,31 It is located in the chromosome 9p24.2 and more broadly expressed than the other superfamily members.32 Binding of PD-L1 to its receptor PD-1 on activated T-cells negatively regulates cytokine secretion and T-cell proliferation.9 Overexpression of PD-L1 in tumor cells allows cancer cells to escape from host immune systems and lead to resistance of cancer cells to antitumor immunity.33,34 PD-L1 was found to be upregulated in many types of tumors and there is a strong association between its expression and the patients’ clinicopathological parameters.13,16,17,35 Oki, in the ASCO Annual Meeting of 2014, reported that HER2 may affect PD-L1 expression in gastric cancer tissue, and therefore PTEN and HER2 may be used as potential biomarkers for PD-1- and PD-L1-targeted drugs in clinical trials.36 Thus, targeting PD-L1 has a bright future in the treatment of tumors.
In the present study, we analyzed the expression of PD-L1 in gastric cancer. In Kaplan–Meier analysis, PD-L1-positive gastric cancers were significantly related to a poor prognosis (P=0.008), which is consistent with a previous study.28 PD-L1 expression was significantly correlated to invasion, differentiation, lymph node metastasis, pathological type, and OS. PD-L1 immunostaining was significantly enhanced when the tumor infiltrated into the deep muscular layers, with lymph node metastasis, high differentiation, or survival time of less than 2 years (P<0.05).
PD-L1 may promote immune escape of cancer cells by activating Treg (regulatory cell) and inactivating antitumor T-cells, leading to poor prognosis.37 Recently, it was reported that factors present in the tumor microenvironment (such as IFN-γ) and antitumor treatment induced PD-L1 expression. Moreover, both in vitro and in vivo experiments showed that blocking PD-L1 or PD-1 could enhance the tumor-specific T-cell responses and hence inhibit tumor cell proliferation.38 However, the intracellular signaling pathways that regulate the expression of PD-L1 remain unclear. A study found that PD-L1 expression requires the activity of transcription factor STAT3 combined with CD274 promoter,39 while the induction of PD-L1 by IFN-γ requires mitogen-activated protein kinases (MAPK). Our results showed that PD-L1 was upregulated in most gastric cancers, suggesting that the MAPK and JAK/STAT signaling pathways may be promising targets for therapy of gastric cancer.
Recent studies have found that APE1 redox activity plays an important role in the immune response.40,41 In a redox-dependent or -independent manner, APE1 may activate several transcription factors, including AP-1, NF-κB, p53, HIF, PEBP-2, Pax-5, Pax-8, and TTF-1, which control different cell biological processes, such as apoptosis, proliferation, and differentiation. APE1 may regulate proliferation and apoptosis by maintaining the intracellular reducing activity status of these transcription factors.42 APE1 is an apurine/apyrimidine endonuclease and key enzyme in DNA base excision repair, which mainly repairs DNA damage caused by alkylating agents and oxidants.43 APE1 levels can be significantly increased under oxidative stress.22 The APE1 levels are consistent with the activity of DNA base excision repair.26 Abnormal expression of APE1 was found to be closely associated with apoptosis, angiogenesis, and tumorigenesis.27 Our study found that 86.9% (93/107) of gastric carcinomas showed positive APE1 expression, and its expression was associated with tumor infiltration depth, lymph node metastasis, histopathologic type, and the prognosis of gastric cancer patients (P<0.05). The APE1 positive expression rate in OS <2 years gastric cancer patients (96.8%) was significantly higher than in OS >2 years patients (73.3%). High expression of APE1 suggested that there might be significant direct DNA damage. Taken together, we speculate that APE1 is involved in the invasion and metastasis of gastric cancer and APE1 expression may have utility in the prognostication of gastric cancer.
Interestingly, we found that PD-L1 and APE1 were coexpressed in 49.5% of gastric cancer patients and this was associated with the extent of tumor invasion (P<0.05). In Kaplan–Meier analysis, PD-L1 and APE1 coexpression was significantly related to a poor prognosis (P=0.003). Further Spearman’s rank correlation analysis showed that the expression of APE1 and PD-L1 in gastric cancer was positively correlated (r=0.336, P<0.336). These results suggest that PD-L1 and APE1 might have some correlation with tumor proliferation and infiltration. Recently, it was found that STAT3 controls APE1 gene expression in the liver.44 Conversely, it has also been shown that APE1 directly regulates STAT3 expression through its REDOX (oxidation reduction) function in pancreatic cancer.45 These facts suggest that APE1 might indirectly control PD-L1 expression through the regulation of STAT3 and inhibition of APE1 may decrease STAT3 and eventually lead to downregulation of PD-L1.
Multivariate analysis using the Cox regression model showed that the depth of invasion could be considered as a significant prognostic factor (risk ratio 19.91; P=0.000). However, there was no significant relationship of PD-L1 and APE1 with other characteristics and prognosis, probably due to the close mutual relationships between APE1, PD-L1, and proliferation or infiltration of tumors. Therefore, depth of infiltration may be an independent factor affecting gastric cancer survival, which has important clinical significance.
The correlation analysis of clinical data characteristics and OS using the Kaplan–Meier method demonstrated that there was no significant correlation between age, sex, and OS of patients. However, the tumor location (P=0.008), depth of invasion (P=0.000), lymph node metastasis (P=0.000), tumor differentiation (P=0.013), and pathological type (P=0.002) were significantly correlated to OS (P<0.05). The median survival time of cardia cancer (22±2.42 months) was lower than that of gastric body (45±3.86 months) in the analysis of tumor location and OS (P=0.003). The median survival time of tubular adenocarcinoma was the longest (41.0 months), while the median survival time of poorly differentiated carcinoma was the shortest (20±2.24 months, P=0.004). Therefore, the high expression of PD-L1 and APE1 is a risk factor of gastric cancer and a new biomarker to predict the prognosis of gastric cancer, further indicating the potential therapeutic targeting value of the combination of PD-L1 and APE1 signaling pathways in advanced gastric cancer patients.
Conclusion
To our knowledge, this is the first study to focus on the association between PD-L1, APE1, and gastric cancer. Our findings suggest that the high expression of PD-L1 and APE1 is a risk factor of gastric cancer and a new biomarker to predict the prognosis of gastric cancer.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (number 81101993). We thank all the people and patients who participated in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Roder DM. The epidemiology of gastric cancer. Gastric Cancer. 2002;5 Suppl 1:5–11. | ||
Yan M, Parker BA, Schwab R, Kurzrock R. HER2 aberrations in cancer: implications for therapy. Cancer Treat Rev. 2014;40(6):770–780. | ||
Kuo CY, Chao Y, Li CP. Update on treatment of gastric cancer. J Chin Med Assoc. 2014;77(7):345–353. | ||
Matsueda S, Graham DY. Immunotherapy in gastric cancer. World J Gastroenterol. 2014;20(7):1657–1666. | ||
Tian Y, Jia X, Wang S, et al. SOX2 oncogenes amplified and operate to activate AKT signaling in gastric cancer and predict immunotherapy responsiveness. J Cancer Res Clin Oncol. 2014;140(7):1117–1124. | ||
Du L, Xiao X, Wang C, et al. Human leukocyte antigen-G is closely associated with tumor immune escape in gastric cancer by increasing local regulatory T-cells. Cancer Sci. 2011;102(7):1272–1280. | ||
Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T-cell tolerance. J Exp Med. 2006;203(4):883–895. | ||
Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. | ||
Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. | ||
Selenko-Gebauer N, Majdic O, Szekeres A, et al. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T-cell anergy. J Immunol. 2003;170(7):3637–3644. | ||
Haspot F, Fehr T, Gibbons C, et al. Peripheral deletional tolerance of alloreactive CD8 but not CD4 T-cells is dependent on the PD-1/PD-L1 pathway. Blood. 2008;112(5):2149–2155. | ||
Ghebeh H, Tulbah A, Mohammed S, et al. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer. 2007;121(4):751–758. | ||
Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–5100. | ||
Wintterle S, Schreiner B, Mitsdoerffer M, et al. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63(21):7462–7467. | ||
Strome SE, Dong H, Tamura H, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63(19):6501–6505. | ||
Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004; 101(49):17174–17179. | ||
Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–2953. | ||
Dinesh RK, Hahn BH, Singh RP. PD-1, gender, and autoimmunity. Autoimmun Rev. 2010;9(8):583–587. | ||
Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66(7):3381–3385. | ||
Mamalis A, Garcha M, Jagdeo J. Targeting the PD-1 pathway: a promising future for the treatment of melanoma. Arch Dermatol Res. 2014;306(6):511–519. | ||
Ansen S, Schultheis AM, Hellmich M, et al. PD-L1 expression and genotype in non-small cell lung cancer (NSCLC). J Clin Oncol. 2014;32: 5s (suppl; abstr 7517). | ||
Xanthoudakis S, Curran T. Identification and characterization of Ref-1, a nuclear protein that facilitates AP-1 DNA-binding activity. EMBO J. 1992;11(2):653–665. | ||
Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The many functions of APE1/Ref-1: not only a DNA repair enzyme. Antioxid Redox Signal. 2009;11(3):601–620. | ||
Liu P, Demple B. DNA repair in mammalian mitochondria: much more than we thought. Environ Mol Mutagen. 2010;51(5):417–426. | ||
Raffoul JJ, Banerjee S, Singh-Gupta V, et al. Down-regulation of apurinic/apyrimidinic endonuclease 1/redox factor-1 expression by soy isoflavones enhances prostate cancer radiotherapy in vitro and in vivo. Cancer Res. 2007;67(5):2141–2149. | ||
Wang D, Zhong ZY, Li MX, Xiang DB, Li ZP. Vector-based Ape1 small interfering RNA enhances the sensitivity of human osteosarcoma cells to endostatin in vivo. Cancer Sci. 2007;98(12):1993–2001. | ||
Tsujie M, Yamamoto H, Tomita N, et al. Expression of tumor suppressor gene p16(INK4) products in primary gastric cancer. Oncology. 2000; 58(2):126–136. | ||
Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108(1): 19–24. | ||
Gu D, Wang M, Wang S, Zhang Z, Chen J. The DNA repair gene APE1 T1349G polymorphism and risk of gastric cancer in a Chinese population. PLoS One. 2011;6(12):e28971. | ||
Loos M, Giese NA, Kleeff J, et al. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268(1):98–109. | ||
Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2(2):116–126. | ||
Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T-cells and APC. J Immunol. 2002;169(10): 5538–5545. | ||
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–12297. | ||
Dong H, Chen L. B7-H1 pathway and its role in the evasion of tumor immunity. J Mol Med (Berl). 2003;81(5):281–287. | ||
Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8(3):190–198. | ||
Eiji Oki, Shinji Okano, Koji Ando, et al. HER2 and programmed death-1 ligand-1 (PD-L1) expression in gastric carcinoma. J Clin Oncol. 2014;32(suppl; abstr e15041). | ||
Muenst S, Hoeller S, Willi N, Dirnhofera S, Tzankov A. Diagnostic and prognostic utility of PD-1 in B cell lymphomas. Dis Markers. 2010; 29(1):47– 53. | ||
Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1( PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T-cell apoptosis. Mol Immunol. 2008;45(5):1470–1476. | ||
Matta BM, Raimondi G, Rosborough BR, Sumpter TL, Thomson AW. IL-27 production and STAT3-dependent upregulation of B7-H1 mediate immune regulatory functions of liver plasmacytoid dendritic cells. J Immunol. 2012;188(11):5227–5237. | ||
Merluzzi S, D’Orlando O, Leonardi A, Vitale G, Pucillo C. TRAF2 and p38 are involved in B cells CD40-mediated APE/Ref-1 nuclear translocation: a novel pathway in B cell activation. Mol Immunol. 2008;45(1): 76–86. | ||
Zou GM, Luo MH, Reed A, Kelley MR, Yoder MC. Ape1 regulates hematopoietic differentiation of embryonic stem cells through its redox functional domain. Blood. 2007;109(5):1917–1922. | ||
Flaherty DM, Monick MM, Hunninghake GW. AP endonucleases and the many functions of Ref-1. Am J Respir Cell Mol Biol. 2001;25(6): 664–667. | ||
Izumi T, Brown DB, Naidu CV, et al. Two essential but distinct functions of the mammalian abasic endonuclease. Proc Natl Acad Sci U S A. 2005;102(16):5739–5743. | ||
Haga S, Terui K, Zhang HQ, et al. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J Clin Invest. 2003;112(7):989–998. | ||
Cardoso AA, Jiang Y, Luo M, et al. APE1/Ref-1 regulates STAT3 transcriptional activity and APE1/Ref-1-STAT3 dual-targeting effectively inhibits pancreatic cancer cell survival. PLoS One. 2012;7(10): e47462. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.