Back to Journals » Cancer Management and Research » Volume 12
Upregulation of DUSP14 Affects Proliferation, Invasion and Metastasis, Potentially via Epithelial–Mesenchymal Transition and Is Associated with Poor Prognosis in Pancreatic Cancer
Authors Wei Y, Wang G, Wang C , Zhou Y, Zhang J, Xu K
Received 26 November 2019
Accepted for publication 27 February 2020
Published 20 March 2020 Volume 2020:12 Pages 2097—2108
DOI https://doi.org/10.2147/CMAR.S240040
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Yajun Wei,* Gang Wang,* Cheng Wang, Yangming Zhou, Jingcheng Zhang, Kai Xu
Department of Biliary and Pancreatic Surgery, Anhui Provincial Hospital Affiliated with Anhui Medical University, Hefei, Anhui 230001, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Cheng Wang
Department of Biliary and Pancreatic Surgery, Anhui Provincial Hospital Affiliated with Anhui Medical University, No. 17 Lujiang Road, Hefei, Anhui Province 230001, People’s Republic of China
Tel +86 51 62283292
Fax +86 51 65327735
Email [email protected]
Background: There is a growing number of evidence which report the relationship of the dual-specificity phosphatases 14 (DUSP14) with physiological and pathological mechanisms in the human body. However, it is still not known what if any role DUSP14 plays in pancreatic cancer.
Materials and Methods: The study evaluates the levels of DUSP14 in the pancreatic cancer tissues and cell lines using Western blotting and qRT-PCR to assess the levels of the DUSP14 and epithelial–mesenchymal transition (EMT) biomarkers. After the DUSP14 was blocked, the following assays were performed: colony formation, assessments of scratch wound and transwell to examine the effects of DUSP14 on the proliferation, migration and invasion of the pancreatic cancer.
Results: Results showed that there was a significant increase in the level of DUSP14 expression both in the pancreatic cancer tissues and cell lines. Experimental downregulation of DUSP14 induced the inhibition of the capacity of proliferation, migration and invasion of the pancreatic cancer cells. Western blotting analyses showed changes in the levels of expression of the EMT biomarkers, which helped to determine the function of DUSP14 in EMT.
Conclusion: In conclusion, we suggest that DUSP14 is a novel molecular target that can be used for the treatment of pancreatic cancer.
Keywords: pancreatic cancer, DUSP14, migration, invasion, epithelial-mesenchymal transition
Introduction
Pancreatic cancer is one of the deadliest malignant tumors in the world.1,2 It is the 4th primary cause of malignant deaths, having a 5-year survival rate of 2% in America.3 Due to the nature of difficult initial diagnosis, strong migratory nature, and high resistance to current cancer therapies, the prognosis remains dismal, which has not changed, regardless of vital progress in medical therapy and surgical techniques during the past few decades.4,5 Thus, a better and more efficient treatment strategy of pancreatic cancer is urgently needed.
The dual-specificity phosphatases (DUSPs) are a set of protein phosphatases with heterogeneity, by which not only phosphotyrosine but also phosphoserine/phosphothreonine residues can be dephosphorylated within the one substrate, which modulates a diversity of cellular processes, like growth, signal transmission, etc. Moreover, DUSPs act critically during the process of cancer cell growth, as well as survival.6,7 To date, up to 43 DUSP genes have been listed in the GeneCards. DUSPs, depending on their sequence similarity, can be categorized into six subgroups, which contains slingshots, PRLs (phosphatases of regenerating liver), Cdc14 phosphatases (Cdc is cell division cycle), PTENs (phosphatase and tensin homologues deleted on chromosome 10), myotubularins, MKPs (mitogen-activated protein kinase phosphatases) and atypical DUSPs.8 Recently, one study9 reported that overexpression of DUSP28 caused a significant increase in the migratory and invasive activity of the pancreatic cancer cells. They also found that PDGF-A enhanced this DUSP28’s effect on the pancreatic cancers. As a homologous gene of DUSP28’s, DUSP14’s role in the progress of pancreatic cancer is not clear. Thus, in the current study, we studied whether DUSP14 can regulate pancreatic cancer malignancy, as well as the latent mechanisms comprised. Furthermore, it is necessary to authenticate whether DUSP14 can play the role of a tumor marker in human pancreatic cancer.
For the past few decades, EMT is regarded as the transdifferentiation of the epithelial cells to the mesenchymal cells after the cells have been subjected to particular physiological and pathological conditions, which is a change accompanied by variations in cell morphology as well as in levels of expression of associated genes. Collective evidence indicates that EMT influences the rates of development and metastasis of pancreatic cancer. EMT refers to a mechanism, wherein tumor cells lose epithelial specialties and acquire a mesenchymal phenotype.10 It relates changes in the degrees of the mesenchymal protein expression, which strengthens migration, invasion, and metastatic ability of pancreatic cancer. Moreover, several important pathways act vitally in the mesenchymal protein expression. The downstream effect of these pathways is to stimulate the expression of the EMT transcription determinants, comprising Snail, Slug, Twist, and Zeb, which ultimately promote epithelial inhibition and the induction of the mesenchymal characteristics. The growing evidence has confirmed the truth that different kinds of small molecule inhibitors and phytochemicals are able to block the progression of EMT, reversing the mechanisms behind EMT, and thereby inducing the re-expression of the epithelial markers. The understanding of the association existing between EMT and pancreatic cancer is likely to make contributions to the establishment of new remedial targets for pancreatic cancer.
Materials and Methods
Clinical Tissue Specimens
In total, six pancreatic cancer samples, as well as the consistent adjacent tissues from patients who were diagnosed at the Anhui Provincial Hospital (Hefei, China), were collected. The samples and tissues were analyzed to examine the mRNA degrees of DUSP14. Elaborate pathological and clinical information (containing gender, age, tumor size, tumor position, vascular invasion, level of differentiation and TNM stage) from 84 patients between June 2013 and June 2016 were obtained from the medical records. Based on the 8th edition of the Union for International Cancer Control TNM structure, these specimens were involved in the current research. Those who had been treated with radiotherapy or chemotherapy methods before the operation were excluded. The samples were preserved in 4% formalin at the temperature of 37°C for 2 hrs. Apart from that, they were also embedded in paraffin to be analyzed in pathological aspects, and further to be used to confirm the diagnosis. The subsequent patients’ clinical information was collected from the pancreatic cancer database of the Anhui Provincial Hospital. The ethics committee has endorsed this research to waive the informed consent of the patients.
Cell Lines and Culture
Attained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, People’s Republic of China), the normal and pancreatic cancer cell lines, that is, HPDE6-C7, Capan-2, PANC-1, AsPC-1, BxPC-3, were used in this study. The cell culturing was conducted in the DMEM or RPMI 1640 media (BI, Haemek, Israel), using 10% fetal bovine serum and 100 U/mL penicillin-streptomycin solution (Gibco, North Andover, MA, USA) in an atmosphere which contained 5% CO2 at a temperature of 37°C.
Stable Transfection of the Pancreatic Cancer Cell
DUSP14 shRNA (short-hairpin RNA) and control sh-NC synthesized by Sigma Company (Shanghai, China) were used in the experiments. For the construction of the β-catenin vector, we cloned the β-catenin cDNA into the Flag-tagged-pcDNA3.1 (GenePharma, China). For the transfection of the DUSP14-shRNA, guided by the manufacturer’s instructions, the controlled transfection of shNC and the β-catenin-vector in the PANC-1 and the BxPC-3 cell samples were carried out with Lipofectamine 2000 Transfection Reagent (Invitrogen, USA).
Quantitative Real-Time PCR (qRT-PCR)
In accordance with the manufacturer’s authentication protocols (Invitrogen, Carlsbad, CA, USA), the total RNA was separated from the cell samples applying the TRIzol reagent. The first sequence of cDNA was synthesized using cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). RT-PCR and SYBR Green PCR Master Mix (Applied Biosystems) were used to detect the degrees of transcription of DUSP14. We used the following primer set for analysis of the levels of expression of DUSP14: forward primer 5′-GGACTCTTGAGGAAGAAGGAGAC-3′ and reverse primer 5′- GGAATAGAGAGGAGGTGATTTGAG −3′. For GAPDH the primer set was: forward 5′-AGGTCGGTGTGAACGGATTTG-3′ and reverse 5′-GGGGTCGTTGATGGCAACA-3′. Determination of the levels of interrelated levels of quantification expression was carried out in accordance with the 2−ΔΔCt methodology.
Western Blot Analysis
Western blotting was adopted during the process to determine the degrees of protein expression. The antibodies included are DUSP14 (cat. no. ab134265, 1:800, Abcam, Cambridge, UK), E-cadherin (cat. no. SAB4503751, 1:500, Sigma-Aldrich, Chicago, IL, USA); Vimentin (cat. no. SAB1305433, 1:1000, Sigma-Aldrich, Chicago, IL, USA); Snail (cat. no. SAB4502825, 1:800, Sigma-Aldrich, Chicago, IL, USA); β-catenin (cat. no. SAB4500541, 1:800, Sigma-Aldrich, Chicago, IL, USA) and/or GAPDH (cat. no. 10494-1-AP, 1:5000, Proteintech Group, Chicago, IL, USA). Signals were detected in accordance with the standard methodologies for chemoluminescence.
Immunohistochemistry (IHC)
Immunohistochemical analyses were conducted with the standard streptavidin-biotin-peroxidase complex-based methodology following the manufacturer’s guidelines (Boster Biological Technology, SA2010). Briefly, we incubated the tissue samples slides in a moist chamber overnight with the DUSP14 (cat. no. ab110938, 1:300, Abcam, Cambridge, UK) antibody at 4°C. We quantified the level of DUSP14 expression through determining the percentage which positive tumor cells comprised and the severity of the positive staining. We scored the severity of staining as follows: negative=0, bordering=1, weak=2, moderate=3, and strong=4. Additionally, we scored the level of staining in accordance with the percentage of positively stained tumor cells in the field: negative=0, 0–25%=1, 26–50%=2, 51–75%=3, and 76–100%=4. Also, we used the resultant multiplicative product of the severity score and percentage of positively stained cells to be the overall IHC score (value range: 0 to 16). Two independent pathologists observed and evaluated the staining process and results.
Colony Formation Assay
Six-well plates were used to contain cells, which are at 1.0*103/well approximately, and cultured in DMEM/10% FBS medium at 37°C. After a two-week treatment, the cells were processed using 10% formaldehyde and stained by 0.1% crystal violet (Sigma, USA)., the observation and calculation of colonies formed were conducted through a microscope (Olympus, Tokyo, Japan).
Scratch Wound Assay
Cell seeding was carried out in 24-well plates at a density of 80%. Following overnight culturing of the cells, a pipette tip was used to scrape an area in the middle of the bottom of the sample well. The floating cells were washed away from the mix with PBS. After 0 and 24 hrs subsequent to the wound assay, pictures were taken to monitor the wound-healing mechanism using a light-based microscope (Nikon, Japan).
Cell Migration Assay
A transwell assay (8 µm pore size, Millipore, MA, USA) was used to investigate the impact of DUSP14 on rate and level of metastasis of cancer. Briefly, the cells were cultured in the upper culture chambers, which had matrix gel coating. The bottoms of the culture chambers were filled with DMEM/10% FBS by volume. Subsequent to an incubation period of 48 hrs, the level of staining of the invaded cells were determined with a 0.5% crystal violet stain and light-based microscope (Nikon, Japan).
Data Processing
Data processing of the collected information was conducted with the SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) software. Determination of the levels of variation and significance of differences for values among cohorts was determined using a paired 2-tailed Student’s t-test. Values for P<0.05, which was regarded as an essential process statistically.
Results
Expression Degrees of DUSP14 in the Human Pancreatic Cancer Tissue and Cell Lines
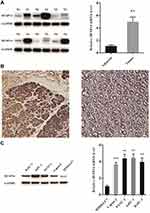
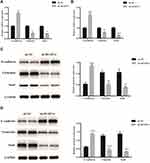
As is stated above, both pancreatic cancer samples and cell lines were verified to evaluate the expression of DUSP14 in pancreatic cancer. As is demonstrated in Figure 1A, DUSP14 mRNA and protein were more notably expressed in the pancreatic tumors, while the normal pancreatic samples showed less significance. Immunohistochemical staining for DUSP14 in pancreatic cancer also showed a likely tendency (Figure 1B). As is depicted in Figure 1C, compared with normal pancreatic ductal epithelial cell (HPDE6-C7), the protein and mRNA levels in four pancreatic cancer cell lines (Capan-2, PANC-1, AsPC-1 and BxPC-3) possessed a remarkable lager scale.
High Levels of DUSP14 Expression Is Associated with the Pancreatic Cancer’s Clinicopathological Parameters and Prognosis
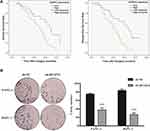
For the purpose of evaluating the biological significance of DUSP14 in pancreatic cancer, we analyzed the association combining the pancreatic cancer tissue DUSP14 degrees, as well as clinicopathological parameters, which is illustrated in Table 1. The enhanced DUSP14 expression was related to T3 and T4 (P=0.030), N2 (P=0.004), and stage III and IV (P=0.037). The Kaplan-Meier survival analysis plotted compared the overall survival (OS) and disease-free survival (DFS) of the patients who had pancreatic cancer, according to their DUSP14 expression levels (Figure 2A). Patients with higher expression of DUSP14 had a poorer prognosis compared to those with lower DUSP14 expression (OS, P=0.005; DFS, P=0.004). Multivariate survival analysis further demonstrated the outcome that intra-tumoral DUSP14 expression (OS, P=0.041; DFS, P=0.025) degree was an independent poor prognostic biomarker for OS and DFS (Tables 2 and 3).
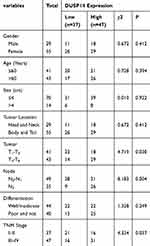 |
Table 1 Relationships Between DUSP14 Protein Expressions (Immunohistochemical Staining) in PDAC and Various Clinicopathological Variables |
 |
Table 2 Univariate and Multivariate Analysis of the Correlation Between Clinicopathological Parameters and Overall Survival Time of Patients with PDAC |
 |
Table 3 Univariate and Multivariate Analysis of the Correlation Between Clinicopathological Parameters and Disease-Free Survival Time of Patients with PDAC |
Establishment of DUSP14 Silencing the Pancreatic Cancer Cell
In order to investigate the potential role of DUSP14 in the malignant pancreatic cancer, we specifically reduced the expression of DUSP14 in the pancreatic cancer cell lines PANC-1 and BxPC-3 with the use of shRNA interference. ShRNA and negative control were constructed by GenePharma (Shanghai, China). Transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Impact of DUSP14’s Downregulation in the Pancreatic Cancer Cells
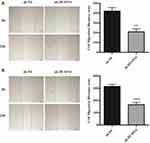
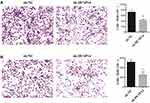
The following step was to study whether the decrease of DUSP14 expression by shRNA transfection caused the bluntness of the activity of pancreatic cancer’s proliferation, migration, and invasion rates. As expected, the colony formation assay revealed that the downregulation of DUSP14 considerably suppressed the cellular proliferation of not only the PANC-1 but also the BxPC-3 cells (Figure 2B). Moreover, the wound-healing assay, together with the transwell assay, showed the fact that inhibition of the DUSP14 expression markedly weakened the activity of the migration and invasion of both the Panc-1 and BxPC-3 (Figures 3 and 4).
DUSP14 Improved the EMT in the Pancreatic Cancer Cell
Increasingly accumulating evidence has indicated that the migration and invasive abilities of the pancreatic cancer cells are regulated by the epithelial–mesenchymal transition (EMT) process.11 In order to investigate whether or not DUSP14 is associated with EMT, we studied the levels of the epithelial (E-cadherin) expression, as well as the mesenchymal markers (Vimentin, Snail) in the PANC-1 and BxPC-3 pancreatic cancer cell lines transfected with sh-DUSP14. Western blotting and qRT-PCR analyses showed an increase in the levels of expression of the epithelial markers (E-cadherin) in the cell lines that were transfected with sh-DUSP14. Comparatively, a noticeable decline appeared in the levels of expression of the mesenchymal markers (Vimentin, Snail) in both the sh-DUSP14 transfected cell lines (Figure 5). In summary, the results can be hypothesized that DUSP14 is a novel marker that can be used to improve the development of EMT in pancreatic cancer cells.
Discussion
Absence of specific symptoms usually delays diagnosis, which is worsened by the lack of reliable methods approaching to initial diagnosis. As a consequence, most patients are diagnosed at an advanced stage of the disease, making fraction of patients eligible for resection. Also, pancreatic cancer is not sensitive to current treatments. According to the previous report, the non-responsiveness may derive from the resistance internally or externally to chemotherapies through different genetic and cellular mechanisms,12,13 epithelial–mesenchymal transformation,14,15 hypoxia,16,17 as well as pancreatic cancer stem cells.18,19
The dual-specificity phosphatases (DUSPs) are protein phosphatase which regulates the activities of mitogen-activated protein kinases (MAPKs), which mediates various physiological activities of the cell, such as growth, signal transmission, etc. Typically, MAPK cascades are triple kinase pathways in which a MAPK kinase activates a MAPK kinase which in turn activates a terminal MAPK (ERK1/2, P38, JNK1/2) via serial phosphorylation to allow for signal amplification, modulation, and specificity.20 DUSPs act pivotally in the pathological process of multiple diseases, as well as in some kinds of cancers. Additionally, some atypical DUSPs are noted to affect potential regulatory on the growth and apoptosis of the cell.21,22 Moreover, several reports have shown that some atypical DUSPs are becoming relevant targets as anti-cancer therapies. Scientists are dedicated to the development of specific and effective DUSP enzyme inhibitors as cancer-targeted drugs.23,24 However, there are difficulties in the comprehension of the system of ERK1/2 initiation, as well as the specific characteristic of DUSPs, which is the moderator of dephosphorylation in the MAPK semaphoring pathway. A small group of atypical DUSPs is noted in a variety of cancer phenotypes, like apoptosis, proliferation, cell cycle, as well as chemoresistance.25,26 As is illustrated in another research, the increased DUSP12 induced cellular migration and defensive action against apoptosis via the up-regulation of both oncogenes validated, which are integrin alpha 1 and the hepatocyte growth element receptor, c-met.27 According to previous report, DUSP26 is a phosphatase that represses the p53 tumor and inhibits p53 action.22 Also, DUSP26 suppresses the p53 pro-apoptosis effect in the neural tumor during the treatment of Adriamycin.28 A recent study showed that the blockade of DUSP28 decreases chemoresistance and migration in human pancreatic cancer cells.29
DUSP14, a member of the atypical DUSPs subgroup, initially was cloned as a novel CD28 cytoplasmic tail interacting protein.30 Although DUSP14 does not contain the kinase interaction sequence constituted by alkaline amino acids, it can still play the same role as other MAKP phosphatases, dephosphorylating extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and P38 kinases.30,31 Data presented in this study suggest that DUSP14 is implicated in the phenotype of pancreatic cancer. First, we verified that DUSP14 is highly expressed in both pancreatic cancer samples and cell lines. And then, the relevance between DUSP14 and pancreatic cancer was illustrated. Data show that high level of DUSP14 expression correlates to advanced T classification (T3 and T4), more lymph node migration (N2) and later tumor stage (III and IV). By using shRNA, we downregulated DUSP14 expression in PANC-1 and BxPC-3. As a consequence, we observed that the abilities of cell proliferation, migration, and invasion are considerably suppressed, proving that high level of DUSP14 affects pancreatic cancer’s clinicopathological parameters and prognosis. Meanwhile, blocking DUSP14 increase the expression level of epithelial biomarker (E-cadherin), reducing that of mesenchymal markers (Vimentin, Snail) in both cell lines. This indicates the effect of DUSP14 may work via EMT. However, we need further study to reveal the potential mechanism in it.
Conclusion
Through this report, we aim to illustrate that pancreatic cancers reflect a considerably higher level of expression of DUSP14 compared to the normal pancreatic samples. Moreover, it is validated in this report that human pancreatic cancer cell lines do notably contain various degrees of DUSP14 mRNA and protein. There is a prominent positive pertinence between DUSP14 expression and human pancreatic cancer biological behaviors, according to our study. The inhibition of DUSP14 made the human pancreatic cells to become less active in migration, invasion, and proliferation. The outcomes above suggest that DUSP14 may act as a “messenger” molecule in cancer through regulating signal transduction in the human pancreatic cancers. In general, the outcomes show that DUSP14 has a particular mission in biological behaviors in human pancreatic cancer cells, which indicated that DUSP14 may be a crucial molecule to adjust phenotypes in pancreatic cancers. Nevertheless, it is necessary for more analysis to be conducted to reflect the specific conditions and mechanisms involved in the process.
In the present research work, we also investigated whether DUSP14 promoted pancreatic cancer’s malignancy via EMT. Downregulation of the DUSP14’s expression led to a reduction in the metastasis and invasion of pancreatic cancer. DUSP14 interference improved the expression of E-cadherin when limiting the expression of Vimentin and Snail. Collectively, the outcomes stated above indicate that DUSP14 is a likely EMT-inducer and an excellent prospective target for treatment, targeting cancer’s metastasis in pancreatic cancer.
Ethical Statement
I declare that this study is in accordance with the Declaration of Helsinki, the ICH GCP, and other relevant regulations and guidelines to ensure the dignity, safety and rights of the patients. This study will not cause physical and mental suffering to patients, or increase the financial burden on patients and their families. All data were anonymized and maintained with confidentiality, and all data and samples were used only for this study. The Ethics Committee of Anhui Provincial Hospital has endorsed this research to waive the informed consent of the patients (The Certification NO. 2019-P-047).
Data Sharing Statement
All data included in this study are available upon request by contact with the corresponding author.
Acknowledgments
This study was supported by the Anhui Provincial Natural Science Foundation (No. 1708085MH228 to Cheng Wang).
Author Contributions
C. W. has provided constructing the idea for research. Y. J. W., G. W., and Y. M. Z. have done experiments to get this conclusion. Taking responsibility in the execution of the experiments and edition of pictures have been provided by J. C. Z. and K. X. Reviewing the article before submission not only for spelling and grammar but also for its intellectual content have been provided by Y. J. W., G. W. and C. W. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Anhui Provincial Natural Science Foundation (No. 1708085MH228 to Cheng Wang).
Disclosure
The authors declare that they have no competing interests.
References
1. Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. doi:10.1016/j.stem.2007.06.002
2. Smith K. Pancreatic cancer: fASCINating insights into the metastatic nature of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2014;11(3):139. doi:10.1038/nrgastro.2014.15
3. Polireddy K, Chen Q. Cancer of the pancreas: molecular pathways and current advancement in treatment. J Cancer. 2016;7(11):1497–1514. doi:10.7150/jca.14922
4. Koay EJ, Truty MJ, Cristini V, et al. Transport properties of pancreatic cancer describe gemcitabine delivery and response. J Clin Invest. 2014;124(4):1525–1536. doi:10.1172/JCI73455
5. Li D, Xie K, Wolff R, Abbruzzese, JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–1057. doi:10.1016/S0140-6736(04)15841-8
6. Caunt CJ, Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs): shaping the outcome of MAP kinase signalling. FEBS J. 2013;280(2):489–504. doi:10.1111/febs.2013.280.issue-2
7. Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802(4):396–405. doi:10.1016/j.bbadis.2009.12.009
8. Patterson KI, Brummer T, O’brien P, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418(3):475–489. doi:10.1042/BJ20082234
9. Lee J, Lee J, Yun JH, et al. Autocrine DUSP28 signaling mediates pancreatic cancer malignancy via regulation of PDGF-A. Sci Rep. 2017;7(1):12760. doi:10.1038/s41598-017-13023-w
10. Nieto MA, Huang R-J, Jackson R, Thiery JP. Emt: 2016. Cell. 2016;166(1):21–45. doi:10.1016/j.cell.2016.06.028
11. Rodriguez-aznar E, Wiesmüller L, Sainz B, Hermann PC. EMT and stemness-key players in pancreatic cancer stem cells. Cancers (Basel). 2019;11(8):1136. doi:10.3390/cancers11081136
12. Edwards SL, Brough R, Lord CJ, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451(7182):1111–1115. doi:10.1038/nature06548
13. Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8(1):67–113. doi:10.1146/annurev.cb.08.110192.000435
14. Plentz R, Park J, Rhim AD, et al. Inhibition of gamma-secretase activity inhibits tumor progression in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology. 2009;136(5):1741–1749. doi:10.1053/j.gastro.2009.01.008
15. Wang Z, Li Y, Kong D, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69(6):2400–2407. doi:10.1158/0008-5472.CAN-08-4312
16. Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68(3):918–926. doi:10.1158/0008-5472.CAN-07-5714
17. Vonlaufen A, Phillips PA, Xu Z, et al. Pancreatic stellate cells and pancreatic cancer cells: an unholy alliance. Cancer Res. 2008;68(19):7707–7710. doi:10.1158/0008-5472.CAN-08-1132
18. Dembinski JL, Krauss S. Characterization and functional analysis of a slow cycling stem cell-like subpopulation in pancreas adenocarcinoma. Clin Exp Metastasis. 2009;26(7):611–623. doi:10.1007/s10585-009-9260-0
19. Simeone DM. Pancreatic cancer stem cells: implications for the treatment of pancreatic cancer. Clin Cancer Res. 2008;14(18):5646–5648. doi:10.1158/1078-0432.CCR-08-0584
20. Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90(4):1507–1546. doi:10.1152/physrev.00054.2009
21. Klinger S, Poussin C, Debril M-B, et al. Increasing GLP-1-induced beta-cell proliferation by silencing the negative regulators of signaling cAMP response element modulator-alpha and DUSP14. Diabetes. 2008;57(3):584–593. doi:10.2337/db07-1414
22. Shang X, Vasudevan SA, Yu Y, et al. Dual-specificity phosphatase 26 is a novel p53 phosphatase and inhibits p53 tumor suppressor functions in human neuroblastoma. Oncogene. 2010;29(35):4938–4946. doi:10.1038/onc.2010.244
23. Prabhakar S, Asuthkar S, Lee W, et al. Targeting DUSPs in glioblastomas - wielding a double-edged sword? Cell Biol Int. 2014;38(2):145–153. doi:10.1002/cbin.v38.2
24. Rios P, Nunes-xavier CE, Tabernero L, Köhn M, Pulido R. Dual-specificity phosphatases as molecular targets for inhibition in human disease. Antioxid Redox Signal. 2014;20(14):2251–2273. doi:10.1089/ars.2013.5709
25. Kozarova A, Hudson JW, Vacratsis PO. The dual-specificity phosphatase hYVH1 (DUSP12) is a novel modulator of cellular DNA content. Cell Cycle. 2011;10(10):1669–1678. doi:10.4161/cc.10.10.15641
26. MacKeigan JP, Murphy LO, Blenis J. Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nat Cell Biol. 2005;7(6):591–600.
27. Cain EL, Braun SE, Beeser A, Polymenis M. Characterization of a human cell line stably over-expressing the candidate oncogene, dual specificity phosphatase 12. PLoS One. 2011;6(4):e18677. doi:10.1371/journal.pone.0018677
28. Lokareddy RK, Bhardwaj A, Cingolani G. Atomic structure of dual-specificity phosphatase 26, a novel p53 phosphatase. Biochemistry. 2013;52(5):938–948. doi:10.1021/bi301476m
29. Lee J, Hun Yun J, Lee J, Choi C, Kim JH. Blockade of dual-specificity phosphatase 28 decreases chemo-resistance and migration in human pancreatic cancer cells. Sci Rep. 2015;5(1):12296. doi:10.1038/srep12296
30. Marti F, Krause A, Post NH, et al. Negative-feedback regulation of CD28 costimulation by a novel mitogen-activated protein kinase phosphatase, MKP6. J Immunol. 2001;166(1):197–206. doi:10.4049/jimmunol.166.1.197
31. Marie-claire C, Benturquia N, Lundqvist A, Courtin C, Noble F. Characteristics of dual specificity phosphatases mRNA regulation by 3,4-methylenedioxymethamphetamine acute treatment in mice striatum. Brain Res. 2008;1239:42–48. doi:10.1016/j.brainres.2008.08.050
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.