Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Update on the Etiology, Assessment, and Management of COPD Cachexia: Considerations for the Clinician
Authors De Brandt J , Beijers RJHCG, Chiles J , Maddocks M, McDonald MLN, Schols AMWJ , Nyberg A
Received 27 May 2022
Accepted for publication 31 October 2022
Published 18 November 2022 Volume 2022:17 Pages 2957—2976
DOI https://doi.org/10.2147/COPD.S334228
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Jana De Brandt,1 Rosanne JHCG Beijers,2 Joe Chiles,3 Matthew Maddocks,4 Merry-Lynn N McDonald,3 Annemie MWJ Schols,2 André Nyberg1
1Faculty of Medicine, Department of Community Medicine and Rehabilitation, Section of Physiotherapy, Umeå University, Umeå, Sweden; 2Department of Respiratory Medicine, NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht University Medical Centre+, Maastricht, the Netherlands; 3Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA; 4Cicely Saunders Institute of Palliative Care, Policy and Rehabilitation, King’s College London, London, UK
Correspondence: Jana De Brandt, Section of Physiotherapy, Department of Community Medicine and Rehabilitation, Faculty of Medicine, Umeå University, Umeå, 901 87, Sweden, Tel +32 477 73 98 11, Email [email protected]
Abstract: Cachexia is a commonly observed but frequently neglected extra-pulmonary manifestation in patients with chronic obstructive pulmonary disease (COPD). Cachexia is a multifactorial syndrome characterized by severe loss of body weight, muscle, and fat, as well as increased protein catabolism. COPD cachexia places a high burden on patients (eg, increased mortality risk and disease burden, reduced exercise capacity and quality of life) and the healthcare system (eg, increased number, length, and cost of hospitalizations). The etiology of COPD cachexia involves a complex interplay of non-modifiable and modifiable factors (eg, smoking, hypoxemia, hypercapnia, physical inactivity, energy imbalance, and exacerbations). Addressing these modifiable factors is needed to prevent and treat COPD cachexia. Oral nutritional supplementation combined with exercise training should be the primary multimodal treatment approach. Adding a pharmacological agent might be considered in some, but not all, patients with COPD cachexia. Clinicians and researchers should use longitudinal measures (eg, weight loss, muscle mass loss) instead of cross-sectional measures (eg, low body mass index or fat-free mass index) where possible to evaluate patients with COPD cachexia. Lastly, in future research, more detailed phenotyping of cachectic patients to enable a better comparison of included patients between studies, prospective longitudinal studies, and more focus on the impact of exacerbations and the role of biomarkers in COPD cachexia, are highly recommended.
Keywords: chronic obstructive pulmonary disease, muscle, weight loss, nutrition, pulmonary rehabilitation
Introduction
Chronic obstructive pulmonary disease (COPD) is a common, preventable, and irreversible respiratory disease accompanied by extra-pulmonary manifestations that significantly impact morbidity and mortality.1 Cachexia is prevalent in many chronic diseases (eg, cancer, chronic kidney disease, chronic heart failure)2 and is an important extra-pulmonary manifestation observed in patients with COPD.2–4 In COPD, cachexia is a multifactorial syndrome characterized by severe loss of body weight, muscle, and fat, as well as increased protein catabolism.5 An estimated 1.4 million patients with COPD in Europe suffer from cachexia;2,4 these patients have a 2- to 3-fold higher mortality risk3,4 and suffer from a greater disease burden (eg, reduced lung function3,6,7 or more emphysema)4 than those without cachexia. In addition to its impact on patients, cachexia poses an increased load on the healthcare system in terms of number, length, and cost of hospitalizations.8,9 The considerable impact of COPD cachexia on patients and the healthcare system highlights the clinical relevance of the COPD cachexia syndrome and underscores the need to improve prevention and treatment.
In the past decade, several international working groups focusing on chronic diseases and cancer have proposed definitions of cachexia to facilitate diagnosis and management (Figure 1).5,10–12 However, scientific and clinical respiratory communities have not systematically incorporated the diagnosis and management of cachexia in patients with COPD in research and practice.3 This oversight has led to an underappreciation of the prevalence and a suboptimal or neglected clinical management of the COPD cachexia syndrome. Understanding the factors that contribute to COPD cachexia is necessary to conceptualize effective prevention and treatment strategies. Therefore, the primary objective of this narrative review is to provide an update on the current scientific evidence on COPD cachexia etiology, assessment, and management. In addition, recommendations for clinical practice and future directions for research are provided. Given the variability in the definition of cachexia (Figure 1), it is important to note that this review covers a wide spectrum of patients and is not limited to a single definition of COPD cachexia.
COPD Cachexia
Diagnosis and Clinical Phenotype
In the 15 years since Evans et al published their consensus definition of cachexia,11 research on the diagnosis and presentation of cachexia in COPD, including the importance of treating comorbid conditions, has evolved significantly. However, COPD cachexia is still primarily characterized using cross-sectional anthropometric measurements that are readily available in clinical offices. Low body mass index (BMI) and low fat-free mass index (FFMI) have long been recognized as risk factors for increased mortality in patients with COPD;13,14 however, patients with COPD who are experiencing weight loss, even among those who are overweight or obese, have a higher risk of death in comparison to those with stable weight.4 So, it is important to recognize that, despite the classically cachectic patient being seen as severely underweight, cachexia can develop in patients with COPD across the BMI spectrum.4 For this reason, cross-sectional measurements of BMI and FFMI are often inferior to longitudinal measures of loss of body weight and fat-free mass (FFM) for predicting mortality.3
Screening for COPD cachexia can be as straightforward as tracking a patient’s weight across clinical visits; active monitoring of weight trends over time is essential, even among patients with obesity. However, in obese patients, unintentional weight loss may be applauded instead of recognized as a harbinger of worsened outcomes. Two large studies have found that the BMI category (normal, overweight, or obese) is a modifier of the risk of mortality among patients with COPD cachexia.4,15 However, several other studies of COPD have shown no statistically significant increased mortality contribution from BMI category above that associated with weight-loss.3,14,16 Concurrent monitoring of the loss of FFM is essential as patients may lose FFM independent of BMI.16 Measurement of FFM typically requires techniques such as dual x-ray absorptiometry (DXA) and bioelectrical impedance (BIA), which are often used in research but not generally accessible in pulmonary clinics.17,18
Routine body composition monitoring for cachexia is most evolved in cancer, where patients frequently receive whole body scans. Cancer cachexia studies typically rely on assessment of muscle mass at the L3 vertebrae which is not typically available on chest computed tomography (CT) scans in COPD designed to capture lung abnormalities. However, pectoralis muscle area (PMA) and first lumbar level (L1) muscle mass can be measured on chest CT scans, which are commonly acquired during acute hospitalizations or as part of a lung cancer screening program in patients with COPD.19 PMA is more highly correlated with COPD disease severity and functional outcomes than BMI,20 and loss of PMA is associated with increased mortality in patients with COPD.16 Research in patients with non-small cell lung cancer showed that L1 muscle mass correlates very strongly (r = 0.90) with L3 muscle mass, which is considered the reference but is often not evident on chest CT, and L1 muscle mass loss predicts overall survival.21 Screening for longitudinal changes in FFM based on PMA or L1 muscle mass on routine chest CT scans is feasible. A large study of longitudinal PMA in more than 10,000 current and former smokers demonstrated increased risk of mortality independent of baseline BMI and disease severity which indicates therapies targeting muscle maintenance may be of benefit early in the disease course.16
Clinical studies indicate that patients with COPD cachexia suffer from more severe disease in terms of pulmonary and physical functioning outcomes compared to those without cachexia. More specifically, patients with COPD cachexia have worse airway obstruction3,4,7,13,14,22, and more severe impairment in exercise capacity3,23 and functional outcomes such as walk distance and handgrip strength.24,25 In addition, cachexia is more prevalent in patients with more extensive emphysema on CT imaging. However, it is important to note that cachexia can develop in patients with COPD without emphysema.26
Further, the multifactorial components of COPD cachexia contribute to the increased likelihood of other extra-pulmonary manifestations in cachectic patients or those at risk of becoming cachectic. For example, metabolically, patients with COPD and cachexia have elevated inflammation and growth hormone resistance compared to non-cachectic patients,6,27,28 while the presence of insulin resistance in cachectic patients remains debatable.6,29 Additionally, low body weight (defined as BMI <21 kg/m2) has also been associated with a higher likelihood of reporting symptoms of depression in patients with COPD.30,31 In contrast, a higher risk of anxiety symptoms has not been reported.30,32
Overall, patients with COPD and cachexia (defined as low muscle mass) appear to have a worse quality of life than patients without cachexia;20,24,33 however, some studies have not reported this association.3,32
Pathophysiological Mechanisms and Etiology of COPD Cachexia
Pathophysiological Mechanisms
The molecular triggers and mechanisms contributing to weight loss and muscle mass loss in COPD cachexia have become increasingly evident in the last decade. We provide a brief summary here, as these mechanisms have been reviewed in detail elsewhere.27,34–36
Inflammation and oxidative/nitrosative stress are well-established triggers of muscle wasting and dysfunction in cachexia27,37 that catalyze an imbalance in protein and myonuclear turnover, leading to weight and muscle loss (atrophy) in cachectic patients with COPD.27,34 Protein breakdown is increased,34 with a compensatory increase in protein synthesis.38 In addition, cachectic patients with COPD have a more pronounced loss of oxidative muscle capacity than patients without cachexia.34,39–41 Recent research has further demonstrated that sarcopenic patients with COPD have more endoplasmic reticulum stress (a possible driver of muscle atrophy) compared to non-sarcopenic patients.42 Additionally, cachectic patients with COPD have dysregulated micro-RNA expression related to muscle proliferation and differentiation during myogenesis compared to patients without cachexia.43
The molecular and cellular mechanisms responsible for fat mass loss, mostly observed in patients with advanced COPD and particularly emphysematous COPD,26 have received less attention. Most scientific work has focused on the role of adipokines, cell-signaling molecules (cytokines), produced by the adipose tissue. For example, leptin, a major influencer of energy balance through its effects on regulation of appetite and food intake, was found to be lower in the circulation of emphysematous patients compared to chronic bronchitis patients.44 Further, an association was noted between reduced serum leptin levels, increased serum adiponectin levels, and increased resting energy expenditure in patients with COPD, though this analysis was not limited to patients with cachexia.45 More recent research has investigated other adipokines, including adiponectin and zinc alpha 2-glycoprotein (ZAG), and reported that serum levels of both adipokines were significantly higher in patients with COPD with cachexia compared to those without cachexia.46 Adiponectin and ZAG were also associated with weight loss.46 Brown adipose tissue (BAT) activity has been suggested as a possible mechanism leading to a hypermetabolic state in patients with emphysema;47 however, Sanders et al recently discovered that BAT activity was not different between hypermetabolic patients with COPD and age, sex, and BMI-matched healthy controls.48 Lastly, upregulation of inflammatory and proapoptotic adipose tissue markers has not been observed in cachectic patients with COPD.29
Other proposed COPD cachexia triggers and mechanisms include altered brain responses to food stimuli, altered gut integrity, and reduced splanchnic extraction.34
Etiology
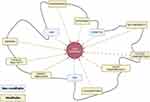
The etiology of COPD cachexia involves a complex interplay of non-modifiable and modifiable factors,49 depicted in Figure 2. Which factors cause, accelerate or impair the recovery of cachexia remains to be determined. Identifying modifiable factors, however, is crucial as it drives the development of therapeutic approaches and may permit early detection of cachexia and initiation of treatment.
Non-Modifiable Factors
Age and sex – Neither age and sex have been reproducibly shown to differ between COPD patients with and without cachexia.3,4,7,9,14,25,32
Genetics – Genetic predisposition to COPD and/or addiction to tobacco smoking are well-established, including monogenic and complex genetic etiologies.50–54 Among the monogenic etiologies, individuals with alpha-1 antitrypsin deficiency (AATD), caused by genetic variation in the SERPINA1 gene, are at increased risk of developing COPD as well as cachexia. This emphasises the importance of early AATD diagnosis and counseling for smoking cessation.55 Yet the majority of AATD patients go undiagnosed: in a recent study of 458,164 European-ancestry participants from the UK Biobank, only 6.4% of subjects with the heterozygous recessive PIZZ genotype, the most common genetic variation causing AATD, had been diagnosed with AATD.56 In addition to being at high risk for worsened airway obstruction, carriers of the PIZZ genotype are more likely to have cachexia and have a higher mortality risk. One challenge in investigating and identifying genetic predictors of COPD cachexia is that genetic variants typically have low to moderate effect sizes, which requires large samples of patients with COPD with and without cachexia to achieve the necessary power to scan the genome. For this reason, early genetic studies focused on candidate genes in patients with COPD who were not thoroughly phenotyped for cachexia. Candidate genes with variants associated with cachexia traits in COPD include angiotensin-converting enzyme (ACE),57 bradykinin receptor,58 vitamin D receptor,59 and secretory phospholipase-A2,60 as well as genes responsible for initiating the inflammatory cascade such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α).60 The first genome-wide association study of longitudinal weight loss in 4308 patients with COPD identified significant gene-level associations with EFNA1 and BAIAP2.61 Interestingly, EFNA2 encodes the membrane-bound protein ephrin-A2, which is involved in regulating developmental processes and homeostasis in adult tissue such as skeletal muscle. BAIAP2 encodes the 53 kD insulin-responsive protein (IRSp53), a negative regulator of myogenic differentiation. At the single gene variant level, the rs35368512 variant, intergenic to GRXCR1 and LINC02383, was associated with an increased likelihood of weight loss but only among African-American patients with COPD. The study also used an integrative approach to mine the underlying proteome network and found that genetic variation associated with weight loss in COPD may influence skeletal muscle regeneration and tissue remodeling. However, additional genetic investigations in larger samples of patients of diverse ancestry with COPD with and without cachexia are needed.
Modifiable Factors
Smoking – Chief among the modifiable risk factors for COPD cachexia is continued tobacco smoking. Even among adults without COPD, longitudinal fat mass and muscle mass loss patterns are associated with tobacco smoking.62 Among patients with COPD, low BMI and muscle wasting are frequently, but not universally, associated with active smoking.3,7,9,32,63,64 The cellular mechanisms of the effects of smoking on skeletal muscle are not completely clear, but greater tobacco smoke exposure is associated with greater evidence of mitochondrial DNA damage, and the muscle from patients with COPD show increased levels of cytochrome c oxidase (COX) deficiency and blunted compensatory transcriptional responses.65 There is also evidence to suggest that m. vastus lateralis fibers of patients with COPD cachexia may undergo repeated cycles of denervation suggestively caused by decades of tobacco smoke exposure,66 although studies on the m. rectus abdominis in patients with cancer-associated cachexia show no evidence of denervation.67 Degens et al previously reviewed the extensive literature regarding skeletal muscle dysfunction secondary to active smoking (human and animal model) and concluded that smoking contributes to the development of muscle dysfunction, and that future studies need to investigate whether smoking cessation restores muscle function in patients with COPD.68 Other studies have suggested that physical activity may modulate tobacco smoke’s effects on skeletal muscle in mouse models.69,70 There may also be a differential temporal association of tobacco smoking with wasting in different tissues, as recent mouse and tissue culture data indicate earlier onset of adipose tissue wasting when compared to wasting of skeletal muscle. Future in vivo studies characterizing longitudinal changes in body composition in response to tobacco smoke exposure could help to identify temporal heterogeneity in the wasting of various tissue types.71
Hypoxemia – Cachexia is more likely to be present in patients with COPD who demonstrate more severe airway obstruction and emphysema, which limits the body’s access to oxygen, contributing to alveolar hypoxia and consequent hypoxemia. For these reasons, hypoxia and hypoxemia are suspected to play a crucial role in the pathogenesis of COPD cachexia.49,72 Hypoxia influences relevant cachexia mechanisms such as appetite and maintenance of skeletal muscle mass and function.73 Hypoxemia can be monitored clinically, and lower resting oxygen saturation has been associated with lower body weight in patients with COPD.74,75 A study in healthy young male volunteers found that hypoxemia (14.1% inspired oxygen) and forced inactivity, compared with normoxic forced inactivity, resulted in more significant thigh muscle cross-sectional area (CSA) loss, a greater increase in the proportion of type IIx muscle fibers, and a greater decrease in the proportion of type I muscle fibers.76 A similar fiber type shift is seen in patients with COPD and is associated with greater disease severity.77
Hypercapnia – The chronic buildup of carbon dioxide in blood, called hypercapnia, is associated with worse nutritional status in COPD.74 Hypercapnia is found to be more likely among patients with COPD and BMI <20 kg/m2 with respiratory failure.78 Interestingly, after receiving non-invasive positive pressure ventilation (NPPV) overnight, those with BMI <20 kg/m2 experienced weight gain independent of changes in blood gas and lung function levels. The mechanisms behind this beneficial effect of NPPV on body weight remain unclear. A reduction in the energy requirements by nocturnal unloading of the respiratory musculature is a plausible hypothesis to explain this improvement.78 Recent data supports this hypothesis, indicated by weight gain in patients with severe COPD and BMI < 21 kg/m2 after undergoing bronchoscopic lung volume reduction.79,80 Further studies to replicate these results are merited.
Physical inactivity – Physical inactivity contributes to a decline in muscle mass and function, and indirectly contributes to cachexia. While physical inactivity is the strongest predictor of all-cause mortality in patients with COPD,81 its role in COPD cachexia is surprisingly scarcely investigated. Muscle dysfunction in patients with COPD, however, has been extensively characterized.36 Physical inactivity is seen as a major driver of muscle dysfunction in patients with COPD, with many muscle characteristics similar to those observed after immobilization (disuse of the muscles).36 Additionally, studies of exercise training interventions have reproducibly shown improvements in muscle mass in patients with COPD.82 However, the number of exercise training studies including cachectic patients with COPD is limited. Observational evidence investigating the relationship between physical inactivity and muscle mass loss leads to contradictory conclusions. A recent systematic review33 showed that patients with COPD and lower-limb sarcopenia had significantly lower levels of subjectively (self-reported)83–86 and objectively (accelerometry)83 measured physical activity. Further, Matkovic et al demonstrated that patients with COPD and poor physical activity have a lower FFMI and lean mass index; however, these were not independently associated with poor physical activity in a multivariate analysis.87 Observational cross-sectional evidence has shown weak positive associations between FFMI, rectus femoris CSA, and physical activity level (r = 0.134 to 0.286) in patients with COPD.88,89 Multivariate analysis showed that FFMI was not an independent predictor of physical activity in patients with COPD;90 however, in GOLD stage I patients, physical activity was associated (independently from airflow limitation) by rectus femoris CSA,91 suggesting that muscle wasting is present even in early disease. Until now, observational studies investigating physical inactivity in patients with COPD and cachexia have been limited by cross-sectional data and small sample sizes. Longitudinal observational studies of physical activity in patients with COPD with cachexia are needed to elaborate on the dynamic process of cachexia and the role of physical inactivity to address causation. For example, it is unclear whether inactive patients with COPD are more prone to develop cachexia or whether patients with cachexia are more likely to be inactive. Lastly, low physical activity is associated with increased systemic inflammation, an essential trigger of cachexia in patients with COPD.92–94 Conversely, improvements in systemic inflammation have been reported in patients with COPD who were able to increase their physical activity.95
Energy imbalance – Factors contributing to the observed energy imbalance (energy expenditure vs energy availability) in cachectic patients with COPD is described in a comprehensive review by Sanders et al.34 An increased resting energy expenditure is reported in patients with COPD cachexia.96,97 Additionally, cachectic patients with COPD have higher energy demands when performing physical activity in comparison to healthy controls.98,99 When combined with the observed respiratory mechanical inefficiency, due to hyperinflation, which increases the work of breathing, the higher energy demand might lead to increased activity-induced energy expenditure (AEE). Research regarding AEE in cachectic patients with COPD is, however, lacking. Compensation for the elevated energy expenditure is necessary to maintain energy balance and to avoid wasting of muscle and fat mass. Dietary intake in patients with COPD who are losing weight are reported to be comparable to weight-stable patients but insufficient to counterbalance the elevated energy expenditure.100 Unfortunately, extensive research focusing on dietary intake in patients with cachexia is currently scarce. In relation to dietary intake, nutrition impact symptoms (eg, constipation, changes in the taste of food, loss of appetite) can be assessed and those symptoms are more frequently reported in patients with low FFMI compared to patients with normal FFMI.6 Additionally, nutrition impact symptoms and reduced food intake (measured as <75% food intake compared to usual) have shown to be associated with involuntary weight loss in patients with COPD,101 suggesting that nutrition impact symptoms and reduced food intake could significantly contribute to the development of cachexia in this population.
Alcohol consumption – Excessive alcohol consumption might also contribute to COPD cachexia. Animal models of COPD102,103 and cancer,104,105 as well as human106,107 studies, have repeatedly shown that excessive alcohol consumption leads to muscle loss as protein synthesis is inhibited and protein breakdown is enhanced.103,108 In addition, patients with COPD may have increased rates of excessive alcohol use compared to age-matched controls,109 suggesting that excessive alcohol consumption could contribute to the development of COPD cachexia. However, this hypothesis has not been tested, and future research is needed to explore the contribution of alcohol consumption to COPD cachexia.
Exacerbations – The number of COPD exacerbations a patient has experienced in the prior year is associated with an increased risk of cachexia (defined as unintentional weight loss of >5% over the preceding 6 months and with reduced FFMI).3 Gene expression studies on muscle tissue from the m. vastus lateralis of patients with COPD during acute exacerbation versus those with stable disease showed increased expression of genes associated with ubiquitin-dependent protein catabolism, one of the leading culprits in the pathophysiology of COPD cachexia, and down-regulation of genes in the mitochondrial respiratory chain.110 The proximal cause of these changes is uncertain as corticosteroids are frequently prescribed for acute exacerbations, and systemic corticosteroid exposure has been directly linked to reduced quadriceps strength after a COPD exacerbation111 and during stable disease.112
Social-demographic factors – Social factors such as access to care, ability to afford medication and food, proximity to environmental pathogens, and other social determinants of health show significant effects across the medical spectrum.113 Little data is available to describe the impact of these factors on the development and prognosis of cachexia in patients with COPD. Attaway et al used a national database in the United States to demonstrate increased prevalence of COPD in more rural and economically deprived areas; however, geographic differences between COPD patients with and without a muscle loss phenotype were relatively minor.9
Multimorbidity – Finally, it is important to conceptualize cachexia within a broader context of multimorbidity in patients with COPD. Vanfleteren et al described a pattern of comorbidity clustering in 215 patients with COPD, including a cluster of cachectic patients that showed associations with osteoporosis and renal disease.32 These associations were not borne out in a later clustering analysis conducted by the same group.7 Within the scientific community, there is a movement towards recognizing extra-pulmonary manifestations of COPD as related symptoms of an overarching systemic disease centered in the lungs but inextricably linked to the function of many other organs.114,115 Recognizing the burden of multimorbidity in COPD, and screening patients for these extra-pulmonary manifestations, could allow for earlier identification and treatment of comorbidities and more holistic care for the patient.115
Therapeutic Approaches to COPD Cachexia
The deleterious effects of cachexia on morbidity and mortality in patients with COPD underscore the importance of identifying effective therapeutic approaches. After cachexia is diagnosed in a patient with COPD, an initial structured nutritional assessment is required, which is ideally repeated regularly along with longitudinal measurements of body weight, body composition, and nutritional intake impact symptoms. These nutritional assessments should prompt multimodal intervention strategies encompassing nutritional support, muscle activation by exercise training, and/or targeted pharmacological interventions in select subpopulations.
Nutritional Therapy
Poor dietary quality may accelerate disturbances in body composition in patients with COPD and contribute to the development of cachexia. In terms of caloric content, patients with COPD generally have a normal dietary intake compared with healthy individuals.116 Nevertheless, this intake may be inadequate for meeting the elevated energy requirements of patients with COPD.117–119 Exacerbation of COPD may further impair energy balance due to decreased dietary intake combined with increased resting energy expenditure during acute illness.120 To compensate for increased energy requirements and prevent weight loss, patients with COPD may need to adapt their dietary intake to an energy-enriched diet. However, since a high caloric load may stress the impaired ventilatory capacity, smaller meals spread throughout the day should be recommended.121 In addition, muscle protein synthesis should be stimulated to maintain muscle mass. Muscle protein synthesis depends on the availability of amino acids in the bloodstream. Therefore, a diet with sufficient proteins amounting to at least 1.2 g per kg body weight might be necessary to achieve an anabolic response.122 High-quality proteins, such as casein, leucine, and whey protein, elicit an increased anabolic response after acute or short-term ingestion in patients with COPD.123–126 However, the long-term benefits of high-quality protein ingestion and the beneficial effects of its metabolites (eg, beta-hydroxy-beta-methylbutyrate) still need to be investigated.127
Nutritional supplementation – To treat cachexia, oral nutritional supplements (medical drinks/snacks) could supplement the diet when nutrient requirements cannot be satisfied through habitual dietary intake or when a temporary boost is needed. A recent Cochrane review on supplementation with medical nutrition in patients with COPD showed moderate-quality evidence that nutritional supplementation promotes weight gain among patients with COPD, especially if malnourished.128 With high-quality proteins to stimulate the regenerative response of muscles, these supplements might need to be enriched with additional vitamins, minerals, and trace elements to combat nutrient deficiencies. Vitamin D deficiency, measured as serum levels of 25-hydroxyvitamin D, is frequently reported in patients with COPD and worsens with disease severity.129 Supplementing vitamin D in patients with COPD may positively affect bone health and improve muscle strength by regulating mitochondrial dynamics and enzyme production;130,131 however, the specific effects of vitamin D in the cachectic patient with COPD remain unclear. Low intake of omega-3 polyunsaturated fatty acids (n-3 PUFAs) is also reported in patients with COPD.132 In addition to their anti-inflammatory effects, PUFAs are the natural ligands of peroxisome proliferator-activated receptors (PPARs) and may potentially boost muscle mitochondrial metabolism by stimulating PPAR and PPAR gamma coactivator-1 alpha (PGC-1α) signaling or inhibiting classic nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling. In addition, a placebo-controlled randomized controlled trial (RCT) in cachectic patients with COPD showed decreased exercise fatigue and dyspnea after 12 weeks of a targeted medical nutrition therapy133 enriched with whey protein, n-3 PUFAs, and vitamin D. Nutrition therapy was compared with an isocaloric diet including milk protein instead of whey protein and sunflower oil instead of fish oil with high levels of n-3 PUFAs.
Nutrition supplementation combined with exercise – Combining nutritional supplementation with pulmonary rehabilitation (PR) might enhance the beneficial effects of PR. For example, PUFA supplementation during an 8-week rehabilitation program significantly improved endurance exercise capacity in comparison to placebo in patients with COPD, even after adjustment for FFM.134 A more recent RCT—the NUTRAIN trial—investigated whether targeted nutritional supplementation enhances outcomes of exercise training in patients with COPD with low muscle mass.135 In the trial, a multimodal drink enriched with leucine, vitamin D, and n-3 PUFAs significantly improved or maintained body weight, inspiratory muscle strength, and physical activity but did not enhance the effects of a 12-week rehabilitation program on muscle mass, muscle strength, and physical performance.
Opportunities for nutritional therapeutics – While most studies of nutritional interventions for COPD cachexia have focused on short-term efficacy (1–3 months) in clinically stable disease or as an adjunct to PR, only a few studies have investigated the benefits of nutritional supplementation during the maintenance phase after PR. For example, the NUTRAIN trial and the INTERCOM trial showed that during the 12- to 24-month maintenance phase after PR, nutritional interventions did not seem to enhance the long-term outcome of exercise training on physical capacity but did improve plasma levels of the supplemented nutrients, total body weight, physical activity, and generic health status. Notably, these outcomes were achieved at an acceptable cost for patients with high disease burden.136,137 Lastly, the effect of nutrition therapy during and after an acute exacerbation still is a wholly neglected area of research. This is noteworthy as, during an acute exacerbation, disease-related factors such as inflammation, hypoxia, inactivity, and glucocorticosteroid treatment converge and intensify.138 Additionally, the gap between energy intake and energy expenditure becomes wider during acute exacerbations, then slowly decreases upon recovery.120,139,140 Only one study has proven the feasibility and efficacy of nutritional supplementation in maintaining energy balance and increasing protein intake in hospitalized patients with COPD.139 Still, the therapeutic window of opportunity after an acute exacerbation warrants further investigation.
Exercise Training
Exercise training is recommended in many diseases associated with skeletal muscle wasting.141 Exercise should be routinely offered to patients with COPD as part of PR, supported by a significant and robust evidence base in both stable and acute COPD populations. The established benefits of PR in general COPD populations include improved exercise capacity, symptom burden, and health status, and, in the post-acute setting, a lower risk of hospital admission.142 Exercise training also clearly improves skeletal muscle function and morphology in patients with COPD36,82,142,143 through upregulation of factors governing skeletal muscle hypertrophy and regeneration, with a variable effect on systemic or local muscle inflammation.144,145 Increased fiber CSA, reduced proportion of type IIx fibers, increased oxidative capacity, and reduced exercise-induced lactic acid production are also observed after exercise training in patients with COPD.36,146 Notably, independent of the exercise modality used, programs using training at higher intensity or with longer duration produce greater physiological training effects.
Endurance training modalities – While the optimal program length is unknown, 8 weeks of endurance training, with at least 2–3 one-hour sessions per week, at >40% of maximal work rate (cycling) is recommended to achieve meaningful benefits.36,142 The goal should be to provide a potent training stimulus at the limit of the patient’s capacity.147 Many patients with COPD, including those with cachexia, find it challenging to sustain a moderate-to-high-intensity training load for the target training duration, for example due to intolerable breathlessness. Different training strategies can allow symptomatic patients to better tolerate training loads that offer an effective stimulus for adaptation. These include interval training, where repeated bouts of high-intensity exercise are interspersed with recovery periods (passive or active recovery)148, and partitioned training, where exercising a smaller muscle mass (eg, single-leg cycling or knee extension) reduces the load on the respiratory system.149–152
Resistance training modalities – A systematic review of resistance training in patients with COPD found increases in muscle strength and mass after short-term programs; typically, these programs involved 12 weeks of training, with at least 2–3 sessions each week, at progressive loads of 30–90% of one-repetition maximum.153–155 Catabolic, anabolic and transcription factor protein expression responses to resistance training are blunted in patients with COPD compared to healthy controls, but these seem not tightly coupled to gains in lean mass.156 Hypoxemia has been suggested to play a role in the variable adaptive response of the skeletal muscle in patients with COPD.157 As with endurance training, limiting symptoms may prevent patients with COPD from undertaking planned training protocols, and alternative training modalities can be helpful to increase the total work done. Neuromuscular electrical stimulation offers an effective form of resistance training,158 where an external current is applied over the target muscles to induce contractions.159 It places a low metabolic load on the respiratory system, and is thus particularly suited to severely breathless patients.
Combined exercise modalities – In practice, combining endurance and resistance training would optimize overall gains, eg, in functional capacity, along with increases in muscle mass and function to counteract the adverse effects of cachexia. Careful tailoring of the exercise prescription with attention paid to exercise modality can improve patient outcomes, including the most severe cases.160 A recent case report in a patient with very severe COPD, chronic respiratory failure, and cachexia showed impressive gains in weight (including lean mass), function (strength, exercise capacity, mobility), health status, and psychological well-being from comprehensive and personalized PR.161 The effects of exercise, anabolic and beyond, rapidly wane once the training stimulus is stopped,162 so attention to behavior changes and/or maintenance strategies is paramount. This requires a consideration of exercise program features that may improve longer-term access, adherence, and efficacy. Low-cost, home-based and telerehabilitation models may have a role; however, these models also have a smaller evidence base and lack the well-defined process and outcomes of center-based PR.163 Finally, as described previously exercise should be combined with nutritional intervention in a multimodal approach, as exemplified in the NUTRAIN and INTERCOM trials,136,137 to target the wide range of etiological factors involved in cachexia, including energy imbalance and low nutrient availability.34
Exercise responses in COPD cachexia – Despite the impressive gains in the abovementioned case report,161 individual responses to exercise training in COPD are variable and cannot be readily predicted by any clinical phenotype.83,164 Some, but not all, cachectic patients with COPD retain the capacity to improve functional exercise performance with exercise training. In an in-depth physiological study, Vogiatzis et al compared 10 cachectic and 19 non-cachectic patients with COPD before and after high-intensity cycling training (45 min, 3x weekly) over 10 weeks.165 The endurance, high-intensity interval program improved peak work rate and 6-min walk distance (6MWD) in both groups. Mean muscle fiber CSA also increased in both groups, though significantly less in cachectic patients. A blunted muscle remodeling response was found at the group level, with less reduction in the proportion of type IIb fibers, less increased muscle capillary/fiber ratio, and less increased insulin-like growth factor I (IGF-I) protein levels in the cachectic group.165 This blunted response to high-intensity endurance exercise in COPD cachexia supports the inclusion of resistance training in managing COPD cachexia. Indeed, combined endurance and resistance training approaches seem highly relevant and should focus on the critical features of the cachexia syndrome (eg, reduced muscle mass, reduced FFMI, and/or reduced muscle strength).16,58,166
Pharmacological Intervention
In addition to nutritional support and exercise training, pharmacological agents have been identified as potential therapeutic options for cachectic patients with COPD. Even though research is sparse,167–171 the efficacy of different pharmacological agents has been investigated and is listed below.
Ghrelin – A 28-amino acid peptide hormone,36 ghrelin, has received specific attention as a pharmacologic agent in COPD cachexia. Studies show that ghrelin has potential benefits in reversing the breakdown of proteins and weight loss in catabolic states like cancer cachexia172,173 and it is thought to affect several vital pathways in the regulation of appetite and body composition. However, less is known about the effects of ghrelin in COPD cachexia, and available study results are conflicting. In a small sample of 7 cachectic individuals with COPD, Nagaya et al168 found that a 3-week treatment with exogenously administered ghrelin resulted in improvements in body weight, food intake, lean body mass, peripheral and respiratory muscle strength, and clinically relevant improvement in 6MWD. In contrast, in a multicenter, randomized, double-blind, placebo-controlled trial, the same research group167 evaluated a similar intravenous treatment of high-dose ghrelin (2 µg/kg) twice daily for 3 weeks but did not see any significant difference in 6MWD, quality of life, symptoms, body weight, food intake, or peripheral muscle strength when compared to placebo. Nevertheless, ghrelin analogues are regarded as a novel potential therapeutic option in COPD cachexia that warrants further investigation despite the somewhat conflicting and limited findings reported in the literature.34,167,168
Megestrol acetate and testosterone – Another potentially relevant pharmacological agent for COPD cachexia is megestrol acetate, a progestational appetite stimulant with anti-inflammatory effects.36,174 In a large RCT including 145 individuals with COPD, megestrol acetate administration improved appetite, body weight, and body image but not exercise tolerance as measured by 6MWD.169 In a 12-week pilot trial in 2015, Casaburi et al171 treated 9 cachectic patients with COPD with oral megestrol acetate (800 mg/day) plus weekly testosterone injections (initially 125 mg in men and 40 mg in women). They found that the treatment reversed weight loss trajectories and resulted in significant gains in both lean and fat mass. Though the small sample size and lack of a control group should be noted when interpreting these findings, the promising findings warrant further research. Moreover, adding testosterone supplementation to men with COPD with low serum testosterone levels during a resistance training intervention have, when compared to resistance training alone, enabled more profound abundances of all major myosine heavy chain (MyHC) isoforms, enhanced expression of muscle IGF-1 and other components of the muscle IGF system, as well as improved maximal leg muscle strength and lean body mass.175,176
Roflumilast – Recent research shows that the phosphodiesterase (PDE)-4 inhibitor roflumilast may revert proteolysis and increase the antioxidant defense in cultured myotubes obtained from cachectic patients with COPD.177 As this PDE-4 inhibitor is available for treating systemic inflammation and exacerbations in patients with COPD, these findings have potential clinical implications for treating muscle wasting in cachectic patients with COPD.
Targeting key pathways – Sanders et al34 emphasizes that muscle wasting may result from protein and myonuclear turnover alterations;27,34 therefore, targeting key pathways in these processes (eg, ubiquitin-proteasome system) will likely be required to combat muscle wasting in cachectic patients with COPD.34 In 2019, Polkey et al178 investigated the effect of 2 doses of intravenously administered bimagrumab, a novel and fully human monoclonal antibody that inhibits myostatin (a negative skeletal muscle regulator), in patients with COPD with reduced skeletal muscle mass (without documented recent weight loss). Although the patients in the study were not diagnosed with cachexia, treatment led to increased thigh muscle volume over 24 months but had no effect on 6MWD.
In summary, the benefit of single modality pharmacological interventions for the cachectic patient with COPD is unclear, research is sparse, and the results are mixed.167–171 Therefore, cachexia cannot be managed entirely by any single modality treatment intervention, including current pharmacological options. Still, pharmacological interventions may be relevant as part of a multimodal approach for some, but not all, cachectic patients with COPD, although the latter requires further investigation.170,179
Recommendations for Practice
Diagnosing (Pre-)Cachexia in Patients with COPD
Longitudinal assessment of weight in patients with COPD meets Wilson and Jungner’s principles of screening.180 Though weight measurements are routinely collected, they require the clinician’s close attention in order to spark recognition and therapeutic action. In addition to weight, information from routine clinical imaging, such as lung screening CTs or DXA scans, present opportunities for monitoring muscle mass or FFM over time, generating longitudinal assessments of a patient’s body composition. If clinical imaging is not available, improving clinical access to other reliable and validated body composition assessment tools (eg BIA) is advised. Additionally, protocolized inquiries about weight loss or changes in strength or functional status could be incorporated into a disease-specific review of systems, along with more classical questions about sputum production and recent exacerbations. Automated generation of alerts by the electronic medical record could help augment the vigilance of clinicians and highlight weight loss when it is first evident. Future clinical studies should quantify the effects of heightened clinical attention and novel information technology solutions for early recognition of cachexia in patients with COPD to facilitate early therapeutic intervention.
Minimizing Modifiable Etiological Factors and Improving Patient Referral to PR
In theory, the prevention and management of COPD cachexia should start with minimizing modifiable etiological factors (Box 1). Generally, a multidisciplinary and holistic PR program considers all proposed minimization steps.181 Unfortunately, PR programs are not equally extensive worldwide, and poor healthcare professional awareness and knowledge about PR is a significant barrier to patient referral.182,183 In practice, therapeutic nihilism about the role of PR in the management of cachexia may prevent some healthcare professionals not recommending it to patients. Given the physiological and psychosocial benefits of PR for patients with stable and acute COPD, clinicians need to engage with patients to talk about barriers and enablers and support the uptake of PR.
 |
Box 1 Ways to Minimize Modifiable Etiological Factors |
Moving Towards Multimodal Therapeutic Approaches
There is a clear consensus that single therapies are insufficient to stabilize or reverse cachexia.170,179 Considering the significant etiologic complexities of COPD cachexia, a multimodal approach that combines nutritional, exercise, and (possibly) pharmacological components is likely necessary. A recent review170 summarized the effect of multimodal treatment strategies tested in 5 individual studies in cachectic patients with COPD.133,136,184–186 All studies that combined exercise with an oral nutritional supplement reported significant improvements in favor of the treatment groups. Thus, building on current evidence, oral nutritional supplementation combined with exercise should be the primary multimodal approach to treating the cachectic patient with COPD. Adding a pharmacological agent might be considered in some, but not all, cachectic patients with COPD170 (Box 2).
 |
Box 2 Multimodal Interventions for Cachexia in COPD |
Future Directions for Research
While acknowledging the challenges in COPD cachexia research studies, the following steps are recommended when designing future research studies to investigate the etiology, assessment, and management of COPD cachexia.
Enable/Facilitate Comparisons Between Studies
A key challenge in interpreting and comparing the results of observational and interventional studies is the use of different definitions of cachexia across studies, resulting in somewhat diverse patient groups. Therefore, we propose that researchers use a longitudinal definition of cachexia and recognize that cross-sectional measurements are not optimal. Furthermore, more detailed phenotyping of patients included in studies will assist in comparing patient cohorts between studies.
Provide Stronger Scientific Evidence
Observational studies on the etiology of COPD cachexia are often cross-sectional and retrospective. Instead, large-scale prospective and longitudinal studies should be conducted to provide more robust etiological evidence. In addition, multi-arm RCTs are needed to fully establish the efficacy of a specific or multimodal intervention.
Select the “Right” Outcome(s)
In previous work on COPD cachexia, functional outcomes, such as 6MWD, are commonly used, but does not seem to be responsive to cachexia interventions, including resistance training.167,169,178,187 The latter, thought to have a particular role in managing COPD cachexia considering the known consequences of the disease (eg, reduced muscle mass, reduced FFMI and/or reduced muscle strength).16,58,166 However, the efficacy of resistance training (at least when designed to improve muscle mass and strength)188,189 would not be reflected in the functional outcome of walking distance on the 6MWT.187 Instead, to evaluate resistance training intervention effects in the cachectic patient with COPD, muscle-related outcomes, including direct measurements of strength and power properties of the quadriceps, should be used due to their relevance to muscle mass; these measurements are particularly responsive to resistance training interventions.82,190–192 If a functional outcome measure is desirable, a sit-to-stand test would be more relevant, considering the requirements of this test compared to the 6MWT. For example, strength and muscle mass properties which often are affected in the cachectic patient with COPD are more closely linked to 5-time sit-to-stand performance than to 6MWT performance.193,194 Sit-to-stand tests also respond to resistance training interventions designed to increase muscle mass and strength.153
Lastly, although selecting the proper functional test, and focusing on muscle-specific outcome measures is essential to capture treatment adaptations, multidimensional outcome assessment including objective and patient-centered outcomes such as quality of life should not be neglected. For example, quality of life is often worsened in patients with COPD cachexia compared to patients without cachexia.24,33 Additionally, although it remains to be determined in the cachectic patient COPD, it is likely that specialized nutrition and exercise can restore muscle mass and strength through metabolic crosstalk between body organs,195 highlighting the need of multidimensional outcome assessments to fully capture the treatment response in the cachectic patient with COPD.
Evaluate Exacerbations
Only limited studies have focused on the role of cachexia during and after severe exacerbations. Disease-related detrimental factors such as systemic inflammation, hypoxia, physical inactivity, malnutrition, and glucocorticosteroid treatment converge and intensify during a severe exacerbation. Therefore, the period of an exacerbation and the recovery phase immediately following might be an excellent opportunity to intervene to prevent or treat cachexia. More studies are needed to investigate the potential and optimal therapeutic strategies for the period around COPD exacerbations.
Discover New Biomarkers Prognostic of Cachexia Development
A major challenge with cachexia is that it remains largely discovered in end-stage COPD. By the time a patient with COPD exhibits weight loss the ideal interventional window for reversing cachexia’s course may have already been missed. Additional research is needed to identify biomarkers of pre-cachexia to guide cachexia risk stratification and/or serve as sensitive endpoints in clinical trials.
Next to future steps described above, the scoping review by Orsso et al also provides excellent recommendations for future trials investigating nutrition or multimodal interventions to prevent or treat cachexia in clinical and non-clinical conditions.196
Acknowledgments
The authors greatly acknowledge Stacey Tobin for editing and improving the English language of the work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Jana De Brandt is funded by the Swedish Heart and Lung Foundation (20200139 and 20210146). Joe Chiles is funded by the NIH NHLBI T32HL105346. Matthew Maddocks is funded by a National Institute for Health and Care Research (NIHR) Career Development Fellowship (CDF-2017-10-009) and NIHR Applied Research Collaboration South London (NIHR ARC South London) at King’s College Hospital NHS Foundation Trust. The views expressed in this article are those of the authors and not necessarily those of the NIHR, or the Department of Health and Social Care. Merry-Lynn N. McDonald is funded by the National Institutes for Health (NIH), National Heart, Lung and Blood Institute (NHLBI), R01HL153460. André Nyberg is funded by the Swedish Research Council (2020-01296) and the Swedish Heart and Lung Foundation (20210146).
Disclosure
The authors report no conflicts of interest in this work.
References
1. GOLD. The global strategy for diagnosis, management and prevention of COPD, 2022 report; 2022. Available from: https://goldcopd.org/2022-gold-reports-2/.
2. von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle. 2016;7(5):507–509. doi:10.1002/jcsm.12167
3. Kwan HY, Maddocks M, Nolan CM, et al. The prognostic significance of weight loss in chronic obstructive pulmonary disease-related cachexia: a prospective cohort study. J Cachexia Sarcopenia Muscle. 2019;10(6):1330–1338. doi:10.1002/jcsm.12463
4. McDonald MN, Wouters EFM, Rutten E, et al. It’s more than low BMI: prevalence of cachexia and associated mortality in COPD. Respir Res. 2019;20(1):100. doi:10.1186/s12931-019-1073-3
5. Muscaritoli M, Anker SD, Argiles J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29(2):154–159. doi:10.1016/j.clnu.2009.12.004
6. Koehler F, Doehner W, Hoernig S, Witt C, Anker SD, John M. Anorexia in chronic obstructive pulmonary disease--association to cachexia and hormonal derangement. Int J Cardiol. 2007;119(1):83–89. doi:10.1016/j.ijcard.2006.07.088
7. Triest FJJ, Franssen FME, Reynaert N, et al. Disease-specific comorbidity clusters in COPD and accelerated aging. J Clin Med. 2019;8(4):511. doi:10.3390/jcm8040511
8. Arthur ST, Noone JM, Van Doren BA, Roy D, Blanchette CM. One-year prevalence, comorbidities and cost of cachexia-related inpatient admissions in the USA. Drugs Context. 2014;3:212265. doi:10.7573/dic.212265
9. Attaway AH, Welch N, Hatipoglu U, Zein JG, Dasarathy S. Muscle loss contributes to higher morbidity and mortality in COPD: an analysis of national trends. Respirology. 2021;26(1):62–71. doi:10.1111/resp.13877
10. Berardi E, Madaro L, Lozanoska-Ochser B, et al. A pound of flesh: what cachexia is and what it is not. Diagnostics. 2021;11(1):116. doi:10.3390/diagnostics11010116
11. Evans WJ, Morley JE, Argiles J, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–799. doi:10.1016/j.clnu.2008.06.013
12. Schols AM, Ferreira IM, Franssen FM, et al. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respir J. 2014;44(6):1504–1520. doi:10.1183/09031936.00070914
13. Wilson DO, Rogers RM, Wright EC, Anthonisen NR. Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am Rev Respir Dis. 1989;139(6):1435–1438. doi:10.1164/ajrccm/139.6.1435
14. Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82(1):53–59. doi:10.1093/ajcn/82.1.53
15. Prescott E, Almdal T, Mikkelsen KL, Tofteng CL, Vestbo J, Lange P. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J. 2002;20(3):539–544. doi:10.1183/09031936.02.00532002
16. Mason SE, Moreta-Martinez R, Labaki WW, et al. Longitudinal association between muscle loss and mortality in ever smokers. Chest. 2022;161(4):960–970. doi:10.1016/j.chest.2021.10.047
17. Steiner MC, Barton RL, Singh SJ, Morgan MD. Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J. 2002;19(4):626–631. doi:10.1183/09031936.02.00279602
18. Cruz Rivera PN, Goldstein RL, Polak M, Lazzari AA, Moy ML, Wan ES. Performance of bioelectrical impedance analysis compared to dual X-ray absorptiometry (DXA) in Veterans with COPD. Sci Rep. 2022;12(1):1946. doi:10.1038/s41598-022-05887-4
19. Sanders KJC, Ash SY, Washko GR, Mottaghy FM, Schols A. Imaging approaches to understand disease complexity: chronic obstructive pulmonary disease as a clinical model. J Appl Physiol. 2018;124(2):512–520. doi:10.1152/japplphysiol.00143.2017
20. McDonald ML, Diaz AA, Ross JC, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc. 2014;11(3):326–334. doi:10.1513/AnnalsATS.201307-229OC
21. Sanders KJC, Degens J, Dingemans AC, Schols A. Cross-sectional and longitudinal assessment of muscle from regular chest computed tomography scans: L1 and pectoralis muscle compared to L3 as reference in non-small cell lung cancer. Int J Chron Obstruct Pulmon Dis. 2019;14:781–789. doi:10.2147/COPD.S194003
22. Mokari-Yamchi A, Jabbari M, Sharifi A, Barati M, Kheirouri S. Low FEV1 is associated with increased risk of cachexia in COPD patients. Int J Chron Obstruct Pulmon Dis. 2019;14:2433–2440. doi:10.2147/COPD.S221466
23. Fermoselle C, Rabinovich R, Ausin P, et al. Does oxidative stress modulate limb muscle atrophy in severe COPD patients? Eur Respir J. 2012;40(4):851–862. doi:10.1183/09031936.00137211
24. Mostert R, Goris A, Weling-Scheepers C, Wouters EF, Schols AM. Tissue depletion and health related quality of life in patients with chronic obstructive pulmonary disease. Respir Med. 2000;94(9):859–867. doi:10.1053/rmed.2000.0829
25. Vermeeren MA, Creutzberg EC, Schols AM, et al. Prevalence of nutritional depletion in a large out-patient population of patients with COPD. Respir Med. 2006;100(8):1349–1355. doi:10.1016/j.rmed.2005.11.023
26. Engelen MP, Schols AM, Lamers RJ, Wouters EF. Different patterns of chronic tissue wasting among patients with chronic obstructive pulmonary disease. Clin Nutr. 1999;18(5):275–280. doi:10.1016/S0261-5614(98)80024-1
27. Remels AH, Gosker HR, Langen RC, Schols AM. The mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol. 2013;114(9):1253–1262. doi:10.1152/japplphysiol.00790.2012
28. Van Helvoort HA, Heijdra YF, Thijs HM, Vina J, Wanten GJ, Dekhuijzen PN. Exercise-induced systemic effects in muscle-wasted patients with COPD. Med Sci Sports Exerc. 2006;38(9):1543–1552. doi:10.1249/01.mss.0000228331.13123.53
29. Skyba P, Ukropec J, Pobeha P, et al. Metabolic phenotype and adipose tissue inflammation in patients with chronic obstructive pulmonary disease. Mediators Inflamm. 2010;2010:173498. doi:10.1155/2010/173498
30. Janssen DJ, Spruit MA, Leue C, et al. Symptoms of anxiety and depression in COPD patients entering pulmonary rehabilitation. Chron Respir Dis. 2010;7(3):147–157. doi:10.1177/1479972310369285
31. Yao HM, Xiao RS, Cao PL, Wang XL, Zuo W, Zhang W. Risk factors for depression in patients with chronic obstructive pulmonary disease. World J Psychiatry. 2020;10(4):59–70. doi:10.5498/wjp.v10.i4.59
32. Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–735. doi:10.1164/rccm.201209-1665OC
33. Sepulveda-Loyola W, Osadnik C, Phu S, Morita AA, Duque G, Probst VS. Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11(5):1164–1176. doi:10.1002/jcsm.12600
34. Sanders KJC, Kneppers AEM, van de Bool C, Langen RCJ, Schols AM. Cachexia in chronic obstructive pulmonary disease: new insights and therapeutic perspective. J Cachexia Sarcopenia Muscle. 2016;7(1):5–22. doi:10.1002/jcsm.12062
35. Langen RC, Gosker HR, Remels AH, Schols AM. Triggers and mechanisms of skeletal muscle wasting in chronic obstructive pulmonary disease. Int J Biochem Cell Biol. 2013;45(10):2245–2256. doi:10.1016/j.biocel.2013.06.015
36. Maltais F, Decramer M, Casaburi R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–e62. doi:10.1164/rccm.201402-0373ST
37. Puig-Vilanova E, Rodriguez DA, Lloreta J, et al. Oxidative stress, redox signaling pathways, and autophagy in cachectic muscles of male patients with advanced COPD and lung cancer. Free Radic Biol Med. 2015;79:91–108. doi:10.1016/j.freeradbiomed.2014.11.006
38. Kneppers AEM, Langen RCJ, Gosker HR, et al. Increased myogenic and protein turnover signaling in skeletal muscle of chronic obstructive pulmonary disease patients with sarcopenia. J Am Med Dir Assoc. 2017;18(7):637e1–637 e11. doi:10.1016/j.jamda.2017.04.016
39. Rabinovich RA, Bastos R, Ardite E, et al. Mitochondrial dysfunction in COPD patients with low body mass index. Eur Respir J. 2007;29(4):643–650. doi:10.1183/09031936.00086306
40. Remels AH, Gosker HR, Schrauwen P, et al. TNF-alpha impairs regulation of muscle oxidative phenotype: implications for cachexia? FASEB J. 2010;24(12):5052–5062. doi:10.1096/fj.09-150714
41. Remels AH, Schrauwen P, Broekhuizen R, et al. Peroxisome proliferator-activated receptor expression is reduced in skeletal muscle in COPD. Eur Respir J. 2007;30(2):245–252. doi:10.1183/09031936.00144106
42. Barreiro E, Salazar-Degracia A, Sancho-Munoz A, Gea J. Endoplasmic reticulum stress and unfolded protein response profile in quadriceps of sarcopenic patients with respiratory diseases. J Cell Physiol. 2019;234(7):11315–11329. doi:10.1002/jcp.27789
43. Barreiro E, Sancho-Munoz A, Puig-Vilanova E, et al. Differences in micro-RNA expression profile between vastus lateralis samples and myotubes in COPD cachexia. J Appl Physiol. 2019;126(2):403–412. doi:10.1152/japplphysiol.00611.2018
44. Schols AM, Creutzberg EC, Buurman WA, Campfield LA, Saris WH, Wouters EF. Plasma leptin is related to proinflammatory status and dietary intake in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(4):1220–1226. doi:10.1164/ajrccm.160.4.9811033
45. Brusik M, Ukropec J, Joppa P, et al. Circulatory and adipose tissue leptin and adiponectin in relationship to resting energy expenditure in patients with chronic obstructive pulmonary disease. Physiol Res. 2012;61(5):469–480. doi:10.33549/physiolres.932306
46. Mokari-Yamchi A, Sharifi A, Kheirouri S. Increased serum levels of S100A1, ZAG, and adiponectin in cachectic patients with COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3157–3163. doi:10.2147/COPD.S172996
47. Petruzzelli M, Schweiger M, Schreiber R, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20(3):433–447. doi:10.1016/j.cmet.2014.06.011
48. Sanders KJC, Wierts R, van Marken Lichtenbelt WD, et al. Brown adipose tissue activation is not related to hypermetabolism in emphysematous chronic obstructive pulmonary disease patients. J Cachexia Sarcopenia Muscle. 2022;13:1329–1338. doi:10.1002/jcsm.12881
49. Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J. 2008;31(3):492–501. doi:10.1183/09031936.00074807
50. Wain LV, Shrine N, Artigas MS, et al. Genome-wide association analyses for lung function and chronic obstructive pulmonary disease identify new loci and potential druggable targets. Nat Genet. 2017;49:416–425. doi:10.1038/ng.3787
51. Prokopenko D, Sakornsakolpat P, Fier HL, et al. Whole-genome sequencing in severe chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2018;59(5):614–622. doi:10.1165/rcmb.2018-0088OC
52. Lutz SM, Cho MH, Hersh CP, et al. A genome-wide association study of spirometric measures of pulmonary function in the COPDGene study. BMC Genet. 2013;16:1–11.
53. Lutz SM, Cho MH, Young K, et al. A genome-wide association study identifies risk loci for spirometric measures among smokers of European and African ancestry. BMC Genet. 2015;16:138. doi:10.1186/s12863-015-0299-4
54. Silverman EK. Genetics of COPD. Annu Rev Physiol. 2020;82:413–431. doi:10.1146/annurev-physiol-021317-121224
55. Strnad P, McElvaney NG, Lomas DA. Alpha1-antitrypsin deficiency. N Engl J Med. 2020;382(15):1443–1455. doi:10.1056/NEJMra1910234
56. Nakanishi T, Forgetta V, Handa T, et al. The undiagnosed disease burden associated with alpha-1 antitrypsin deficiency genotypes. Eur Respir J. 2020;56(6):2001441. doi:10.1183/13993003.01441-2020
57. Hopkinson NS, Nickol AH, Payne J, et al. Angiotensin converting enzyme genotype and strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(4):395–399. doi:10.1164/rccm.200304-578OC
58. Hopkinson NS, Eleftheriou KI, Payne J, et al. +9/+9 Homozygosity of the bradykinin receptor gene polymorphism is associated with reduced fat-free mass in chronic obstructive pulmonary disease. Am J Clin Nutr. 2006;83(4):912–917. doi:10.1093/ajcn/83.4.912
59. Hopkinson NS, Li KW, Kehoe A, et al. Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am J Clin Nutr. 2008;87(2):385–390. doi:10.1093/ajcn/87.2.385
60. Broekhuizen R, Grimble RF, Howell WM, et al. Pulmonary cachexia, systemic inflammatory profile, and the interleukin 1beta −511 single nucleotide polymorphism. Am J Clin Nutr. 2005;82(5):1059–1064. doi:10.1093/ajcn/82.5.1059
61. Lakshman Kumar P, Wilson AC, Rocco A, et al. Genetic variation in genes regulating skeletal muscle regeneration and tissue remodelling associated with weight loss in chronic obstructive pulmonary disease. J Cachexia Sarcopenia Muscle. 2021;12(6):1803–1817. doi:10.1002/jcsm.12782
62. Park HK, Lee SH, Lee SY, Kim SS, Park HW. Relationships between lung function decline and skeletal muscle and fat mass changes: a longitudinal study in healthy individuals. J Cachexia Sarcopenia Muscle. 2021;12(6):2145–2153. doi:10.1002/jcsm.12821
63. Divo MJ, Cabrera C, Casanova C, et al. Comorbidity distribution, clinical expression and survival in COPD patients with different body mass index. Chronic Obstr Pulm Dis. 2014;1(2):229–238. doi:10.15326/jcopdf.1.2.2014.0117
64. Ji Z, de Miguel-diez J, Castro-Riera CR, et al. Differences in the outcome of patients with COPD according to body mass index. J Clin Med. 2020;9(3):710. doi:10.3390/jcm9030710
65. Konokhova Y, Spendiff S, Jagoe RT, et al. Failed upregulation of TFAM protein and mitochondrial DNA in oxidatively deficient fibers of chronic obstructive pulmonary disease locomotor muscle. Skelet Muscle. 2016;6:10. doi:10.1186/s13395-016-0083-9
66. Kapchinsky S, Vuda M, Miguez K, et al. Smoke-induced neuromuscular junction degeneration precedes the fibre type shift and atrophy in chronic obstructive pulmonary disease. J Physiol. 2018;596(14):2865–2881. doi:10.1113/JP275558
67. Boehm I, Miller J, Wishart TM, et al. Neuromuscular junctions are stable in patients with cancer cachexia. J Clin Invest. 2020;130(3):1461–1465. doi:10.1172/JCI128411
68. Degens H, Gayan-Ramirez G, van Hees HW. Smoking-induced skeletal muscle dysfunction: from evidence to mechanisms. Am J Respir Crit Care Med. 2015;191(6):620–625. doi:10.1164/rccm.201410-1830PP
69. Cielen N, Maes K, Heulens N, et al. Interaction between physical activity and smoking on lung, muscle, and bone in mice. Am J Respir Cell Mol Biol. 2016;54(5):674–682. doi:10.1165/rcmb.2015-0181OC
70. Toledo-Arruda AC, Sousa Neto IV, Vieira RP, et al. Aerobic exercise training attenuates detrimental effects of cigarette smoke exposure on peripheral muscle through stimulation of the Nrf2 pathway and cytokines: a time-course study in mice. Appl Physiol Nutr Metab. 2020;45(9):978–986. doi:10.1139/apnm-2019-0543
71. Wang L, van Iersel LEJ, Pelgrim CE, et al. Effects of cigarette smoke on adipose and skeletal muscle tissue: in vivo and in vitro studies. Cells. 2022;11(18):2893. doi:10.3390/cells11182893
72. Raguso CA, Luthy C. Nutritional status in chronic obstructive pulmonary disease: role of hypoxia. Nutrition. 2011;27(2):138–143. doi:10.1016/j.nut.2010.07.009
73. Kent BD, Mitchell PD, McNicholas WT. Hypoxemia in patients with COPD: cause, effects, and disease progression. Int J Chron Obstruct Pulmon Dis. 2011;6:199–208. doi:10.2147/COPD.S10611
74. Schols A, Mostert R, Soeters P, Greve LH, Wouters EF. Inventory of nutritional status in patients with COPD. Chest. 1989;96(2):247–249. doi:10.1378/chest.96.2.247
75. Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147(5):1151–1156. doi:10.1164/ajrccm/147.5.1151
76. Debevec T, Ganse B, Mittag U, Eiken O, Mekjavic IB, Rittweger J. Hypoxia aggravates inactivity-related muscle wasting. Front Physiol. 2018;9:494. doi:10.3389/fphys.2018.00494
77. Gosker HR, Zeegers MP, Wouters EF, Schols AM. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax. 2007;62(11):944–949. doi:10.1136/thx.2007.078980
78. Budweiser S, Heinemann F, Meyer K, Wild PJ, Pfeifer M. Weight gain in cachectic COPD patients receiving noninvasive positive-pressure ventilation. Respir Care. 2006;51(2):126–132.
79. Fernandez-Bussy S, Kornafeld A, Labarca G, Abia-Trujillo D, Patel NM, Herth FJF. Endoscopic lung volume reduction in relation to body mass index in patients with severe heterogeneous emphysema. Respiration. 2020;99(6):477–483. doi:10.1159/000507591
80. O’Corragain OA, Townsend R, Lashari B, Sinha T, van der Rijst N, Criner GJ. Weight gain following bronchoscopic lung volume reduction. Am J Respir Crit Care Med. 2022;2022:A2134.
81. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi:10.1378/chest.10-2521
82. De Brandt J, Spruit MA, Hansen D, et al. Changes in lower limb muscle function and muscle mass following exercise-based interventions in patients with chronic obstructive pulmonary disease: a review of the English-language literature. Chron Respir Dis. 2018;15(2):182–219. doi:10.1177/1479972317709642
83. Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70(3):213–218. doi:10.1136/thoraxjnl-2014-206440
84. Koo HK, Park JH, Park HK, Jung H, Lee SS. Conflicting role of sarcopenia and obesity in male patients with chronic obstructive pulmonary disease: Korean National Health and Nutrition Examination Survey. PLoS One. 2014;9(10):e110448. doi:10.1371/journal.pone.0110448
85. Lee DW, Jin HJ, Shin KC, Chung JH, Lee HW, Lee KH. Presence of sarcopenia in asthma-COPD overlap syndrome may be a risk factor for decreased bone-mineral density, unlike asthma: Korean National Health and Nutrition Examination Survey (KNHANES) IV and V (2008–2011). Int J Chron Obstruct Pulmon Dis. 2017;12:2355–2362. doi:10.2147/COPD.S138497
86. Lee DW, Choi EY, Wu Q. Sarcopenia as an independent risk factor for decreased BMD in COPD patients: Korean National Health and Nutrition Examination Surveys IV and V (2008–2011). PLoS One. 2016;11(10):e0164303. doi:10.1371/journal.pone.0164303
87. Matkovic Z, Cvetko D, Rahelic D, et al. Nutritional status of patients with chronic obstructive pulmonary disease in relation to their physical performance. COPD. 2017;14(6):626–634. doi:10.1080/15412555.2017.1386643
88. Andersson M, Slinde F, Gronberg AM, Svantesson U, Janson C, Emtner M. Physical activity level and its clinical correlates in chronic obstructive pulmonary disease: a cross-sectional study. Respir Res. 2013;14:128. doi:10.1186/1465-9921-14-128
89. Matkovic Z, Tudoric N, Cvetko D, et al. Easy to perform physical performance tests to identify COPD patients with low physical activity in clinical practice. Int J Chron Obstruct Pulmon Dis. 2020;15:921–929. doi:10.2147/COPD.S246571
90. Waschki B, Spruit MA, Watz H, et al. Physical activity monitoring in COPD: compliance and associations with clinical characteristics in a multicenter study. Respir Med. 2012;106(4):522–530. doi:10.1016/j.rmed.2011.10.022
91. Shrikrishna D, Patel M, Tanner RJ, et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J. 2012;40(5):1115–1122. doi:10.1183/09031936.00170111
92. Bossenbroek L, de Greef MH, Wempe JB, Krijnen WP, Ten Hacken NH. Daily physical activity in patients with chronic obstructive pulmonary disease: a systematic review. COPD. 2011;8(4):306–319. doi:10.3109/15412555.2011.578601
93. Moy ML, Teylan M, Weston NA, Gagnon DR, Danilack VA, Garshick E. Daily step count is associated with plasma C-reactive protein and IL-6 in a US cohort with COPD. Chest. 2014;145(3):542–550. doi:10.1378/chest.13-1052
94. Watz H, Pitta F, Rochester CL, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–1537. doi:10.1183/09031936.00046814
95. Kantorowski A, Wan ES, Homsy D, Kadri R, Richardson CR, Moy ML. Determinants and outcomes of change in physical activity in COPD. ERJ Open Res. 2018;4(3):00054–2018. doi:10.1183/23120541.00054-2018
96. Schols AM, Fredrix EW, Soeters PB, Westerterp KR, Wouters EF. Resting energy expenditure in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 1991;54(6):983–987. doi:10.1093/ajcn/54.6.983
97. Green JH, Muers MF. The thermic effect of food in underweight patients with emphysematous chronic obstructive pulmonary disease. Eur Respir J. 1991;4(7):813–819.
98. Lahaije AJ, van Helvoort HA, Dekhuijzen PN, Heijdra YF. Physiologic limitations during daily life activities in COPD patients. Respir Med. 2010;104(8):1152–1159. doi:10.1016/j.rmed.2010.02.011
99. Vaes AW, Wouters EFM, Franssen FME, et al. Task-related oxygen uptake during domestic activities of daily life in patients with COPD and healthy elderly subjects. Chest. 2011;140(4):970–979. doi:10.1378/chest.10-3005
100. Schols AM, Soeters PB, Mostert R, Saris WH, Wouters EF. Energy balance in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1991;143(6):1248–1252. doi:10.1164/ajrccm/143.6.1248
101. Christensen T, Mikkelsen S, Geisler L, Holst M. Chronic obstructive pulmonary disease outpatients bear risks of both unplanned weight loss and obesity. Clin Nutr ESPEN. 2022;49:246–251. doi:10.1016/j.clnesp.2022.04.010
102. Rom O, Reznick AZ. The role of E3 ubiquitin-ligases MuRF-1 and MAFbx in loss of skeletal muscle mass. Free Radic Biol Med. 2016;98:218–230. doi:10.1016/j.freeradbiomed.2015.12.031
103. Steiner JL, Lang CH. Dysregulation of skeletal muscle protein metabolism by alcohol. Am J Physiol Endocrinol Metab. 2015;308(9):E699–712. doi:10.1152/ajpendo.00006.2015
104. Li Y, Zhang F, Modrak S, Little A, Zhang H. Chronic alcohol consumption enhances skeletal muscle wasting in mice bearing cachectic cancers: the role of tnfalpha/myostatin axis. Alcohol Clin Exp Res. 2020;44(1):66–77. doi:10.1111/acer.14221
105. Wang B, Zhang F, Zhang H, et al. Alcohol intake aggravates adipose browning and muscle atrophy in cancer-associated cachexia. Oncotarget. 2017;8(59):100411–100420. doi:10.18632/oncotarget.22243
106. Preedy VR, Adachi J, Ueno Y, et al. Alcoholic skeletal muscle myopathy: definitions, features, contribution of neuropathy, impact and diagnosis. Eur J Neurol. 2001;8(6):677–687. doi:10.1046/j.1468-1331.2001.00303.x
107. Dasarathy J, McCullough AJ, Dasarathy S. Sarcopenia in alcoholic liver disease: clinical and molecular advances. Alcohol Clin Exp Res. 2017;41(8):1419–1431. doi:10.1111/acer.13425
108. Prokopidis K, Witard OC. Understanding the role of smoking and chronic excess alcohol consumption on reduced caloric intake and the development of sarcopenia. Nutr Res Rev. 2021;1–10. doi:10.1017/S0954422421000135
109. Hansen H, Johnsen NF, Molsted S. Time trends in leisure time physical activity, smoking, alcohol consumption and body mass index in Danish adults with and without COPD. BMC Pulm Med. 2016;16(1):110. doi:10.1186/s12890-016-0265-6
110. Crul T, Testelmans D, Spruit MA, et al. Gene expression profiling in vastus lateralis muscle during an acute exacerbation of COPD. Cell Physiol Biochem. 2010;25(4–5):491–500. doi:10.1159/000303054
111. Decramer M, Lacquet LM, Fagard R, Rogiers P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150(1):11–16. doi:10.1164/ajrccm.150.1.8025735
112. Bernard S, LeBlanc P, Whittom F, et al. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(2):629–634. doi:10.1164/ajrccm.158.2.9711023
113. Snyder-Mackler N, Burger JR, Gaydosh L, et al. Social determinants of health and survival in humans and other animals. Science. 2020;368(6493). doi:10.1126/science.aax9553
114. Barnes PJ, Baker J, Donnelly LE. Cellular senescence as a mechanism and target in chronic lung diseases. Am J Respir Crit Care Med. 2019;200(5):556–564. doi:10.1164/rccm.201810-1975TR
115. Divo M, Celli BR. Multimorbidity in patients with chronic obstructive pulmonary disease. Clin Chest Med. 2020;41(3):405–419. doi:10.1016/j.ccm.2020.06.002
116. Goris AH, Vermeeren MA, Wouters EF, Schols AM, Westerterp KR. Energy balance in depleted ambulatory patients with chronic obstructive pulmonary disease: the effect of physical activity and oral nutritional supplementation. Br J Nutr. 2003;89(5):725–731. doi:10.1079/BJN2003838
117. Baarends EM, Schols AM, Westerterp KR, Wouters EF. Total daily energy expenditure relative to resting energy expenditure in clinically stable patients with COPD. Thorax. 1997;52(9):780–785. doi:10.1136/thx.52.9.780
118. Hugli O, Schutz Y, Fitting JW. The daily energy expenditure in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153(1):294–300. doi:10.1164/ajrccm.153.1.8542132
119. Slinde F, Ellegard L, Gronberg AM, Larsson S, Rossander-Hulthen L. Total energy expenditure in underweight patients with severe chronic obstructive pulmonary disease living at home. Clin Nutr. 2003;22(2):159–165. doi:10.1054/clnu.2002.0618
120. Vermeeren MA, Schols AM, Wouters EF. Effects of an acute exacerbation on nutritional and metabolic profile of patients with COPD. Eur Respir J. 1997;10(10):2264–2269. doi:10.1183/09031936.97.10102264
121. Broekhuizen R, Creutzberg EC, Weling-Scheepers CA, Wouters EF, Schols AM. Optimizing oral nutritional drink supplementation in patients with chronic obstructive pulmonary disease. Br J Nutr. 2005;93(6):965–971. doi:10.1079/BJN20051437
122. Deutz NE, Bauer JM, Barazzoni R, et al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–936. doi:10.1016/j.clnu.2014.04.007
123. Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Supplementation of soy protein with branched-chain amino acids alters protein metabolism in healthy elderly and even more in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2007;85(2):431–439. doi:10.1093/ajcn/85.2.431
124. Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Casein protein results in higher prandial and exercise induced whole body protein anabolism than whey protein in chronic obstructive pulmonary disease. Metabolism. 2012;61(9):1289–1300. doi:10.1016/j.metabol.2012.03.001
125. Jonker R, Deutz NE, Erbland ML, Anderson PJ, Engelen MP. Hydrolyzed casein and whey protein meals comparably stimulate net whole-body protein synthesis in COPD patients with nutritional depletion without an additional effect of leucine co-ingestion. Clin Nutr. 2014;33(2):211–220. doi:10.1016/j.clnu.2013.06.014
126. Jonker R, Deutz NEP, Schols A, et al. Whole body protein anabolism in COPD patients and healthy older adults is not enhanced by adding either carbohydrates or leucine to a serving of protein. Clin Nutr. 2019;38(4):1684–1691. doi:10.1016/j.clnu.2018.08.006
127. Engelen M, Deutz NEP. Is beta-hydroxy beta-methylbutyrate an effective anabolic agent to improve outcome in older diseased populations? Curr Opin Clin Nutr Metab Care. 2018;21(3):207–213. doi:10.1097/MCO.0000000000000459
128. Ferreira IM, Brooks D, White J, Goldstein R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD000998. doi:10.1002/14651858.CD000998.pub3
129. Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179(8):630–636. doi:10.1164/rccm.200810-1576PP
130. Ryan ZC, Craig TA, Folmes CD, et al. 1alpha, 25-dihydroxyvitamin D3 regulates mitochondrial oxygen consumption and dynamics in human skeletal muscle cells. J Biol Chem. 2016;291(3):1514–1528. doi:10.1074/jbc.M115.684399
131. Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22(3):859–871. doi:10.1007/s00198-010-1407-y
132. Ahmadi A, Haghighat N, Hakimrabet M, Tolide-ie H. Nutritional evaluation in chronic obstructive pulmonary disease patients. Pak J Biol Sci. 2012;15(10):501–505. doi:10.3923/pjbs.2012.501.505
133. Calder PC, Laviano A, Lonnqvist F, Muscaritoli M, Öhlander M, Schols A. Targeted medical nutrition for cachexia in chronic obstructive pulmonary disease: a randomized, controlled trial. J Cachexia Sarcopenia Muscle. 2018;9(1):28–40. doi:10.1002/jcsm.12228
134. Broekhuizen R, Wouters EF, Creutzberg EC, Weling-Scheepers CA, Schols AM. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax. 2005;60(5):376–382. doi:10.1136/thx.2004.030858
135. van de Bool C, Rutten EPA, van Helvoort A, Franssen FME, Wouters EFM, Schols A. A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J Cachexia Sarcopenia Muscle. 2017;8(5):748–758. doi:10.1002/jcsm.12219
136. van Beers M, Rutten-van Mölken M, van de Bool C, et al. Clinical outcome and cost-effectiveness of a 1-year nutritional intervention programme in COPD patients with low muscle mass: the randomized controlled NUTRAIN trial. Clin Nutr. 2020;39(2):405–413. doi:10.1016/j.clnu.2019.03.001
137. van Wetering CR, Hoogendoorn M, Broekhuizen R, et al. Efficacy and costs of nutritional rehabilitation in muscle-wasted patients with chronic obstructive pulmonary disease in a community-based setting: a prespecified subgroup analysis of the INTERCOM trial. J Am Med Dir Assoc. 2010;11(3):179–187. doi:10.1016/j.jamda.2009.12.083
138. Lainscak M, Gosker HR, Schols AM. Chronic obstructive pulmonary disease patient journey: hospitalizations as window of opportunity for extra-pulmonary intervention. Curr Opin Clin Nutr Metab Care. 2013;16(3):278–283. doi:10.1097/MCO.0b013e328360285d
139. Vermeeren MA, Wouters EF, Geraerts-Keeris AJ, Schols AM. Nutritional support in patients with chronic obstructive pulmonary disease during hospitalization for an acute exacerbation; a randomized controlled feasibility trial. Clin Nutr. 2004;23(5):1184–1192. doi:10.1016/j.clnu.2004.03.008
140. Creutzberg EC, Wouters EF, Vanderhoven-Augustin IM, Dentener MA, Schols AM. Disturbances in leptin metabolism are related to energy imbalance during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1239–1245. doi:10.1164/ajrccm.162.4.9912016
141. Zinna EM, Yarasheski KE. Exercise treatment to counteract protein wasting of chronic diseases. Curr Opin Clin Nutr Metab Care. 2003;6(1):87–93. doi:10.1097/00075197-200301000-00013
142. Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64. doi:10.1164/rccm.201309-1634ST
143. Marillier M, Bernard AC, Verges S, Neder JA. Locomotor muscles in COPD: the rationale for rehabilitative exercise training. Front Physiol. 2019;10:1590. doi:10.3389/fphys.2019.01590
144. Vogiatzis I, Stratakos G, Simoes DC, et al. Effects of rehabilitative exercise on peripheral muscle TNFalpha, IL-6, IGF-I and MyoD expression in patients with COPD. Thorax. 2007;62(11):950–956. doi:10.1136/thx.2006.069310
145. Vogiatzis I, Terzis G, Nanas S, et al. Skeletal muscle adaptations to interval training in patients with advanced COPD. Chest. 2005;128(6):3838–3845. doi:10.1378/chest.128.6.3838
146. De Brandt J, Spruit MA, Derave W, Hansen D, Vanfleteren LE, Burtin C. Changes in structural and metabolic muscle characteristics following exercise-based interventions in patients with COPD: a systematic review. Expert Rev Respir Med. 2016;10(5):521–545. doi:10.1586/17476348.2016.1157472
147. Troosters T, Blondeel A, Janssens W, Demeyer H. The past, present and future of pulmonary rehabilitation. Respirology. 2019;24(9):830–837. doi:10.1111/resp.13517
148. Beauchamp MK, Nonoyama M, Goldstein RS, et al. Interval versus continuous training in individuals with chronic obstructive pulmonary disease--a systematic review. Thorax. 2010;65(2):157–164. doi:10.1136/thx.2009.123000
149. Dolmage TE, Goldstein RS. Effects of one-legged exercise training of patients with COPD. Chest. 2008;133(2):370–376. doi:10.1378/chest.07-1423
150. Bronstad E, Rognmo O, Tjonna AE, et al. High-intensity knee extensor training restores skeletal muscle function in COPD patients. Eur Respir J. 2012;40(5):1130–1136. doi:10.1183/09031936.00193411
151. Nyberg A, Martin M, Saey D, et al. Effects of low-load/high-repetition resistance training on exercise capacity, health status, and limb muscle adaptation in patients with severe COPD: a randomized controlled trial. Chest. 2020;159:1821–1832. doi:10.1016/j.chest.2020.12.005
152. Nyberg A, Saey D, Martin M, Maltais F. Cardiorespiratory and muscle oxygenation responses to low-load/high-repetition resistance exercises in COPD and healthy controls. J Appl Physiol. 2018;124(4):877–887. doi:10.1152/japplphysiol.00447.2017
153. O’Shea SD, Taylor NF, Paratz JD. Progressive resistance exercise improves muscle strength and may improve elements of performance of daily activities for people with COPD: a systematic review. Chest. 2009;136(5):1269–1283. doi:10.1378/chest.09-0029
154. Schoenfeld BJ, Grgic J, Ogborn D, Krieger JW. Strength and hypertrophy adaptations between low- vs. high-load resistance training: a systematic review and meta-analysis. J Strength Cond Res. 2017;31(12):3508–3523. doi:10.1519/JSC.0000000000002200
155. Schoenfeld BJ, Grgic J, Van Every DW, Plotkin DL. Loading recommendations for muscle strength, hypertrophy, and local endurance: a re-examination of the repetition continuum. Sports. 2021;9(2):32. doi:10.3390/sports9020032
156. Constantin D, Menon MK, Houchen-Wolloff L, et al. Skeletal muscle molecular responses to resistance training and dietary supplementation in COPD. Thorax. 2013;68(7):625–633. doi:10.1136/thoraxjnl-2012-202764
157. Costes F, Gosker H, Feasson L, et al. Impaired exercise training-induced muscle fiber hypertrophy and Akt/mTOR pathway activation in hypoxemic patients with COPD. J Appl Physiol. 2015;118(8):1040–1049. doi:10.1152/japplphysiol.00557.2014
158. Hill K, Cavalheri V, Mathur S, et al. Neuromuscular electrostimulation for adults with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2018;5(5):Cd010821. doi:10.1002/14651858.CD010821.pub2
159. Maddocks M, Nolan CM, Man WD, et al. Neuromuscular electrical stimulation to improve exercise capacity in patients with severe COPD: a randomised double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4(1):27–36. doi:10.1016/s2213-2600(15)00503-2
160. Wouters EFM, Augustin IML. Process of pulmonary rehabilitation and program organization. Eur J Phys Rehabil Med. 2011;47(3):475–482.
161. Franssen FME, Vanfleteren LE, Janssen DJA, Wouters EFM, Spruit MA. Effects of a comprehensive, inpatient pulmonary rehabilitation programme in a cachectic patient with very severe COPD and chronic respiratory failure. Breathe. 2019;15(3):227–233. doi:10.1183/20734735.0186-2019
162. Bolton CE, Broekhuizen R, Ionescu AA, et al. Cellular protein breakdown and systemic inflammation are unaffected by pulmonary rehabilitation in COPD. Thorax. 2007;62(2):109–114. doi:10.1136/thx.2006.060368
163. Holland AE, Cox NS, Houchen-Wolloff L, et al. Defining modern pulmonary rehabilitation. An official American Thoracic Society workshop report. Ann Am Thorac Soc. 2021;18(5):e12–e29. doi:10.1513/AnnalsATS.202102-146ST
164. Kneppers AEM, Haast RAM, Langen RCJ, et al. Distinct skeletal muscle molecular responses to pulmonary rehabilitation in chronic obstructive pulmonary disease: a cluster analysis. J Cachexia Sarcopenia Muscle. 2019;10(2):311–322. doi:10.1002/jcsm.12370
165. Vogiatzis I, Simoes DC, Stratakos G, et al. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur Respir J. 2010;36(2):301–310. doi:10.1183/09031936.00112909
166. Wan ES, Cho MH, Boutaoui N, et al. Genome-wide association analysis of body mass in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2011;45(2):304–310. doi:10.1165/rcmb.2010-0294OC
167. Miki K, Maekura R, Nagaya N, et al. Ghrelin treatment of cachectic patients with chronic obstructive pulmonary disease: a multicenter, randomized, double-blind, placebo-controlled trial. PLoS One. 2012;7(5):e35708. doi:10.1371/journal.pone.0035708
168. Nagaya N, Itoh T, Murakami S, et al. Treatment of cachexia with ghrelin in patients with COPD. Chest. 2005;128(3):1187–1193. doi:10.1378/chest.128.3.1187
169. Weisberg J, Wanger J, Olson J, et al. Megestrol acetate stimulates weight gain and ventilation in underweight COPD patients. Chest. 2002;121(4):1070–1078. doi:10.1378/chest.121.4.1070
170. McKeaveney C, Maxwell P, Noble H, Reid J. A critical review of multimodal interventions for cachexia. Adv Nutr. 2021;12(2):523–532. doi:10.1093/advances/nmaa111
171. Casaburi R, Nakata J, Bistrong L, Torres E, Rambod M, Porszasz J. Effect of megestrol acetate and testosterone on body composition and hormonal responses in COPD cachexia. Chronic Obstr Pulm Dis. 2015;3(1):389–397. doi:10.15326/jcopdf.3.1.2015.0128
172. Garcia JM, Boccia RV, Graham CD, et al. Anamorelin for patients with cancer cachexia: an integrated analysis of two Phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol. 2015;16(1):108–116. doi:10.1016/s1470-2045(14)71154-4
173. Currow DC, Abernethy AP. Anamorelin hydrochloride in the treatment of cancer anorexia-cachexia syndrome. Future Oncol. 2014;10(5):789–802. doi:10.2217/fon.14.14
174. Mantovani G, Macciò A, Lai P, Massa E, Ghiani M, Santona MC. Cytokine involvement in cancer anorexia/cachexia: role of megestrol acetate and medroxyprogesterone acetate on cytokine downregulation and improvement of clinical symptoms. Crit Rev Oncog. 1998;9(2):99–106. doi:10.1615/critrevoncog.v9.i2.10
175. Casaburi R, Bhasin S, Cosentino L, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(8):870–878. doi:10.1164/rccm.200305-617OC
176. Lewis MI, Fournier M, Storer TW, et al. Skeletal muscle adaptations to testosterone and resistance training in men with COPD. J Appl Physiol. 2007;103(4):1299–1310. doi:10.1152/japplphysiol.00150.2007
177. Barreiro E, Puig-Vilanova E, Salazar-Degracia A, Pascual-Guardia S, Casadevall C, Gea J. The phosphodiesterase-4 inhibitor roflumilast reverts proteolysis in skeletal muscle cells of patients with COPD cachexia. J Appl Physiol. 2018;125(2):287–303. doi:10.1152/japplphysiol.00798.2017
178. Polkey MI, Praestgaard J, Berwick A, et al. Activin type II receptor blockade for treatment of muscle depletion in chronic obstructive pulmonary disease. A randomized trial. Am J Respir Crit Care Med. 2019;199(3):313–320. doi:10.1164/rccm.201802-0286OC
179. Di Girolamo FG, Guadagni M, Fiotti N, Situlin R, Biolo G. Contraction and nutrition interaction promotes anabolism in cachectic muscle. Curr Opin Clin Nutr Metab Care. 2019;22(1):60–67. doi:10.1097/mco.0000000000000527
180. Wilson JMG, Jungner G; World Health Organization. Principles and practice of screening for disease; 2022. Available from: https://apps.who.int/iris/handle/10665/37650.
181. Spruit MA, Wouters EFM. Organizational aspects of pulmonary rehabilitation in chronic respiratory diseases. Respirology. 2019;24(9):838–843. doi:10.1111/resp.13512
182. Rochester CL, Vogiatzis I, Holland AE, et al. An official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. 2015;192(11):1373–1386. doi:10.1164/rccm.201510-1966ST
183. Vogiatzis I, Rochester CL, Spruit MA, Troosters T, Clini EM. Increasing implementation and delivery of pulmonary rehabilitation: key messages from the new ATS/ERS policy statement. Eur Respir J. 2016;47(5):1336–1341. doi:10.1183/13993003.02151-2015
184. van Wetering CR, Hoogendoorn M, Mol SJ, Rutten-van molken MP, Schols AM. Short- and long-term efficacy of a community-based COPD management programme in less advanced COPD: a randomised controlled trial. Thorax. 2010;65(1):7–13. doi:10.1136/thx.2009.118620
185. Pison CM, Cano NJ, Cherion C, et al. Multimodal nutritional rehabilitation improves clinical outcomes of malnourished patients with chronic respiratory failure: a randomised controlled trial. Thorax. 2011;66(11):953–960. doi:10.1136/thx.2010.154922
186. Baldi S, Aquilani R, Pinna GD, Poggi P, De martini A, Bruschi C. Fat-free mass change after nutritional rehabilitation in weight losing COPD: role of insulin, C-reactive protein and tissue hypoxia. Int J Chron Obstruct Pulmon Dis. 2010;5:29–39. doi:10.2147/copd.s7739
187. Liao WH, Chen JW, Chen X, et al. Impact of resistance training in subjects with COPD: a systematic review and meta-analysis. Respir Care. 2015;60(8):1130–1145. doi:10.4187/respcare.03598
188. American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. doi:10.1249/MSS.0b013e3181915670
189. Kraemer WJ, Ratamess NA. Fundamentals of resistance training: progression and exercise prescription. Med Sci Sports Exerc. 2004;36(4):674–688. doi:10.1249/01.mss.0000121945.36635.61
190. Chen L, Nelson DR, Zhao Y, Cui Z, Johnston JA. Relationship between muscle mass and muscle strength, and the impact of comorbidities: a population-based, cross-sectional study of older adults in the United States. BMC Geriatr. 2013;13(1):74. doi:10.1186/1471-2318-13-74
191. Menon MK, Houchen L, Singh SJ, Morgan MD, Bradding P, Steiner MC. Inflammatory and satellite cells in the quadriceps of patients with COPD and response to resistance training. Chest. 2012;142(5):1134–1142. doi:10.1378/chest.11-2144
192. Ramos D, Bertolini GN, Leite MR, et al. Is dynamometry able to infer the risk of muscle mass loss in patients with COPD? Int J Chron Obstruct Pulmon Dis. 2015;10:1403–1407. doi:10.2147/copd.s69829
193. Gephine S, Frykholm E, Nyberg A, et al. Specific contribution of quadriceps muscle strength, endurance, and power to functional exercise capacity in people with chronic obstructive pulmonary disease: a multicenter study. Phys Ther. 2021;101(6). doi:10.1093/ptj/pzab052
194. Zanini A, Aiello M, Cherubino F, et al. The one repetition maximum test and the sit-to-stand test in the assessment of a specific pulmonary rehabilitation program on peripheral muscle strength in COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:2423–2430. doi:10.2147/COPD.S91176
195. Argilés JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Mañas L. Skeletal muscle regulates metabolism via interorgan crosstalk: roles in health and disease. J Am Med Dir Assoc. 2016;17(9):789–796. doi:10.1016/j.jamda.2016.04.019
196. Orsso CE, Montes-Ibarra M, Findlay M, et al. Mapping ongoing nutrition intervention trials in muscle, sarcopenia, and cachexia: a scoping review of future research. J Cachexia Sarcopenia Muscle. 2022;13(3):1442–1459. doi:10.1002/jcsm.12954
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.