Back to Archived Journals » Botanics: Targets and Therapy » Volume 4
Update on the efficacy and safety of Petadolex®, a butterbur extract for migraine prophylaxis
Authors Prieto JM
Received 4 September 2013
Accepted for publication 25 November 2013
Published 10 March 2014 Volume 2014:4 Pages 1—9
DOI https://doi.org/10.2147/BTAT.S54023
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Jose M Prieto
Department of Pharmaceutical and Biological Chemistry, UCL School of Pharmacy, London, UK
Abstract: Migraine has a heavy socioeconomic impact in terms of lost productivity and burden on the health care system. The efficacy of current drug regimens in migraine prophylaxis is limited, and therapeutic alternatives are needed. These include a range of herbal medicines based on butterbur, feverfew, St John's wort, and Ginkgo. Of these, Petadolex®, an extract of the butterbur root, is the most promising. Petadolex® has been investigated in four studies, including one good quality clinical trial involving 202 patients, two randomized controlled trials with smaller cohorts including adults and children, and a large observational, open-label study. However, post-marketing surveillance only supports its safety at lower doses and over treatment durations shorter than those used in the clinical trials. Moreover, the long-term safety of the product has been called into question, leading to withdrawal in some European countries. This review draws an overall picture of this complex set of data. The safety and efficacy of Petadolex® remains a matter of debate by a number of clinical, regulatory, and professional bodies.
Keywords: Petasites hybridus, migraine, herbal medicine, clinical evidence, safety
Introduction to herbal therapies in migraine prophylaxis
Migraine affects an estimated 324 million patients worldwide, and has a heavy socioeconomic impact in terms of both lost productivity and burden to health care systems.1,2 Once considered a psychologic problem due to comorbidity with other neurologic and psychiatric illnesses,3 migraine is nowadays a well recognized idiopathic condition, although many of its pathophysiologic aspects still remain obscure.4 The lack of well defined targets, the multifactorial nature of the condition (involving both genetic and environmental factors), and variability in symptoms reported by patients hampers drug discovery efforts.5
Migraine is typically characterized by severe headache, photophobia, phonophobia, and/or nausea, and may be preceded by a series of visual symptoms known as “aura”. The “attacks” may last from hours to days, and recur at intervals of varying duration. Symptoms generally occur less frequently and become less severe as the patient gets older.6 In North America and Europe, the one-year prevalence of migraine is 6% in men and 15%–18% in women.1
Migraine may affect any age group. In children, there is a worrying shift in peak incidence towards younger age groups,7–9 potentially affecting future career opportunities by reducing school attendance.10,11 Until puberty, boys and girls are equally likely to develop migraine, but in adulthood it is more common in women than men in a 3:2 ratio, which is generally consistent across countries.9
Prescription medications commonly used to treat migraine headache include common pain killers, triptans, and ergot derivatives. Prevention in patients with more than three migraine attacks per month may involve a beta-blocker, an antidepressant, or antiseizure medication, depending on the history of the patient.5,12–14 However, these prophylactic strategies have limited efficacy, with response rates ranging from only 20% to 40%.13,15 In this context, the importance of alternative pharmacologic treatments or even nonpharmacologic approaches to migraine prophylaxis,16 such as behavioral strategies,17 complementary therapies (relaxation, sport, music therapy), and changes in diet and lifestyle is clinically recognized,12 and these strategies continue to be explored in an attempt to provide better quality of life for migraine sufferers.11,18
In Europe and North America, popular herbal remedies for migraine include butterbur (Petasites hybridus, Asteraceae, root) and feverfew (Tanacetum parthenium, Asteraceae, leaves).19 Both have a common chemical profile dominated by sesquiterpenes.19,20 There are also some reports on the use of St John’s wort (Hypericum perforatum, Hypericaceae, flowering aerial parts) for the treatment of migraine attacks. Its chemistry is characterized by the naphthodianthrone hypericine21 but it is now known that both butterbur and St John’s wort contain significant amounts of melatonin, a deficit of which has been related to migraine.22 Ginkgo (Ginkgo biloba, Ginkgoaceae, leaves) has been also used in clinical interventions and its efficacy may be related to its active principles, ie, Ginkgolides,23 which are diterpene trilactones and potent inhibitors of platelet-activating factor activity,24 and its anti-inflammatory, antiallergic, antioxidant, and neuroprotective effects.25 This pharmacologic profile is important in minimizing the effects of ischemia-reperfusion and reducing synthesis of platelet-activating factor, levels of which are increased during migraine attacks and may account for persistent platelet activation during migraine crises.26
A number of clinical studies have been conducted using these herbal medicines, yielding mixed results for feverfew27 (possibly due to wide variation in the products tested), some preliminary insights into St John’s wort,28 promising preliminary results for Ginkgo components,29 and quite convincing evidence for a special butterbur root extract called Petadolex®, a proprietary herbal medicinal product that is the subject of this paper.11,30–34
Butterbur: the plant and its uses
The traditional herbal medicinal drug is the root or whole herb of Petasites hybridus (L.) Gaertn P, Mey B, et Scherb (Asteraceae).35 Synonyms include P. officinalis Moench, P. ovatus Hill, P. sabaudus Beauverd,36 P. vulgaris Desf, and Tussilago petasites L.35 The common name butterbur may be ambiguous in North America, where it is known as “European butterbur”, whilst the name butterbur is linked to Petasites japonicus (Siebold and Zucc) Maxim.37 Other common names are petasites, butterbur, bog rhubarb, umbrella plant (English), farfaraccio maggiore (Italian), chapeau du diable (French), and gewöhnliche pestwurz (German).38 It can be found throughout the northern hemisphere (Asia, Europe, and parts of North America) along rivers, ditches, and marshy areas. Stalks of reddish flowers appear before the very large heart-shaped leaves. Its chemistry is characterized by essential oils (up to 0.4%), sesquiterpenes (notably petasine and isopetasine), related sesquiterpene lactones (bakkenolides and eremopetasitenins), and the notorious pyrrolizidine alkaloids, eg, senecionine20 (Figure 1).
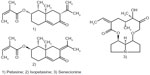  | Figure 1 Representative secondary metabolites of butterbur. |
Traditional indications include the treatment of painful spasm, chiefly urinary tract pain and asthma.20,35 Although some research has demonstrated the antispasmodic properties of petasites extracts, which may underlie their popularity, relevant clinical evidence for their use remains limited. However, use of butterbur as an antiasthmatic agent is supported by some clinical data.39,40 Interestingly, asthma is comorbid with migraine in both children41 and adults.42 Proinflammatory lipid mediators, including leukotrienes, have been identified as important mediators in asthma and are also known to contribute to headache and migraine.43,44 Interestingly, there is evidence that petasines inhibit the biosynthesis of leukotrienes.45–47 An open-label study of montelukast, an eicosanoid receptor antagonist, reported promising results in migraine.48 Unfortunately, a further randomized controlled study with a more robust design failed to find any significant efficacy for this treatment.49
On the other hand, the two major modern indications for medicinal butterbur products, ie, migraine and allergic rhinitis, are supported by a number of trials using two different but technically similar proprietary plant extracts devoid of toxic alkaloids. It is known that allergy and migraine are often comorbid conditions, with many patients suffering from “sinus headache” or migraine-associated food intolerance.42,50,51 Shared biochemical pathways between migraine and allergy are a matter of debate, with histamine playing a key role in triggering migraine via vasodilatation and inflammation.52 Therefore, the antihistamine properties of butterbur extract may be a common mechanism explaining at least in part its modern therapeutic indications.
Quality and safety of butterbur products and their components
Ensuring consistent quality of herbal medicinal products is a major challenge in the pharmaceutical industry. The complex and often variable chemistry of these products, as well as the risk of botanical misidentification or adulteration, requires use of state-of-the-art analytical techniques.53 This makes it difficult to compare different products containing the same active herbal ingredient, given that they are likely to have been processed in different ways, resulting in different chemical profiles.
Butterbur is a paradigm of these challenges. On the one hand, the quality of butterbur products must be ensured by providing a constant content of petasines, ie, the sesquiterpenes believed to be the active compounds. On the other hand, safety must be assured by removing the extremely toxic pyrrolizidine alkaloids to avoid hepatotoxicity.20,54
The pyrrolizidine alkaloids are generally found in young and metabolically active parts of the plant, including the rhizomes and flower stalks, whereas the leaf buds, petioles, and leaf blades are nearly free of pyrrolizidine alkaloids. In contrast, there is not much variation in sesquiterpene content across the plant. The most common manufacturing process involves extracting parts of the plant with a limited pyrrolizidine alkaloid content, such as the roots and leaves, using very apolar solvents such as chloroform or supercritical CO2. These solvents selectively extract the sesquiterpenes, with only traces of the very polar pyrrolizidine alkaloids, which mostly remain within the plant material. Chemical features determining the toxicity of these alkaloids are the presence of a double bond in the 1,2 position of the pyrrolizidine moiety, a hydroxymethyl substituent (C-1 position) in the pyrrolizidine moiety, preferably with a second hydroxyl group in the C-7 position, and esterification of the primary hydroxymethyl group with a branched monocarbolic or dicarbolic acid containing at least five carbon atoms (necic acid).55
In 2012, the European Medicines Agency published an extended account of the toxicity of these alkaloids and their implications for herbal medicines.55 All European partners recognize them as a great danger to public health and strictly limit their presence in medicinal products. However, there is a certain lack of consensus, with some countries (Belgium, Austria) enforcing a “zero tolerance principle” with regard to medicinal products containing pyrrolizidine alkaloids intended for internal use and others requiring very low exposure limits. For example, in Germany, the maximum daily dose for internal use of unsaturated pyrrolizidine alkaloids and their N-oxides is set at 1 μg for a maximum duration of 6 weeks per year or 0.1 μg without any limit on duration.55 In the Netherlands, the final product must contain less than one part per billion (μg/L).20 A maximum daily exposure of 10 μg is considered the limit for external use.35 There is still a concern about long-term exposure to these low levels of pyrrolizidine alkaloids, which are believed to induce tumors via a genotoxic mechanism mediated by formation of 6,7-dihydro-7-hydroxy-1-hydroxymethyl-5H-pyrrolizin, a derived DNA adduct.56 Even if a product meets these requirements, the advice is that it should not be used by pregnant or nursing women, young children, or people with severe kidney or liver disease until further safety testing has been performed.55 Manufacturers are usually required to warn the public to discontinue consumption should any symptom of liver toxicity appear (see Table 1).57
  | Table 1 Symptoms of possible drug-induced hepatitis associated with administration of pyrrolizidine alkaloids |
There are some preliminary studies on the quality of the petasites-based dietary supplements freely available on the market. In a study conducted by Bauer,58 analysis of the petasine/isopetasine content of six such products showed that only one complied with the labeling, another product contained double the claimed dose and the rest contained approximately 10,000-fold less than the content specified on the label. In a similar way, Avula et al sampled 21 different butterbur-containing dietary supplements, finding eleven to be in the dose range of 5–11.6 mg, four to contain traces only (<0.1 mg per dosage unit), and six without any trace of the active principle. Importantly, and of concern, is that pyrrolizidine alkaloids were detected in seven of these dietary supplements.59 Although fraudulent, underdosing of such products is not a health issue, but the existence of pyrrolizidine alkaloid-containing products on the market may result in serious toxicity and eventually cause a fatal outcome.
A closer look at the identity of the surveyed products reveals that only original Petadolex®-based products (Weber and Weber GmbH and Co, KG, Inning/Ammersee, Germany; Linpharma Inc., Oldsmar, FL, USA) consistently “pass” these quality screenings. Petadolex® is a proprietary extract standardized to provide a minimum of 15% petasines and to be “virtually free of pyrrolizidine alkaloids”.60 It has been manufactured since 1988 according to a patented process61 involving extraction of underground butterbur plant material using supercritical CO2 and further purification with acidic water. This results in a product with a pyrrolizidine alkaloid content of less than 0.1 parts per billion and a total petasine content of around 40%. These levels are measured by the manufacturer using enzyme-linked immunosorbent assays which are 1,000 times more sensitive than instrumental techniques such as gel chromatography. Still, there are some contradictory data in the literature referring to Petadolex® containing less than 0.088%62 or less than 0.088 parts per million.63
It is worth mentioning that Ze339® (Tesalin®, Max Zeller Söhne AG, Romanshorn, Switzerland) is another technologically and chemically similar butterbur extract which differs only in that it is sourced from the leaves of the plant.64 This proprietary extract has been clinically tested for the treatment of allergic rhinitis.65
Clinical studies using Petadolex®
Efficacy
A search in PubMed for clinical trials involving butterbur products and migraine yielded only four reports, all using the proprietary extract, Petadolex® (Table 2). The last systematic review was published in 2006,63 when only two randomized controlled studies were available. The first was a study in a small cohort designed and run by German researchers, which appears to be duplicated in the literature.31,32 Its findings were re-evaluated in 2004 by Diener et al using state-of-the-art statistical methods to meet the International Guidelines E9.30 This exploratory trial was followed by a larger confirmatory study conducted in primary care and specialty centers in both the US and Germany.33 It reported moderate evidence of effectiveness in the prophylaxis of adult migraine when using a higher than the recommended dose of the proprietary petasites root extract, Petadolex® (150 mg versus 100 mg).63 In 2005, Pothmann et al published a prospective observational study on the efficacy of Petadolex® in children and adolescents with severe migraine. The authors reported that Petadolex® 50–150 mg/day reduced the frequency of attacks and increased the percentage of responders, with a low rate of adverse events.34 However, its open-label, uncontrolled design and the wide age range of the cohort do not allow comparison of its results with those of the previous two multicenter, placebo-controlled studies in adults. It was only in 2008 that a randomized controlled trial investigated the use of Petadolex® in childhood migraine, which may be considered exploratory in nature due to the small cohort involved. The outcome was a significantly better effect in the Petadolex® completer group compared with placebo. Interestingly, increasing the dose in nonresponders was associated with an even smaller response. This suggests that an insufficient response to Petadolex® over 8 weeks might be indicative of “nonresponsiveness”. In general, the response to this extract was evident during the first weeks of treatment, although a full response only comes after a few months of treatment.11
Although the number of trials is rather small, and the individual trials are too different in design to draw comparisons, a recent meta-analysis by the American Academy of Neurology and the American Headache Society concluded that the existence of at least two “good” clinical trials, ie, those published by Grossmann and Schmidramsl31,32 and by Lipton et al in 2004,33 are enough to warrant endorsement of butterbur as a prophylactic agent in migraine.66 However, this has been received with polemic. First, the authors literally suggest that “petasites (butterbur)” is effective for migraine prevention. This is misleading given that the clinical trials were not dealing with butterbur, which is potentially lethal when unprocessed, but with the special extract, Petadolex®, which is free of pyrrolizidine alkaloids and therefore safe. The weak wording and citation of the flawed report by Grossmann and Schmidramsl,31,32 instead of the more correct one reported by Diener et al,30 prompted a series of letters to the editor of Neurology questioning the guidelines.67 Further, some criticisms of the methodology of the whole meta-analysis were raised because some drug treatments dismissed in a previous similar meta-analysis were now better ranked despite the lack of new clinical trials.68
To complicate matters further, the position of the American Academy of Neurology and the American Headache Society disagrees with that of the German, Austrian, and Swiss headache societies and the German Society of Neurology. These European medical societies did not endorse the efficacy of Petadolex® found by Lipton et al because “the multiple test procedure was not taken into account either in the study methodology or the results section of the publication, and the chosen statistical procedure enables no confirmatory proof of efficacy for any of the three endpoints”.69 This is a less favorable analysis than the one reported by Agosti et al,63 who concluded that there was “moderate evidence of effectiveness” for Petadolex®, although still recommending further studies.
Comparison with other herbal products
Feverfew is another important herbal medicine in migraine prophylaxis and/or treatment. Several clinical trials are available and show an overall lack of clear efficacy, possibly due to the great diversity of the tested products, some of which were of poor quality.27 Only one of the trials used a proprietary extract of feverfew (MIG-99®), which is fully characterized and technologically similar to Petadolex®. Despite the negative results of a first exploratory trial,70 it was noted that a very specific subgroup of patients with at least four attacks during the 28-day baseline period experienced significant improvement on a daily dose of three capsules of MIG-99® each containing 6.25 mg of extract. This encouraged a second clinical trial which confirmed these preliminary results.71 Interestingly, this study was run by Diener et al, the same team behind the reinterpretation of the first clinical study of Petadolex®,30 and the patients were recruited on the basis of very similar profiles. Despite MIG-99® showing significant effects, the response rate was just 30.3%, leading to a level B classification by the American Academy of Neurology and the American Headache Society.66
There are no quality studies for other herbal remedies in migraine. However, it is worth mentioning that proprietary extracts of Ginkgo and its component Ginkgolide B, either alone or in combination with vitamins and minerals, are the subject of an increasing number of interventions in adult and young migraineurs.23,29,72,73 Their results seem promising, but the variable composition of the treatments, the open-label trial designs, and the small cohort sizes make it difficult to draw any preliminary conclusions. Accordingly, Ginkgo is generally disregarded as a preventive agent for migraine in the guidelines published by the American and European headache associations.66,69
Safety and tolerability
Preclinical studies based on acute, subchronic, and chronic animal models as well as in vitro mutagenicity studies indicate that Petadolex® has no significant toxic effects.74 The available data from clinical studies (3–4 months of treatment) do not show significant differences compared with placebo regarding adverse events possibly causally related to Petadolex®, except for “burping”. This well known adverse effect is of a mild and transient nature. In addition, no changes were observed in systolic or diastolic blood pressure, heart rate, serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, gamma-glutamyl transpeptidase, or bilirubin during the study by Lipton et al (4 months of treatment).33
Safety in children and adolescents has been actively explored in some 160 patients treated for 3–4 months.11,34 The available data from these studies indicate excellent tolerability in subjects aged 6–17 years at doses of 50–150 mg, depending on age and response. The incidence of adverse effects was similar to placebo, and generally mild and self-limiting. However, better trials of longer duration must be performed prior to more robust assessment of the safety of Petadolex® in this age group. Lipton et al also recognize the need to perform longer studies in adults (treatment for more than one year) to fully evaluate the relative long-term safety and tolerability of this product.33
Quality of life, satisfaction, acceptability, adherence, and uptake
According to a recent meta-analysis, the response to common preventive pharmacologic treatments of choice, including topiramate, divalproex, timolol, propranolol, atenolol, nadolol, acebutolol, captopril, lisinopril, and candesartan, is in the range of 20%–40%.15 Thus, the moderate results seen with herbal proprietary extracts such as Petadolex® and MIG-99® compare very positively and seem to offer patients an effective alternative for improving their quality of life. In fact, Pothmann et al reported an improvement in overall well-being of 91.8% in patients treated with Petadolex®.34
Data on dropouts cannot be easily interpreted in terms of acceptability. It is true that sometimes the benefits of prophylactic treatment may be offset by environmental factors. For example, the dropouts in a group of children reported on by Oelkers-Ax et al were not related to adverse effects but to the “time-consuming” procedures associated with the trial.11 In fact, compliance with Petadolex® was similar to that found with placebo or music therapy, but there were responders in the latter group (>70% versus <60%). This highlights the importance of psychological factors in this particular age group. In terms of absolute numbers, compliance in the clinical trial conducted by Lipton et al33 was slightly higher than that of patients in a regular tertiary headache center,75 although this may be expected in patients who are more closely monitored during clinical trials.
Post-marketing safety data for Petadolex®
According to a review published in 200374 by the manufacturers, post-marketing surveillance for Petadolex®, ie, documentation of spontaneous reports of adverse effects, started in 1976. By 2002, up to 75 reports from Germany and 18 from other countries had been received, and consisted mainly of mild gastrointestinal discomfort. In another review published in the same year “four cases of a reversible cholestatic hepatitis probably associated with long-term administration of butterbur (incidence of 1:175.000)” are mentioned.76 Both reviews concluded that the overall incidence of “generally mild” adverse effects was very low (about 0.02%) in the estimated 500,000 patients treated from 1976 to 2003.
These data have to be interpreted with caution. First, they do not relate to the same product. Until 1988, Petadolex® was manufactured using methylene chloride as the extraction solvent, and thereafter using supercritical CO2 extraction, which is the product used in clinical trials. Second, the authors had an obvious conflict of interest, being employed by either the manufacturer or a commercial distributor of Petadolex® products.
In fact, the safety profile of Petadolex® was first called into question by the Swiss authorities one year before these reviews, following the appearance of three clinical cases in Germany associated with consumption of Petadolex®-based products. As a consequence, products containing Petadolex® had to add a warning about the possible (albeit rare) risk of liver toxicity to the patient information leaflet (Table 1).57
Two years later, there was a Swiss report of six cases of liver toxicity associated with use of Petadolex®-based products for migraine prophylaxis. Importantly, it was also reported that two batches of Petadolex® had to be withdrawn from the market due to a high content of pyrrolizidine alkaloids during this time. On these grounds, the Swiss Agency for Therapeutic Products rated the benefit-risk ratio of Petadolex®-based products as negative, banned their commercialization, and revoked their registration.77 In Germany, these products were still commercialized until 2009, when the BfArM revoked their registration.78 Many countries outside Europe, including the US, still allow the commercialization of Petadolex® and other butterbur-based herbal products as food supplements. However, these products are also sold over the Internet and therefore virtually available worldwide. Interestingly, a similar proprietary butterbur (leaves) supercritical CO2 extract Ze339® is marketed as Tesalin® N in Switzerland, where it is approved for the treatment of hay fever.64
Concluding remarks
The available clinical data include one trial with a large patient population and good a priori power calculations (80%), showing the significant effect of the proprietary extract Petadolex® in the prevention of migraine.33 This is in agreement with case reports of efficacy coming from two smaller clinical trials11,30 involving adults and children and a large observational study.34 This preliminary clinical evidence indicates that Petadolex® may reduce the frequency of attacks in a subgroup of patients responding to this treatment, who only achieve full benefit after 3–4 months of treatment with doses up to 150 mg/day. Post-marketing surveillance data exist, albeit for inferior doses (100 mg/day) and shorter treatment durations (2 months). From the available data, it is difficult to infer if the benefit-risk ratio could be maintained over longer periods of time,33 and in fact its long-term safety profile has been called into question by regulatory authorities, leading to its withdrawal from the European market.77 Safety concerns are compounded by an apparent failure to maintain quality, with the product failing on two occasions to meet the stringent low pyrrolizidine alkaloid content required in Europe.77
To complicate matters further, there is a lack of consensus among the different professional bodies and academic research groups on the real significance of these studies and post-marketing surveillance.63,66–69,77 It seems that only a new independent, randomized, long-term (>7 months), placebo-controlled, double-blind clinical trial, involving a critical mass of adult patients receiving different doses and followed by exquisite statistical analysis, would establish the real efficacy and best posology for supercritical CO2 extracts of butterbur. However, in a complex condition such as migraine, efficacy is difficult to measure and there will be always environmental factors limiting its effectiveness as well as the existence of a subgroup of “nonresponsive” patients. Perhaps more importantly, the manufacturers have to regain the confidence of regulatory bodies by proving that they are able to consistently provide a product devoid of pyrrolizidine alkaloids.
At present, many butterbur products of mixed quality are freely available on the market. According to the published research, it seems that only Petadolex®-based products are of consistent quality, whilst other butterbur-based supplements aimed at migraineurs may be underdosed or even pose unacceptable risks.58,59 The status of “herbal supplement” means that adults suffering from migraine can try this alternative therapy at their own risk. In this case, immediate discontinuation and referral to health care professionals is encouraged at the first sign of adverse effects (see Table 1). Importantly, patients suffering from liver disease, children, and pregnant or lactating women must not use this product until more safety testing is done.
The journey of Petadolex® illustrates how the complexities of herbal remedies are still deeply misunderstood by both herbalists and medical doctors. Herbalists must understand the importance of “highly standardized, state of the art, proprietary products” versus “herbal drugs”, the latter either being of variable quality or, in the case of butterbur, posing unacceptable safety risks. Health care professionals, particularly medical doctors, need to understand that common plant names are not equivalent to generic drugs, and that only proprietary products supported by good quality clinical trials and having a solid safety profile can be endorsed.
Disclosure
The author reports no conflicts of interest in this work. The listing of a medication or supplement herein does not imply endorsement or recommendation by the author.
References
World Health Organization. Global Burden of Disease. Geneva, Switzerland: World Health Organization; 2000. Available from: http://www.who.int/healthinfo/global_burden_disease/en/. Accessed November 29, 2011. | |
World Health Organization. Global Burden of Disease. 2004 Update. Geneva, Switzerland: World Health Organization; 2004. Available from: http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf. Accessed November 29, 2013. | |
Antonaci F, Nappi G, Galli F, Manzoni GC, Calabresi P, Costa A. Migraine and psychiatric comorbidity: a review of clinical findings. J Headache Pain. 2011;12(2):115–125. | |
Charles A. The evolution of a migraine attack – a review of recent evidence. Headache. 2013;53(2):413–419. | |
Bekkelund SI, Alstadhaug KB. Migraine prophylactic drugs – something new under the sun? Expert Opin Investig Drugs. 2011;20(9):1201–1210. | |
National Health Service. Migraine; 2013. Available from: http://www.nhs.uk/Conditions/Migraine/Pages/Introduction.aspx. Accessed November 29, 2013. | |
Laurell K, Larsson B, Eeg-Olofsson O. Prevalence of headache in Swedish schoolchildren, with a focus on tension-type headache. Cephalalgia. 2004;24(5):380–388. | |
Egermark-Eriksson I. Prevalence of headache in Swedish schoolchildren. A questionnaire survey. Acta Paediatr Scand. 1982;71(1):135–140. | |
Merikangas KR. Contributions of epidemiology to our understanding of migraine. Headache. 2013;53(2):230–246. | |
Sillanpaa M, Anttila P. Increasing prevalence of headache in 7-year-old schoolchildren. Headache. 1996;36(8):466–470. | |
Oelkers-Ax R, Leins A, Parzer P, et al. Butterbur root extract and music therapy in the prevention of childhood migraine: an explorative study. Eur J Pain. 2008;12(3):301–313. | |
Schulman E, McGeeney BE. Current concepts in refractory migraine. Curr Treat Options Neurol. 2013;15(1):40–55. | |
Barbanti P, Aurilia C, Egeo G, Fofi L. Migraine prophylaxis: what is new and what we need? Neurol Sci. 2011;32 Suppl 1:S111–S115. | |
Stovner LJ, Tronvik E, Hagen K. New drugs for migraine. J Headache Pain. 2009;10(6):395–406. | |
Shamliyan TA, Choi JY, Ramakrishnan R, et al. Preventive pharmacologic treatments for episodic migraine in adults. J Gen Intern Med. 2013;28(9):1225–1237. | |
Schiapparelli P, Allais G, Castagnoli Gabellari I, Rolando S, Terzi MG, Benedetto C. Non-pharmacological approach to migraine prophylaxis: part II. Neurol Sci. 2010;31 Suppl 1:S137–S139. | |
Grazzi L, Andrasik F. Non-pharmacological approaches in migraine prophylaxis: behavioral medicine. Neurol Sci. 2010;31 Suppl 1:S133–S135. | |
Sun-Edelstein C, Mauskop A. Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache. 2011;51(3):469–483. | |
Evans RW, Taylor FR. “Natural” or alternative medications for migraine prevention. Headache. 2006;46(6):1012–1018. | |
Aydin AA, Zerbes V, Parlar H, Letzel T. The medical plant butterbur (Petasites): analytical and physiological (re)view. J Pharm Biomed Anal. 2013;75:220–229. | |
Galeotti N, Ghelardini C. St John’s wort relieves pain in an animal model of migraine. Eur J Pain. 2013;17(3):369–381. | |
Murch SJ, Simmons CB, Saxena PK. Melatonin in feverfew and other medicinal plants. Lancet. 1997;350(9091):1598–1599. | |
Esposito M, Carotenuto M. Ginkgolide B complex efficacy for brief prophylaxis of migraine in school-aged children: an open-label study. Neurol Sci. 2011;32(1):79–81. | |
Singh P, Singh IN, Mondal SC, Singh L, Garg VK. Platelet-activating factor (PAF)-antagonists of natural origin. Fitoterapia. 2013;84:180–201. | |
Strømgaard K. Medicinal chemistry of ginkgolides from ginkgo biloba. In: Liang X, Fang WS, editors. Medicinal Chemistry of Bioactive Natural Products. Hoboken, NJ, USA: Wiley-Interscience/John Wiley; 2006. | |
Sarchielli P, Alberti A, Coppola F, et al. Platelet-activating factor (PAF) in internal jugular venous blood of migraine without aura patients assessed during migraine attacks. Cephalalgia. 2004;24(8):623–630. | |
Pittler MH, Ernst E. Feverfew for preventing migraine. Cochrane Database Syst Rev. 2004;1:CD002286. | |
Mirzaei MG, Sewell RDE, Kheiri S, Rafieian-Kopaei M. A clinical trial of the effect of St John’s wort on migraine headaches in patients receiving sodium valproate. J Med Plants Res. 2012;6(9):1519–1523. | |
Usai S, Grazzi L, Bussone G. Gingkolide B as migraine preventive treatment in young age: results at 1-year follow-up. Neurol Sci. 2011; 32 Suppl 1:S197–S199. | |
Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51(2):89–97. | |
Grossmann M, Schmidramsl H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Int J Clin Pharmacol Ther. 2000;38(9):430–435. | |
Grossmann W, Schmidramsl H. An extract of Petasites hybridus is effective in the prophylaxis of migraine. Altern Med Rev. 2001;6(3):303–310. | |
Lipton RB, Gobel H, Einhaupl KM, Wilks K, Mauskop A. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63(12):2240–2244. | |
Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache. 2005;45(3):196–203. | |
Williamson EM. Potter’s Herbal Cyclopaedia: Saffron Walden, UK: The CW Daniel Company Limited; 2003. | |
Royal Botanic Garden Edinburgh. Flora Europaea. Available from: http://rbg-web2.rbge.org.uk/FE/fe.html. Accessed November 29, 2013. | |
Duke JA. Dr Duke’s Phytochemical and Ethnobotanical Databases. Available from: http://www.ars-grin.gov/duke/chem-activities.html. Accessed November 29, 2013. | |
US Department of Agriculture. Petasites hybridus (L.) G Gaertn, et al. 2012. Available from: http://www.ars-grin.gov/cgi-bin/npgs/html/tax_search.pl?Petasites%20hybridus. Accessed November 29, 2013. | |
Danesch UC. Petasites hybridus (butterbur root) extract in the treatment of asthma – an open trial. Altern Med Rev. 2004;9(1):54–62. | |
Ziolo G, Samochowiec L. Study on clinical properties and mechanisms of action of Petasites in bronchial asthma and chronic obstructive bronchitis. Pharm Acta Helv. 1998;72(6):378–380. | |
de Souza Carvalho D, Fragoso YD, Coelho FM, Pereira MM. Asthma plus migraine in childhood and adolescence: prophylactic benefits with leukotriene receptor antagonist. Headache. 2002;42(10):1044–1047. | |
Low NC, Merikangas KR. The comorbidity of migraine. CNS Spectr. 2003;8(6):433–434, 437–444. | |
LaMancusa R, Pulcinelli FM, Ferroni P, et al. Blood leukotrienes in headache: correlation with platelet activity. Headache. 1991;31(6):409–414. | |
Parantainen J, Vapaatalo H, Hokkanen E. Clinical aspects of prostaglandins and leukotrienes in migraine. Cephalalgia. 1986;6 Suppl 4:95–101. | |
Thomet OA, Schapowal A, Heinisch IV, Wiesmann UN, Simon HU. Anti-inflammatory activity of an extract of Petasites hybridus in allergic rhinitis. Int Immunopharmacol. 2002;2(7):997–1006. | |
Thomet OA, Simon HU. Petasins in the treatment of allergic diseases: results of preclinical and clinical studies. Int Arch Allergy Immunol. 2002;129(2):108–112. | |
Bickel D, Röder T, Bestmann HJ, Brune K. Identification and characterization of inhibitors of peptido-leukotriene-synthesis from petasites hybridus. Planta Med. 1994;60(4):318–322. | |
Sheftell F, Rapoport A, Weeks R, Walker B, Gammerman I, Baskin S. Montelukast in the prophylaxis of migraine: a potential role for leukotriene modifiers. Headache. 2000;40(2):158–163. | |
Brandes JL, Visser WH, Farmer MV, et al. Montelukast for migraine prophylaxis: a randomized, double-blind, placebo-controlled study. Headache. 2004;44(6):581–586. | |
Ku M, Silverman B, Prifti N, Ying W, Persaud Y, Schneider A. Prevalence of migraine headaches in patients with allergic rhinitis. Ann Allergy Asthma Immunol. 2006;97(2):226–230. | |
Mehle ME. Migraine and allergy: a review and clinical update. Curr Allergy Asthma Rep. 2012;12(3):240–245. | |
Thomas WA, Butler S. Intravenous histamine in the treatment of migraine. Bull N Y Acad Med. 1946;22(3):125–136. | |
Mosihuzzaman M. Herbal medicine in healthcare-an overview. Nat Prod Commun. 2012;7(6):807–812. | |
McLean EK. The toxic actions of pyrrolizidine (senecio) alkaloids. Pharmacol Rev. 1970;22(4):429–483. | |
European Medicines Agency. Working Party on Community Monographs and Community List. Public statement on the use of herbal medicinal products containing toxic, unsaturated pyrrolizidine alkaloids (PAs). 2012. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Public_statement/2013/11/WC500154224.pdf. Accessed November 29, 2013. | |
Fu P, Xia Q, Lin G, Chou M. Genotoxic pyrrolizidine alkaloids – mechanisms leading to DNA adduct formation and tumorigenicity. Int J Mol Sci. 2002;3(9):948–964. | |
SwissMedic. Médicaments contenant de la pétasite (Extractum Petasitidis) et risque de lésions hépatiques graves: procédure de réexamen en vertu de l’art. 16, al. 2, et de l’art. 58, al. 3, de la loi sur les produits thérapeutiques (LPTh). [Medicines containing Petasites (Extractum Petasitidis) and serious liver toxicity risk: revision on the grounds of articles 16_2 and 58_3 of the Therapeutic Products Act.] Swissmedic Journal. 2002(6):392. | |
Bauer R. Recent progress in the research on traditional herbal medicinal products. Revista de Fitoterapia. 2006;6 Suppl 1:31–35. | |
Avula B, Wang YH, Wang M, Smillie TJ, Khan IA. Simultaneous determination of sesquiterpenes and pyrrolizidine alkaloids from the rhizomes of Petasites hybridus (L.) GM et Sch and dietary supplements using UPLC-UV and HPLC-TOF-MS methods. J Pharm Biomed Anal. 2012;70:53–63. | |
Linpharma Inc. Petadolex®. Oldsmar, FL, USA: Linpharma Inc; 2013. Available from: http://www.petadolex.com. Accessed November 4, 2013. | |
Koch V, Rittinghauser R. Composition containing pyrrolizidine-alkaloid-free petasites. US Patent 6,551,626 B1. 2003. | |
Chrubasik JE, Duke RK, Chrubasik S. Mise à jour de la monographie allemande de la commission E sur Petasites hybridus (pétasite officinal). [Update of the German Commission E Monograph on Petasites hybridus (petasites officinal)] Phytothérapie. 2007;5(3):135–136. | |
Agosti R, Duke RK, Chrubasik JE, Chrubasik S. Effectiveness of Petasites hybridus preparations in the prophylaxis of migraine: a systematic review. Phytomedicine. 2006;13(9–10):743–746. | |
Zellermedical. Tesalin™. Romanshorn, Switzerland: Max Zeller Söhne AG, 2012. Available from: http://zellermedical.ch/products/tesalinn/. Accessed November 29, 2013. German. | |
Schapowal A, Petasites Study G. Butterbur Ze339 for the treatment of intermittent allergic rhinitis: dose-dependent efficacy in a prospective, randomized, double-blind, placebo-controlled study. Arch Otolaryngol Head Neck Surg. 2004;130(12):1381–1386. | |
Holland S, Silberstein SD, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1346–1353. | |
Mauskop A. Safety of Petasites (butterbur). Response to “Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society”. Neurology. 2012;80(9):868–869. | |
Tfelt-Hansen PC. Problematic recommendations in ANN’s guidelines on drug prevention of migraine. Response to “Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society”. Neurology. 2012;80(9):869–870. | |
Haag G, Diener HC, May A, et al. Self-medication of migraine and tension-type headache: summary of the evidence-based recommendations of the Deutsche Migrane und Kopfschmerzgesellschaft (DMKG), the Deutsche Gesellschaft fur Neurologie (DGN), the Osterreichische Kopfschmerzgesellschaft (OKSG) and the Schweizerische Kopfwehgesellschaft (SKG). J Headache Pain. 2011;12(2):201–217. | |
Pfaffenrath V, Diener HC, Fischer M, Friede M, Henneicke-von Zepelin HH. The efficacy and safety of Tanacetum parthenium (feverfew) in migraine prophylaxis – a double-blind, multicentre, randomized placebo-controlled dose-response study. Cephalalgia. 2002;22(7):523–532. | |
Diener HC, Pfaffenrath V, Schnitker J, Friede M, Henneicke-von Zepelin HH. Efficacy and safety of 6.25 mg tid feverfew CO2-extract (MIG-99) in migraine prevention – a randomized, double-blind, multicentre, placebo-controlled study. Cephalalgia. 2005;25(11):1031–1041. | |
Usai S, Grazzi L, Andrasik F, Bussone G. An innovative approach for migraine prevention in young age: a preliminary study. Neurol Sci. 2010;31 Suppl 1:S181–S183. | |
D’Andrea G, Bussone G, Allais G, et al. Efficacy of Ginkgolide B in the prophylaxis of migraine with aura. Neurol Sci. 2009;30 Suppl 1:S121–S124. | |
Danesch U, Rittinghausen R. Safety of a patented special butterbur root extract for migraine prevention. Headache. 2003;43(1):76–78. | |
Gaul C, van Doorn C, Webering N, et al. Clinical outcome of a headache-specific multidisciplinary treatment program and adherence to treatment recommendations in a tertiary headache center: an observational study. J Headache Pain. 2011;12(4):475–483. | |
Kalin P. [The common butterbur (Petasites hybridus) – portrait of a medicinal herb]. Forsch Komplementarmed Klass Naturheilkd. 2003;10 Suppl 1:41–44. German. | |
Swissmedic. Revocation de l’Authorisation de mis sur le marche (AMM) de certains medicaments contenant du petasite. [Withdrawal of the Product License (PL) of some medicines containing Petasites] SwissMedic Journal. 2004;3(1):22. French. | |
Weber & Weber. Information: Petadolex® capsules extinguished approval for formal reasons; 2009. Available from: http://www.weber-weber.de. Accessed November 4, 2013. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

