Back to Journals » Drug Design, Development and Therapy » Volume 18
Unveiling the Mechanism of the ChaiShao Shugan Formula Against Triple-Negative Breast Cancer
Authors Fan T, Huang Y, Liu Z, Huang J , Ke B, Rong Y, Qiu H, Zhang B
Received 16 August 2023
Accepted for publication 25 March 2024
Published 10 April 2024 Volume 2024:18 Pages 1115—1131
DOI https://doi.org/10.2147/DDDT.S394287
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Teng Fan,1– 3,* Yuanyuan Huang,1– 3,* Zeyu Liu,1,2,* Jinsheng Huang,1,2 Bin Ke,1– 3 Yuming Rong,1,2 Huijuan Qiu,1,2 Bei Zhang1– 3
1TCM&VIP Department, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China; 2State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China; 3Integrated Traditional Chinese and Western Medicine Research Center, Sun Yat-sen University Cancer Center, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bei Zhang, TCM&VIP Department, Sun Yat-sen University Cancer Center, 651 Dongfeng Road East, Guangzhou, 510060, People’s Republic of China, Tel/Fax +86-20-87342708, Email [email protected]
Background: The ChaiShao Shugan Formula (CSSGF) is a traditional Chinese medicine formula with recently identified therapeutic value in triple-negative breast cancer (TNBC). This study aimed to elucidate the underlying mechanism of CSSGF in TNBC treatment.
Methods: TNBC targets were analyzed using R and data were from The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) databases. The major ingredients and related protein targets of CSSGF were explored via the Traditional Chinese Medicine Systems Pharmacology database, and an ingredient–target network was constructed via Cytoscape to identify hub genes. The STRING database was used to construct the PPI network. GO and KEGG enrichment analyses were performed via R to obtain the main targets. The online tool Kaplan‒Meier plotter was used to identify the prognostic genes. Molecular docking was applied to the core target genes and active ingredients. MDA-MB-231 and MCF-7 cell lines were used to verify the efficacy of the various drugs.
Results: A total of 4562 genes were screened as TNBC target genes. The PPI network consisted of 89 nodes and 845 edges. Our study indicated that quercetin, beta-sitosterol, luteolin and catechin might be the core ingredients of CSSGF, and EGFR and c-Myc might be the latent therapeutic targets of CSSGF in the treatment of TNBC. GO and KEGG analyses indicated that the anticancer effect of CSSGF on TNBC was mainly associated with DNA binding, transcription factor binding, and other biological processes. The related signaling pathways mainly involved the TNF-a, IL-17, and apoptosis pathways. The molecular docking data indicated that quercetin, beta-sitosterol, luteolin, and catechin had high affinity for EGFR, JUN, Caspase-3 and ESR1, respectively. In vitro, we found that CSSGF could suppress the expression of c-Myc or promote the expression of EGFR. In addition, we found that quercetin downregulates c-Myc expression in two BC cell lines.
Conclusion: This study revealed the effective ingredients and latent molecular mechanism of action of CSSGF against TNBC and confirmed that quercetin could target c-Myc to induce anti-BC effects.
Keywords: triple-negative breast cancer, chaishao shugan formula, network pharmacology, molecular docking, c-Myc
Introduction
Breast cancer (BC) is a common cancer among women and has surpassed lung cancer as the most commonly diagnosed cancer; an estimated 2.3 million new cases are diagnosed annually (11.7%).1 BC can be divided into three types according to its immunocytochemical characteristics: HER-2-overexpressing BC, luminal epithelial (luminal) BC, and triple-negative breast cancer (TNBC).2 In addition, TNBC is a more aggressive subtype, constituting 10–15% of patients.3
Currently, the standard treatment for TNBC includes neoadjuvant chemotherapy (NAC), a patterned regimen of taxanes and anthracyclines. As TNBC patients lack expression of the estrogen receptor (ER), progesterone receptor (PR), and HER2 receptor, they are not eligible for hormone or anti-HER2 therapy. Therefore, NAC is effective only in some TNBC patients, and approximately 30–50% of patients develop drug resistance, resulting in decreased overall survival.4 Moreover, NAC treatment results in adverse reactions such as nausea, anorexia, vomiting, diarrhea, constipation, and hair loss.5 Therefore, safer and more effective treatment strategies for TNBC are urgently needed.
Chinese medicine (CM) is based on practical medical experience spanning two thousand years.6 CM refers to drugs used under the guidance of traditional Chinese medical theory. Currently, CM is regarded as a potential drug for cancer treatment. Chinese herbs (CHs) are natural and inexpensive and have few adverse effects. In addition, CHs are effective in treating certain diseases, especially TNBC.7,8 ChaiShao Shugan Formula (CSSGF), a compound of CM prescription, consists of Chaihu (bupleurum root), Baishao (radix paeoniae alba), Fuling (Poria cocos), Baishu (white atractylodes rhizome) and TaiZishen (radix pseudostellariae). The CSSGF prescription is derived from Xiaoyaosan (XYS) and was first applied to treat various diseases in females approximately 700 years ago in China.8 XYS has been used on breast nodules and other estrogen-related diseases, such as BC.9 Given that TNBC has no significant response to estrogen, we revised the XYS prescription to CSSGF by deleting Danggui and adding Taizishen, which has been used clinically for almost 15 years at our hospital. However, the molecular mechanism and biological processes of CSSGF in TNBC need to be clarified. This study focused on discovering the molecular mechanism underlying the multigene regulatory effects of CSSGF on TNBC cells.
Network pharmacology is a novel method based on biological methods that involves the study of the formation of disease, drug characteristics, and drug–disease systems.10,11 This process is used to determine the complex networks among biological processes, drugs, and diseases, which makes it useful for studying CM. Bioinformatics has been successfully used to delineate novel biological mechanisms and drive transitional advances in personalized medicine.12 In this study, bioinformatics analysis and network pharmacology were combined to identify the therapeutic targets of TNBC, identify the ingredients and targets of CSSGF, and construct a network of proteins with CSSGF-TNBC-related targets. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were subsequently performed to uncover the biological mechanisms of CSSGF. In particular, Cytoscape was used to further evaluate the hub genes, and a survival study was performed to determine the interactions among these genes and to predict the prognosis of BC. Finally, molecular docking and experimental data confirmed the hub targets of CSSGF in TNBC. This study offers new insight into the improvement and application of the CSSGF.
Materials and Methods
Ingredient Analysis of the CSSGF
CSSGF was obtained from the Sun Yat-sen University Cancer Center. Briefly, 100 mg of sample (10 g Taizishen (radix pseudostellariae), 10 g ChaiHu (radix bupleuri), 10 g, BaiShao (radix paeoniae albal), 10 g FuLing (Poria cocos) and 10 g BaiZhu (Rhizoma Atractylodis)) was weighed, 500 μL of extract (methanol/water was 4:1, core normal concentration is 10 μg/mL) was added; the sample was vortexed for a total of 30s, homogenized at 45 Hz total 4 mins, and sonicated in ice water bath for 1 h. After incubation at −40°C for 1 h, the sample was centrifuged at 12,000 rpm at 4°C for 15 min; then, the supernatant was filtered through a 0.2 2μm micropore membrane. Liquid chromatography tandem mass spectrometry (LC‒MS/MS) analysis was performed on an ultra high performance liquid chromatography (UHPLC) system (Vanquish, Thermo Fisher Scientific). The flow rate was 0.5 mL/min, and the sample dose was 5 μL. The mobile phase contained 0.1% formic acid in water (A) and acetonitrile (B). The multistep line elution gradient schedule was: 0–11 min, 85–25% A; 11–12 min, 25–2% A; 12–14 min, 2–2% A; 14–14.1 min, 2–85% A; and 14.1–16 min, 85–85% A.
MS and MS/MS data were obtained with an Orbitrap Exploris 120 mass spectrometer and Xcalibur software according to the IDA acquisition mode. Throughout every gain cycle, the amount array was 100–1500, the upper four cycles were selected, and the matching MS/MS data were obtained. The covering gas flow rate was 35 Arb, the aux gas flow rate was 15 Arb, the ion transfer tube temperature was 350°C, the vaporizer temperature was 350°C, the full MS resolution was 60,000, the MS/MS resolution was 15,000, the collision energy was 16/32/48 in the NCE type, and the spray voltage was 5.5 kV (positive)/-4 kV (negative).
Collection and Selection of Candidate Chemical Components of CSSGF
The main chemical component candidates for CSSGF were obtained from the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database (http://lsp.nwu.edu.cn/tcmsp.php). The TCMSP database contains information on the absorption, metabolism, distribution, and drug excretion (ADME) of a wide range of compounds. In this report, the chemical modules of CSSGF were obtained from the literature and two other databases. The selection criteria for the TCMSP database were an oral bioavailability (OB) greater than 30% and a drug likeness (DL) greater than 0.18. In addition, five Lipinski drug traits (molecular weight < 500, <10 hydrogen bond acceptors, fewer than 5 hydrogen bond donors, lipid water partition coefficient <5, and no more than 10 rotatable bonds) were used to identify the effective components of CSSGF from another database called ETCM.
Exploration and Analysis of Compound-Related Targets
The TCMSP database was used to identify and analyze the related targets of the components in CSSGF. The UniProt database (https://www.uniprot.org/) was used to label whole target proteins with Homo sapiens as the selected species. The expected SMILE setups for the selected composites were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov). The targets of the active compounds in CSSGF were predicted with SwissTargetPrediction (http://www.swisstargetprediction.Ch/), and “Homo sapiens” was the source. Targets with a probability greater than 0.5 were selected, duplicates were removed, and the obtained targets were further explored.
Forecast of Helpful Targets in TNBC
The original TNBC target data were extracted from the Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA) databases. The TCGA database was searched using the query “breast cancer”. Gene expression data for BC were obtained from the TCGA database, and 1170 breast cancer samples were downloaded from the database. The GSE39858 dataset (43 samples) derived from the GEO database (Affymetrix GPL4133 platform) was used in this study. To analyze the genetic differences between examples, differentially expressed genes (DEGs) with p < 0.05 were identified via the R package. Latent target genes in TNBC were identified by comparing the two datasets.
Protein–Protein Interaction Data and Network Creation
To further clarify the likely mechanisms of CSSGF against TNBC, the Venny online tool (http://bioinformatics.psb.ugent.be/webtools/Venn/) was used to analyze the similarity between the chosen targets of CSSGF and TNBC targets. In this report, the relationships between usual targets were explored with STRING when the interaction score was greater than 0.4. Cytoscape (version 3.7.2) was applied to construct the network of those targets.
In protein‒protein interaction (PPI) networks, a node characterizes a gene/target/protein/molecule, whereas a connection among nodes symbolizes a link between genes/targets/proteins/molecules. The “degree” rate of the node indicates the number of connections among nodes in this network. The greater the degree value is, the more likely the target is a significant target of a compound.
Estimate of Recognized Hub Genes and Meta-Analysis
Hub genes were selected according to their degree and imaged using Cytoscape (3.7.2) software. K‒M plotter is an online tool (http://kmplot.com/analysis/) used to identify hub genes that can predict patient survival. In all tests, a p value less than 0.05 indicated statistical significance.
GO and KEGG Pathway Enrichment Analysis of the Targets
GO and KEGG enrichment analyses of shared target genes were executed with the Cluster Profiler and Enrich plot package in R software, with FDRs less than 0.05. In addition, SangerBox (http://vip.sangerbox.com/home.html) was used to explore the enrichment between genes and pathways.
Molecular Docking Simulation
The 3D crystal structures of the central targets were obtained from the Protein Data Bank (http://www.rcsb.org/pdb/). Next, all the protein constructions were managed using AutoDock Tools, comprising the elimination of ligands and water molecules, computation of the Gasteiger charge, accumulation of polar hydrogen, and pattern of nonpolar hydrogen. AutoDock Vina software was used to perform the molecular docking. Finally, the receptor–ligand complex was introduced into LigPlot+ software to investigate the hydrogen bonds and hydrophobic interactions between the receptor and ligand.
In vitro Experiment
Reagents and Cell Culture
Quercetin (purity: 98.03%) was diluted in dimethyl sulfoxide (DMSO; HY-18085, MCE, USA) and stored at −20°C.
The MCF-7 cell line and MDA-MB-231 cells were purchased from the National Collection of Authenticated Cell Cultures, Beijing, China.
The cells were cultured in a humidified incubator with 5% CO2 at 37°C in DMEM supplemented with 10% fetal calf serum and 50 U/mL penicillin.
Cell Proliferation Assay
The cells were plated in 96-well microplates (8000 cells/well, 100 μL/well) and cultured for 24 h. The medium was then discarded and replaced with medium containing quercetin (160, 80, 40, 20, 10, or 5 μg/l). After 48 hours of growth, cell viability was tested by a Cell Counting Kit-8 (CCK-8) (Beyotime, Shanghai, China) assay. Six replicates of each sample were subjected to an independent experiment, and the experiment was repeated three times.
Western Blotting
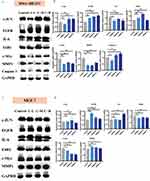
Whole-cell protein lysates were obtained 48 hours after drug treatment as previously reported by utilizing RIPA buffer (Beyotime, Shanghai, China). Proteins from the different groups were separated via electrophoresis on a 10% SDS‒PAGE gel and subsequently transferred to polyvinylidene fluoride (PVDF) membranes (Millipore). The membranes were blocked with 5% skim milk in TBST for 1 h and then incubated overnight with the primary antibody (at 4°C). Then, the membranes were washed with TBST, incubated with secondary antibodies for 1 h at room temperature (RT) and analyzed using an imaging system (LI-COR Biosciences). Each sample was analyzed as a separate independent experiment, and all the WB analyses were repeated three times. Primary antibodies against the following targets were used: C-myc (ab32072) and EGFR (ab52894) (Abcam); IL6 (21865-1-ASP) (Proteintech); MMP1 (AF0209), ESR1 (BF0200) and c-JUN (AF6090) (Affinity Technology); and apopain (caspase-3) (Santa Cruz Biotechnology); and GAPDH (ab8245) (Abcam). The antibodies were diluted with 5% BSA at a ratio of 1:1000.
Statistical Analysis
GraphPad Prism 9.0 software was used for statistical analysis. Student’s t test was used here to compare two groups, while one-way ANOVA was used to compare several groups of data; p < 0.05 indicates that the difference is statistically significant.
Results
CSSGF Quality Inspection
To confirm the quality of the CSSGF, its composition was analyzed using HPLC. A total of 112 compounds in CSSGF were examined by LC–MS/MS (Figure 1a and b).
Differential Gene Expression in TNBC
We identified 6060 differentially expressed genes between healthy individuals and BC patients (based on 112 normal samples and 1070 tumor samples), as shown in Figure 2a and b. Then, we analyzed the GEO dataset GSE38959, which included information on 13 normal samples and 30 tumor samples, and obtained 2540 differentially expressed genes (DEGs) (1319 upregulated genes and 1221 downregulated genes), as shown in Figure 2c and d. Finally, we analyzed the intersecting genes in the GEO and TCGA databases, and 4562 genes were screened, as shown in Figure 2e and f.
Candidate Active Ingredients of CSSGF
The chemical ingredients of CSSGF were obtained from the TCMSP database; an oral bioavailability (OB) of more than 30% and a drug likeness (DL) of more than 0.18 were the screening criteria. Thirteen compounds from Chaihu, seven from Baishao, five from Fuling, four from Baishao, and six from Taizishen were selected for further analyses. These herbs might have some of the same compounds; for example, kaempferol is present in Chaihu and Baishao, and beta-sitosterol is present in Baishao and Taizishen. As presented in Table 1, 33 CSSGF compounds were acquired from the TCMSP.
 |
Table 1 Information on Active Compounds of CSSGF Screened by TCMSP |
PPI Network for the Anti-TNBC Effect of CSSGF and Recognized Hub Genes
In this study, we first performed target screening for 33 active compounds according to their chemical similarities. A total of 192 related targets were obtained through TCMSP and Swiss Target Prediction. To determine the relationships between the possible targets of CSSGF and TNBC-related targets, the Venn tool was used, which identified 93 shared targets between CSSGF and TNBC (Figure 3a). We then performed an ingredient–disease target analysis (Figure 3b).
A novel protein interaction network was created from the STRING database and subsequently constructed to elucidate the latent mechanisms by which CSSGF affects TNBC. Cytoscape was used to visualize the network, and the connectivity of the nodes was proportional to the degree value determined in the pathology analysis. As presented in Figure 3c, the PPI network contained 89 nodes and 845 edges, and the top 30 genes according to degree value are shown in Figure 3d. A topology inquiry showed that IL6, MYC, JUN, CCND1, CASP3, MMP9, ESR1, EGFR, EGF, and PTGS2 were the top ten shared targets in terms of degree centrality (Figure 3e). The degree data for the hub genes are listed in Table 2.
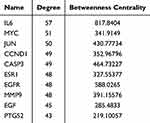 |
Table 2 Top ten Targets Information of PPI Network |
GO and KEGG Pathway Enrichment Analysis of the Effect of CSSGF on TNBC
GO analysis in R was subsequently performed to explore the biological relevance of the identified genes in the CSSGF-TNBC cohort. GO enrichment analysis revealed that these genes were associated with membrane rafts, membrane microdomains, the serine-type peptidase complex, the transcription regulator complex, the chromosomal region, the cyclin-dependent protein kinase holoenzyme complex, and other areas of the cell. The DEGs were involved in energy pathways, apoptosis, metabolism, steroid hormone receptor signaling pathways, neurotransmitter metabolism, the response to xenobiotic stimulus, response to steroid hormones, cellular response to chemical stress, response to metal ions, and other biological processes (Figure 4a and b). Moreover, DNA-binding transcription factor binding, transcription coregulator binding, transcription coactivator binding, RNA polymerase II-specific DNA-binding transcription factor binding, serine-type endopeptidase activity, and kinase regulator activity were the main enriched molecular functions of CSSGF-related genes in TNBC (Figure 4c).
To determine the underlying mechanism of the antitumor effect of CSSGF in TNBC, we performed a KEGG pathway enrichment analysis of 93 targets. The top 20 pathways were selected (Figure 4d); the pathways included the TNF, IL-17, and kappa B and miRNAs in cancer signaling pathways. Using the online SangerBox tool, we determined the relationships between the top 20 KEGG pathways and TNBC-related genes, as shown in Figure 4e.
Survival Analysis and CSSGF Target Proteins
The predictive value of the hub genes was analyzed via Kaplan–Meier plotter. As indicated in Figure 5a-h, JUN, EGFR, IL-6, PTGS2, and ESR1 were favorable prognostic factors, while c-Myc, matrix metallopeptidase (MMP)1, and apopain were poor prognostic factors of BC, but additional studies are needed to verify these associations; moreover, these genes might be regarded as targets by which CSSGF induces anticancer effects in upcoming research.
Furthermore, we performed a Western blot analysis of the proteins harvested after 48 hours of treatment with the CSSGF compound. As indicated in Figure 6a and b, compared with that in the control group, the expression of c-JUN and EGFR was increased, while the expression of c-Myc and MMP1 was decreased in the CSSGF-treated group of MDA-MB-231 cells (p < 0.05).
As indicated in Figure 6c and d, the expression of c-Myc was lower in the CSSGF-treated group than in the control group of MCF-7 cells. However, the expression of EGFR was increased in the low- and medium-dose CSSGF groups compared to the control group in MCF-7 cells (p < 0.05).
Molecular Docking Proof
Then, we used molecular docking to identify the link between the bioactive ingredients of CSSGF and the hub genes. The candidate active ingredients quercetin, beta-sitosterol, luteolin, and catechin, as well as the potential curative targets CASP3, ESR1, EGFR, c-Myc, JUN, and PTGS2, were assessed by network pharmacology. The results revealed that there was a strong binding effect between the ingredients and target proteins, and the binding energies were all <−7 kcal/mol. The strongest interaction types and the specifics of the binding energies are presented in Figure 7a–f. According to our network analysis and molecular docking, the most reliable predicted CSSGF component–hub gene interactions were quercetin with EGFR and MYC, beta-sitosterol with JUN, and luteolin with CASP3; these interactions are strictly linked to apoptosis and the development of vascular mimicry in TNBC.
Quercetin Decreases Cell Viability and Downregulates the Expression of c-Myc
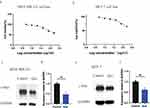
The MDA-MB-231 and MCF-7 cell lines were used to verify the effects of apoptosis via the CCK-8 assay. As shown in Figure 8a and b, the key ingredient of CSSGF, quercetin, decreased cell viability both in the MDA-MB-231 cell line and in the MCF-7 cell line (p < 0.01).
Figure 8c and d shows that the protein expression of c-Myc was downregulated in the quercetin groups compared with the control group in the MDA-MB-231 cell line (p < 0.01). Moreover, Figure 8e and f shows that the protein expression of c-Myc was downregulated in the quercetin groups compared with the control group in the MCF-7 cell line (p < 0.01).
Discussion
CM has been used as a cancer treatment for thousands of years, and its influence has been broadly recognized.13 Generally, CMs originate from plants, are easy to administer, and have fewer adverse effects on cancer treatment.14 In the practice of CM, the common phrase for breast cancer is “breast rock”.15 CSSGF evolved from XYS, which consists of Chaihu (bupleurum root), Baishao (Radix paeoniae alba), Fuling (Poria cocos), Baizhu (white atractylodes rhizome), and TaiZishen (Radix pseudostellariae). It can cause liver qi and resolve depression and is frequently applied as a fundamental treatment for BC. This study aimed to reveal the previously undetermined role of CSSGF and the possible underlying mechanism in BC treatment.
Using the TCGA and GEO databases, we first identified 4562 differentially expressed genes in TNBC vs normal samples, which is a large sample size for reliable data analysis. Then, 33 chemical components of CSSGF were identified via the TCMSP database; these included the key chemical constituents quercetin, beta-sitosterol, luteolin, kaempferol paeoniflorin, and catechin. These core chemical constituents could work on various targets in a network containing additional active components. Among the anticipated active ingredients, quercetin, beta-sitosterol, luteolin, and catechin have been shown to have therapeutic effects on TNBC. Quercetin can prevent cytoplasmic HuR protein release, suggesting that quercetin is an anticancer agent for aggressive TNBCs.16 In addition, quercetin inhibits the proliferation and invasion of TNBC cells by downregulating the expression of MMP2 and MMP9.17,18 Luteolin, another main ingredient of CSSGF, was reported to inhibit MDA-MB-231 cell stemness and increase chemosensitivity.19 In addition, beta-sitosterol affects several cell signaling pathways, including the cell cycle, proliferation, angiogenesis, apoptosis, and anticancer pathways, especially in TNBC cells.20,21 These findings suggest that these components may be critical for the therapeutic effects of CSSGF.
According to the GO and KEGG analyses, CSSGF targets several tumor-related pathways, such as the IL-17, TNF, and NF kappa B signaling pathways. In addition, the network pharmacology analysis shown CSSGF was closely related to BC. Thus, we believe that CSSGF is effective at treating tumors, especially BC. Based on the target ingredient and pathway analysis, these constituents could be critical for the beneficial effect of CSSGF in TNBC and are worthy of further investigation.
Furthermore, we focused on the target analysis of CSSGF in TNBC, as shown in Figure 3. IL6, c-Myc, JUN, CCND1, Caspase-3, MMP9, ESR1, EGFR, EGF, and PTGS2 were the ten hub genes closely associated with the pathogenesis of TNBC. c-Myc plays a crucial role in the progression of TNBC.22 It is well known that c-Myc participates in several physiological processes, including proliferation, apoptosis, and differentiation.23 Targeting c-Myc is a useful strategy for treating TNBC.24 Caspase-3 is an important protein involved in the intrinsic pathway of apoptosis.25 Caspase-3 mediates GSDME-induced pyroptosis in BC cells via the ROS/JNK signaling pathway, which is important for treatment.26,27 EGFR, an oncogene and the primary recognized tyrosine kinase receptor, is involved in various biological processes, such as cell proliferation, differentiation, apoptosis, and transcription. EGFR is also a well-known curative target for TNBC.28,29 Similarly, Livasy reported that 70–78% of patients with TNBC highly express EGFR. Hence, EGFR might be a possible therapeutic target in TNBC.30 PTGS2 is related to amplified cell adhesion, phenotypic changes, resistance to apoptosis, and tumor angiogenesis. Moreover, PTGS2 is a significant factor in the construction of prostaglandin E2 (PGE2), which plays a key role in the recurrence of TNBC and may be useful in deciding treatment strategies for TNBC. Treatment strategies targeting PTGS2 appear to be promising for this aggressive disease.31,32 The estrogen receptor 1 (ESR1) level is correlated with the proliferation and differentiation of target tissues. In addition, TNBC cells exhibit greater resistance to radiation than luminal-type BC cells do, and ESRs mediate the radiosensitivity of TNBC cells.33,34 Based on these data, we concluded that CSSGF may target these genes in patients with TNBC.
Survival analysis revealed a lower survival rate in the higher c-Myc subgroup and lower EGFR subgroup in the TNBC patients, which means that high c-Myc expression and lower EGFR expression was poor prognostic factors in the TNBC patients. Furthermore, we did the WB to figure out the possible drug effect of CSSGF on TNBC. We found that CSSGF downregulated the expression of c-Myc and upregulated the expression of EGFR in both the MDA-MB-231 and MCF-7 cell lines (Figure 6). Based on the survival analysis and WB results, we speculated that CSSGF suppressed the expression of c-Myc and enhanced the expression of EGFR to increase the TNBC patients survival rate. Furthermore, we thought that patients with high c-Myc expression or lower EGFR expression could benefit from CSSGF treatment.
The molecular docking results could be applied to assess CSSGF in the context of the basic CM Jun-Chen-Zuo-Shi theory. According to our molecular docking data, quercetin can target EGFR and c-Myc, beta-sitosterol can target JUN, luteolin can target Caspase-3, and catechin can target ESR1. According to the ingredient analysis of CSSGF, quercetin was the main ingredient of Chaihu, beta-sitosterol was the main ingredient of Baishao and Taizishen, catechin was the main ingredient of Baishao, and luteolin was the main ingredient of Taizishen. These results suggested that Chaihu was the Jun drug, Baishao served as the Chen drug, Taizishen served as the Zuo drug, and Fuling and Baizhu were the Shi drugs used in the CSSGF, perfectly fitting the basic CM (Jun-Chen-Zuo-Shi) theory.
Quercetin, the key ingredient of CSSGF, could induce TNBC cell apoptosis by downregulating the expression of c-Myc. As quercetin is the key ingredient of Jun, we used it to confirm the molecular docking data. As shown in Figure 8, quercetin decreased the viability of both MDA-MB-231 and MCF-7 cells. Apoptosis can lead to a decrease in cell viability. Based on our data, we speculated that quercetin may promote apoptosis to affect cell viability. Mechanistically, we found that quercetin decreased c-Myc expression in two BC cell lines, which was consistent with the molecular docking results. However, we did not found the exact effect of quercetin on EGFR(data not shown). Based on previous data, we concluded that quercetin, as the key ingredient of CSSGF, could induce TNBC cell apoptosis by downregulating the expression of c-Myc.
Based on the network pharmacology and molecular docking results, we concluded that, through direct or indirect interactions, CSSGF could have an anti-TNBC effect on multiple targets and multiple pathways. Through cell experiments, we found that CSSGF could suppress the expression of c-Myc or promote the expression of EGFR to improve TNBC patient survival. Moreover, quercetin, which is the key ingredient of Jun drug (Chaihu), decreased the expression of the c-Myc protein to induce the apoptosis of TNBC cells.
Conclusion
Our study indicated that CSSGF could suppress the expression of c-Myc or promote the expression of EGFR to improve TNBC patient survival. Moreover, quercetin might be the key ingredient of the Jun drug (Chaihu), which can decrease the expression of the c-Myc protein to induce the apoptosis of TNBC cells.
Data Sharing Statement
Data used to support the findings of this study are available from the corresponding author upon request.
Ethics Approval and Consent to Participate
This study already get the approval of Sun Yat-sen University Cancer Center IRB for the exempt from review, based on the following reason:1.Do not deviate from or make changes to the study protocol without prior written IRB approval.2.Before the principle investigator, project source, protocol, informed consent form, questionnaire and recruitment materials change, application for protocol amendment should be submitted to the IRB, which will view the proposal again. As this study did not involve animal experiments, animal ethical approval was not applicable.
Funding
This study was financially supported by the Science and Technology Foundation in Guangzhou (2023A04J2147 to TF), Key Research and Development Plan for Science and Technology in Guangzhou(202206080012 to BZ),National Natural Science Foundation of China (82104951 to YYH).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Ca a Cancer J Clinicians. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Katsura C, Ogunmwonyi I, Kankam HK, et al. Breast cancer: presentation, investigation and management. Br J Hosp Med. 2022;83(2):1–7. doi:10.12968/hmed.2021.0459
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi:10.3322/caac.21551
4. Chaudhary LN, Wilkinson KH. Triple-Negative Breast Cancer: who Should Receive Neoadjuvant Chemotherapy? Surgical Oncol Clin North Am. 2018;27(1):141–153. doi:10.1016/j.soc.2017.08.004
5. Di Maio M, Gallo C, Leighl NB. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J clin oncol. 2015. doi:10.1200/JCO.2014.57.9334
6. Qing J, Fangshi Z, Xuan L, et al. Recent Advance in Applications of Proteomics Technologies on Traditional Chinese Medicine Research. Evidence-Based Complementray Altern Med. 2015:983139. doi:10.1155/2015/983139
7. Chen J, Qin Y, Sun C, et al. Clinical study on postoperative triple-negative breast cancer with Chinese medicine: study protocol for an observational cohort trial. Medicine. 2018;97(25):e11061. doi:10.1097/MD.0000000000011061
8. Li F, Shi Y, Zhang Y, et al. Investigating the mechanism of Xian-ling-lian-xia-fang for inhibiting vasculogenic mimicry in triple negative breast cancer via blocking VEGF/MMPs pathway. ChinMed. 2022;17(1):44. doi:10.1186/s13020-022-00597-5
9. Chen JL, Chang CJ, Wang JY, et al. In Vitro and In Vivo Effects of Jia-Wei-Xiao-Yao-San in Human Breast Cancer MCF-7 Cells Treated With Tamoxifen. Integr Cancer Ther. 2014;13(3):226. doi:10.1177/1534735414520970
10. Li X, Wei S, Niu S, et al. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis. Comput. Biol. Med. 2022;144:105389. doi:10.1016/j.compbiomed.2022.105389
11. Zhou W, Zhang H, Wang X, et al. Network pharmacology to unveil the mechanism of Moluodan in the treatment of chronic atrophic gastritis. Phytomedicine. 2021;95:153837. doi:10.1016/j.phymed.2021.153837
12. Wu D, Wang X. Application of clinical bioinformatics in lung cancer-specific biomarkers. Cancer Metastasis Rev. 2015;34(2):209–216. doi:10.1007/s10555-015-9564-2
13. Xiang Y, Guo Z, Zhu P, et al. Traditional Chinese medicine as a cancer treatment: modern perspectives of ancient but advanced science. Cancer Med. 2019;8(5):1958–1975. doi:10.1002/cam4.2108
14. Xu Z, Jin Y, Gao Z, et al. Autophagic degradation of CCN2 (cellular communication network factor 2) causes cardiotoxicity of sunitinib. Autophagy. 2021:1–22. doi:10.1080/15548627.2021.1965712
15. Zhang Z, Liu J, Liu Y, et al. Virtual screening of the multi-gene regulatory molecular mechanism of Si-Wu-tang against non-triple-negative breast cancer based on network pharmacology combined with experimental validation. J Ethnopharmacol. 2021;269:113696. doi:10.1016/j.jep.2020.113696
16. Umar SM, Patra S, Kashyap A, et al. Quercetin Impairs HuR-Driven Progression and Migration of Triple Negative Breast Cancer (TNBC) Cells. Nutr Cancer. 2022;74(4):1497–1510. doi:10.1080/01635581.2021.1952628
17. Gol M, Kheirouri S. The Effects of Quercetin on the Apoptosis of Human Breast Cancer Cell Lines MCF-7 and MDA-MB-231: a Systematic Review. Nutr Cancer. 2022;74(2):405–422. doi:10.1080/01635581.2021.1897631
18. Ozkan E, Bakar-Ates F. Potentiation of the effect of Lonidamine by Quercetin in MCF-7 human breast cancer cells through downregulation of MMP-2/9 mRNA Expression. Acad Bras Cienc. 2020;92(4):
19. Tsai KJ, Tsai HY, Tsai CC, et al. Luteolin Inhibits Breast Cancer Stemness and Enhances Chemosensitivity through the Nrf2-Mediated Pathway. Molecules. 2021;26(21):6452. doi:10.3390/molecu-les26216452
20. Khan Z, Nath N, Rauf A, et al. Multifunctional roles and pharmacological potential of β-sitosterol: emerging evidence toward clinical applications. Chem Biol Interact. 2022;365:110117. doi:10.1016/j.cbi.2022.110117
21. Wang KN, Hu Y, Han LL, et al. Salvia chinensis Benth Inhibits Triple-Negative Breast Cancer Progression by Inducing the DNA Damage Pathway. Front Oncol. 2022;12:882784. doi:10.3389/fonc.2022.882784
22. Wu SY, Xiao Y, Wei JL, et al. MYC suppresses STING-dependent innate immunity by transcriptionally upregulating DNMT1 in triple-negative breast cancer. J Immunother Cancer. 2021;9(7):e002528. doi:10.1136/jitc-2021-002528
23. Soucek L, Evan GI. The ups and downs of Myc biology. Curr Opin Genet Dev. 2010;20(1):91–95. doi:10.1016/j.gde.2009.11.001
24. Li J, Gao X, Zhang Z, et al. CircCD44 plays oncogenic roles in triple-negative breast cancer by modulating the miR-502-5p/KRAS and IGF2BP2/Myc axes. Mol Cancer. 2021;20(1):138. doi:10.1186/s12943-021-01444-1
25. Beroske L, Van den Wyngaert T, Stroobants S, et al. Molecular Imaging of Apoptosis: the Case of Caspase-3 Radiotracers. Int J Mol Sci. 2021;22(8):3948. doi:10.3390/ijms22083948
26. Zhang Z, Zhang H, Li D, et al. Caspase-3-mediated GSDME induced Pyroptosis in breast cancer cells through the ROS/JNK signalling pathway. J Cell & Mol Med. 2021;25(17):8159–8168. doi:10.1111/jcmm.16574
27. Kim HR, Cho YS, Chung SW, et al. Caspase-3 mediated switch therapy of self-triggered and long-acting prodrugs for metastatic TNBC. J Control Release. 2022;346:136–147. doi:10.1016/j.jconrel.2022.04.014
28. Anbuselvam M, Easwaran M, Meyyazhagan A, et al. Structure-based virtual screening, pharmacokinetic prediction, molecular dynamics studies for the identification of novel EGFR inhibitors in breast cancer. J Biomol Struct Dyn. 2021;39(12):4462–4471. doi:10.1080/07391102.2020.1777899
29. Yin L, Duan JJ, Bian XW, et al. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22(1):61. doi:10.1186/s13058-020-01296-5
30. Livasy CA, Karaca G, Nanda R, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19(2):264–271. doi:10.1038/modpathol.3800528
31. Freitas-Alves DR, Vieira-Monteiro HA, Piranda DN, et al. PTGS2 polymorphism rs689466 favors breast cancer recurrence in obese patients. Endocr Relat Cancer. 2018;25(3):351–365. doi:10.1530/ERC-17-0374
32. Mori N, Mironchik Y, Wildes F, et al. HIF and COX-2 expression in triple negative breast cancer cells with hypoxia and 5-fluorouracil. Curr Cancer Rep. 2020;2(1):54–63. doi:10.25082/CCR.2020.01.005
33. Chen X, Ma N, Zhou Z, et al. Estrogen Receptor Mediates the Radiosensitivity of Triple-Negative Breast Cancer Cells. Med Sci Monit. 2017;23:2674–2683. doi:10.12659/msm.904810
34. Christopoulos PF, Vlachogiannis NI, Vogkou CT, et al. The Role of the Androgen Receptor Signaling in Breast Malignancies. Anticancer Res. 2017;37(12):6533–6540. doi:10.21873/anticanres.12109
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.