Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Unveiling a Shared Precursor Condition for Acne Keloidalis Nuchae and Primary Cicatricial Alopecias
Authors Umar S , Ton D, Carter MJ , Shitabata P
Received 12 June 2023
Accepted for publication 18 August 2023
Published 25 August 2023 Volume 2023:16 Pages 2315—2327
DOI https://doi.org/10.2147/CCID.S422310
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Sanusi Umar,1– 3 Donna Ton,3 Marissa J Carter,4 Paul Shitabata1,2,5
1Department of Medicine, University of California at Los Angeles, Los Angeles, CA, USA; 2Division of Dermatology, Department of Medicine, Harbor-UCLA Medical Center, Torrance, CA, USA; 3Dr. U Hair and Skin Clinic, Manhattan Beach, CA, USA; 4Strategic Solutions, Inc., Bozeman, MT, USA; 5Dermatopathology Institute, Torrance, CA, USA
Correspondence: Sanusi Umar, Dr. U Hair and Skin Clinic, 2121 N. Sepulveda Boulevard, Suite 200, Manhattan Beach, CA, 90266, USA, Tel +1-310-318-1500, Fax +1-310-318-1590, Email [email protected]
Purpose: Small observational studies suggest subclinical disease occurrence in the normal-appearing scalp zones of several primary cicatricial alopecias. To aid patient management, we began routinely evaluating the entire scalp of patients with acne keloidalis nuchae (AKN), including trichoscopy-guided biopsies.
Patients and Methods: This retrospective study evaluated 41 patients sequentially presenting with AKN at a single clinic between June and December 2022. Primary lesions and normal-appearing scalp in the superior parietal scalp at least 5 cm away from AKN-affected zones were clinically evaluated, and areas showing perifollicular erythema or scales/casts on trichoscopy were biopsied and histologically analyzed.
Results: Forty-one men with AKN, including 20 men of African descent, 17 Hispanic, and 4 European-descended Whites, were evaluated. All patients, including 22% with associated folliculitis decalvans, showed scalp-wide trichoscopy signs of perifollicular erythema or scaling in normal-appearing scalp areas. All patients showed histologic evidence of perifollicular infundibulo-isthmic lymphocytoplasmic infiltrates and fibrosis (PIILIF), with 96% showing Vellus or miniaturized hair absence. PIILIF was often clinically mistaken for seborrheic dermatitis (44– 51%). All White patients had mild papular acne keloidalis nuchae lesions mistaken for seborrheic dermatitis.
Conclusion: PIILIF may be a precursor to a wide spectrum of primary cicatricial alopecias, including AKN and folliculitis decalvans. This finding carries implications for the early diagnosis and management of AKN and other primary cicatricial alopecias.
Plain Language Summary: Acne keloidalis nuchae (AKN) is a type of hair loss and scalp condition marked by scarring and inflammation. This condition falls under a group of chronic hair and scalp issues known as primary cicatricial alopecia (PCA). Current treatments for AKN and similar PCAs often do not work well, and the condition tends to return. We have found a hidden scalp condition that could be causing AKN and other PCAs. It’s a subtle disease that affects the entire scalp, even though it might not show noticeable symptoms. We have observed this condition in all 41 AKN patients in our study, and it’s characterized by certain changes in the hair and scalp’s structure and immune system response. Other studies have linked this condition to various other PCAs. We believe this hidden condition could be causing AKN and making it come back after treatment. This study suggests that treating AKN might require a broader approach beyond just treating the visible symptoms. Since this hidden condition exists in other PCAs, it might be a common cause.
Keywords: folliculitis decalvans, lichen planopilaris, subclinical, central centrifugal cicatricial alopecia, frontal fibrosing alopecia, scarring alopecia
Introduction
Acne keloidalis nuchae (AKN) is a form of primary cicatricial alopecia (PCA) that develops mainly in the occipital area of men of color1,2, but rarely in women3 and European-descended White men,4 AKN is characterized by scattered inflamed papules in the early stages, progressing to suppurative papules and nodules that merge to form plaques or masses, with associated hair loss and multi-shafted hair tufting.1,2,5,6 It is histologically characterized by lamellar fibroplasia, mixed inflammatory cells consisting of neutrophils, lymphohistiocytic, and plasma cells mainly involving the isthmus and infundibulum, signs of foreign body reactions to detached hair shafts, and ruptured hair follicles.7 Precipitating factors include scalp microtrauma, hairline carving, and friction from shirt collars and pillows.1,8 The cause of AKN remains unclear and without effective treatments. Although not a true keloidal condition, AKN can form at surgical sites or in new areas separated from the parent lesions.1,7
Previously, a small, prospective, blinded study of histologic material collected from 10 patients with mild AKN6 suggested that an early subclinical disease characterized by perifollicular infundibulo-isthmic lymphocytoplasmic infiltrates lamellar fibrosis (PIILIF), and sebaceous gland destruction may predate classical AKN lesions.6 The finding of a scalp-wide subclinical pathologic condition may influence our understanding of AKN and result in more effective treatments. As such, our clinic began routinely screening the entire scalps of patients with AKN via trichoscopy9,10 for scalp diseases in normal-appearing-scalp (NAS) zones to optimize therapy and prevent a recurrence. Here, we report a retrospective analysis of our experiences.
Materials and Methods
Study Design and Population
All patients sequentially presenting with clinically diagnosed AKN to our Los Angeles area clinic who had their NAS biopsied between June and December 2022 were included. All procedures were performed in accordance with the Declaration of Helsinki (as was revised in 2013). Written informed consent was obtained from patients, including consent for publication and use of photographs. An institutional review board exemption was obtained from the Western Institutional Review Board (IRB) Copernicus Group, which has IRB authority over the site of the study and the source of all patient data – Dr. U Hair and Skin Clinic. The exemption was issued because the study uses deidentified data from procedures that are part of routine clinical care, with no plans to re-identify or contact the subjects.
Study Procedures
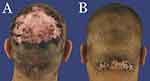
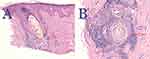
As part of routine clinical care, all patients underwent examination of their NAS zones, which included gross examination for perifollicular scales and erythema to direct further trichoscopy evaluation. In the right or left upper parietal areas, trichoscopy-selected points with perifollicular erythema or cast scaling were biopsied at least 5 cm away from AKN-affected zones (Figure 1A and B). Both vertical and horizontal sections were examined for histologic analysis of NAS biopsies. Diagnosis of AKN (Figures 1A and 2A and B) and folliculitis decalvans (Figures 1A and 2B) was done by clinical examination or previous histopathology reports. However, biopsies were performed in case of uncertain diagnosis or as a post-surgical routine.
Data Collection and Analysis
Collected clinical data included patient age, self-reported race, and sex; the duration of AKN/FD disease (if known), morphology, location, and AKN lesion classification4 and the presence of FD; clinical symptoms in the AKN, FD, and NAS zones, including whether the patient was aware of the AKN or FD; past or concurrent skin conditions of interest (eg, seborrheic dermatitis); history of traumatic hair grooming practices (eg, chemical texturizers, hair dyes, hot combs); the presence of symmetrical non-scarring alopecia signifying androgenic alopecia (AGA) graded using the Norwood scale; and past treatments/interventions and their outcomes.
Histologic data included the presence of inner root sheath desquamation, desquamation without inflammation, Max Joseph spaces, squamatized basal layers, Lichen planus interfollicular changes, absence of sebaceous glands, and absence of Vellus or miniaturized hairs. The presence of fibrosis or lymphocytic, neutrophilic, lymphohistiocytic, and plasma infiltrates in the perifollicular-infundibular, perifollicular-isthmus, perifollicular-bulbar, and interfollicular aspects was noted, as were foreign body granulomas and released hair shafts. Additionally, we examined total hair count, including anagen, catagen/telogen, and vellus/miniaturized hair from patients with documented data who underwent biopsies of both their NAS and AKN zones.
To further evaluate the pathologies of NAS lesions and their relationship to AKN or FD and gain favorable treatment insight, we began assessing CD4 and CD8 immunohistochemistry and CD117 expression analysis for mast cell activity in some patients. Furthermore, because of the common complaints of seborrheic dermatitis, we also performed periodic acid–Schiff (PAS) staining to detect pityrosporum or Demodex in some patients.
The histology and CD4/CD8/CD117 data (positive/negative) were compared between matched AKN and NAS biopsy datasets using the chi-squared test (PASW 28, IBM) and raw p-values adjusted for type I error using the step-up Hochberg procedure.
Results
Forty-one men (mean age, 34.2 years, range 22–57) with AKN were included. Four patients were European-descended Whites (10%), and the remaining patients were either of African descent (n=20, 49%) or Hispanic (n=17, 41%). AKN lesion characteristics were as follows: Class I, n=8, 20%); Class II, n=17, 41%; Class III, n=11, 27%; and Class IV, n=5, 12%. Table 1 summarizes patient demographic and clinical data.
 |
Table 1 Associated Medical Conditions and Past Treatments |
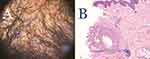
Clinically, perifollicular erythema, with or without scales, is the most common trichoscopy indicator of PIILIF. (Figure 3A and B). All specimens taken from the NAS zones showed histological features of PIILIF (Figure 4A and B), with 96% showing Vellus or miniaturized hair absence. All PIILIF diagnoses were made during the first biopsy attempt, except in one patient whose diagnosis required a second biopsy. Of all patients, 9 (22%) had FD lesions in their vertex zones (Table 1). Thirty-six (88%) patients had prior AKN treatments involving either systemic/topical antibiotics (63%) or steroids (21%; topical, injections, or both) (Table 1). The most common prior AKN treatments were surgery (40%), laser hair removal (24%), or topical multimodal solutions (38%). At presentation, 36% of all patients had a history of AKN recurrence or disease expansion post-treatment. None of the patients had a history of traumatic hair grooming practices, keloids, or hidradenitis suppurativa.
Although most patients were unaware of a disease condition in their NAS zones, 60% reported itchiness with 18/41 (44%) assumed to have seborrheic dermatitis or receiving treatment for it (n=21, 51%), which was noted as PIILIF on histology. No patient had seborrheic dermatitis on histologic examination. Patients commonly reported more severe symptoms in AKN sites, with only 25–35% of these symptoms affecting NAS areas (Table 2). A third of the patients had associated symmetric hair loss clinically consistent with AGA, which they self-reported as male-pattern baldness. Of the nine patients with coexisting FD and AKN, FD was diagnosed much later than AKN; however, the exact interval was unknown.
 |
Table 2 Scalp Clinical Characteristics |
In descending order, the most notable differences in the histological results (Table 3) of the biopsies of AKN vs NAS (PIILIF) that highly favored an AKN diagnosis over PIILIF are (1) lymphohistiocytic infiltrates and granulomas, which were never found in any PIILIF specimen; (2) fibrosis or inflammatory cells outside the follicular zones (absent in PIILIF); (3) released follicles (rare −4% of PIILIF); (4) neutrophilic infiltrates anywhere in the specimen (rare −4% of PIILIF); (5) peri-bulbar fibrosis or inflammatory cells infiltrates (occurred in 29% of PIILIF); (6) the presence of plasma cells (found in 50% of the cases of PIILIF for infundibular and isthmic aspects of perifollicular changes); and (7) absence of sebaceous glands (absent in 45% of cases of PIILIF), suggesting that the least sensitive histologic differentiator between AKN and PIILIF are the presence of plasma cells and the absence of sebaceous glands (Table 3). AKN and PIILIF are histologically indistinguishable by the following: (1) infundibulo-isthmic lymphocytic infiltrates (100% concordance); (2) infundibulo-isthmic fibrosis (100% concordance); (3) absence of Vellus or miniaturized hair (occurring in ≥90% of AKN and PIILIF patients); and (4) root sheath desquamation usually occurring over half the time in both conditions in the presence of inflammatory cells. Max Joseph features and squamatized basal layers were three times more frequent in PIILIF than in AKN and FD, likely due to effacement of the affected zones by severe neutrophilic inflammation in AKN and FD.
 |
Table 3 Histological Analysis of the Biopsies for AKN, Healthy Scalp (NAS), and FD |
Among 12 patients with paired biopsies of NAS and AKN zones, featuring recorded hair counts per 4mm punch biopsy sections, NAS exhibited a significantly higher mean hair count (15.9 vs 10.0 in AKN, p=0.036), and anagen: catagen-telogen ratio (6.4 vs 1.7 in AKN, p=0.001).
Based on immunohistochemistry analysis, AKN and NAS showed CD4>CD8 ratios. The CD117 maker for mast cells was ubiquitously present in AKN and NAS specimens. A similar trend was found in FD lesions, although very few FD specimens were analyzed (Table 4).
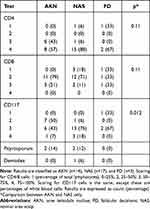 |
Table 4 Immunohistochemistry (CD4 and CD8 Cells), Mast Cell Activity (CD117 Cells), and Pityrosporum/Demodex Tests |
Discussion
Our finding of PIILIF in trichoscopy-guided NAS biopsies in all 41 AKN patients suggests a strong association between the two conditions and the presence of a subclinical disease state (PIILIF) predating AKN. PIILIF was not caused by using chemical texturizers, hot combs, braiding, or other traumatic hair grooming practices since there was no significant history of such involving the NAS of our patient cohort. Our finding of PIILIF in 100% of the cohort contrasts with that of Sperling et al,6 who found PIILIF in 6/10 (60%) patients prospectively studied. Apart from its small sample size, the study did not use trichoscopy to guide its biopsies; rather, biopsies were blindly taken from an area 4 cm above ear level in the parietal zone.6 In addition, Vellus or miniaturized hairs were not investigated, which were noticeably absent from 96% of NAS, 92% of AKN, and 100% of FD specimens in our study. Although they reported reduced sebaceous glands, we could not evaluate this finding in our study as it would have required comparing previously matched biopsies with current ones to assess interval changes. Furthermore, we did not observe definite histological evidence of sebaceous gland destructions in the NAS zones. Our findings suggest that the absence of vellus or miniaturized hair is a more consistent finding than sebaceous gland reduction in AKN-associated PIILIF. Furthermore, the significant difference in sebaceous gland loss between AKN (75%) and PIILIF (42%) biopsies suggests that the destruction of vellus/miniaturized hairs is an earlier feature of AKN than sebaceous gland destruction, which occurs after the disease is fully established. Considering the prevailing view of sebaceous glands having a central role in PCA pathogenesis,11 our finding could result from Vellus or miniaturized hairs being more susceptible to destruction than sebaceous glands. Furthermore, our study revealed a significant reduction in anagen: telogen-catagen ratios, concomitant with a significant decrease in hair count observed between specimens from NAS vs AKN. From our experience, it is critical to evaluate both vertical and transverse sections of the biopsy specimens as some features of PIILIF are best seen in the vertical section (perifollicular lymphocytic infiltrates) than in the transverse section and vice versa for perifollicular fibrosis). In our report, we did not assign a name to the condition in the NAS but used the descriptive term - PIILIF. We did not call it lichen planopilaris (LPP) because biopsies were taken from NAS zones, where alopecia (required to diagnose LPP) was clinically absent.
Sperling et al hypothesized that abnormal histologic changes in the NAS and perilesional biopsies were the same as those of AKN papules, albeit less severe.6 Our finding of PIILIF in five patients’ mild nuchal AKN papules supports this hypothesis. Thus, in contrast to the 2003 North American Hair Research Society’s classification of AKN as a mixed lymphocytic PCA,12 our study and that of Sperling et al suggest that AKN is a lymphocytic PCA that is affected in later stages by secondary influences (eg, microbial and mechanical stimuli) leading to a chronic inflammatory and mixed cellular response manifesting as suppurative lesions. Accordingly, AKN histology is PIILIF with secondary features of neutrophilic and granulomatous reactions.
While PIILIF in NAS may be clinically asymptomatic and may not histologically indicate AKN severity in the affected area, these observations may represent early-stage/mild AKN. Repeated treatments may disrupt the progression.6 However, to what extent medications use influences PIILIF progression to a more advanced condition or the absence of symptoms remains unknown.
The prevalence of combined FD and AKN in our study population was 22%, consistent with other reports that associates found FD in 6.5% (7/108),4 7% (3/42 patients),13 and 21% (12/57 patients)14 of AKN patients. Although the number of histologic specimens for FD was too small to draw conclusions, our findings mirrored those of AKN in all aspects (Table 3). This supports the notion that both diseases are histologically indistinguishable and the two can be differentiated through clinical findings based on scalp location (AKN predominantly on the nape; FD on the crown) and the presence or absence of neutrophilic response mediators (ie, Staphylococcus aureus in FD and not in AKN).14 We observed PIILIF in NAS specimens of all the patients with combined AKN-FD. Furthermore, another recent non-trichoscopy-guided NAS biopsy study of 25 patients with FD found an association between FD and PIILIF-like features in NAS, including perifollicular infundibula-isthmic lymphocytic inflammation (in 64%) and fibrosis (in 48%).15 Consequently, PIILIF may be a precursor of both AKN and FD. We also propose that the entities thus far reported as FD-LPP phenotypes16–18 are likely FD-associated with scalp-wide PIILIF, which in the vicinity of an FD lesion is compounded by the FD-induced alopecia. In those FD-LPP studies, biopsies and clinical assessments were confined to FD lesion vicinities in which alopecia had already resulted from the FD.16–18 Histologically and clinically, PIILIF lesions in an area already manifesting signs of FD-induced cicatricial alopecia will be indistinguishable from a classic LPP lesion; however, further investigation is needed to support our hypothesis.
Given that FD alopecia is typically present on the crown, we propose that genetically predisposed individuals with PIILIF lesions in the vertex zone get influenced by the host’s immune profile and microbiome favor a neutrophilic response (eg, from interleukin 8), accentuated by bacterial superantigen reaction (typically S. aureus).15,18 Furthermore, because AKN and FD in men are PCAs that occur after the onset of puberty,1–4,6,7 androgens and local factors (eg, dihydrotestosterone [DHT] or DHT receptor-related cofactors) likely have a role in determining the phenotypic endpoint of their progenitor—PIILIF. Notably, both androgen receptors and 5a-reductase 1 and 2 enzymes responsible for converting testosterone to DHT are expressed at higher levels in the crown than in the occipital zones.19 As FD primarily affects the crown, this may be more noticeable where there are more DHT-sensitive follicles. Similarly, frontal fibrosing alopecia (FFA) consistently manifests in AGA-prone zones responding to anti-DHT therapeutic agents.20–22
Anecdotally, we observed perifollicular erythema and scaling features in most of our patient cohort’s beard (Figure 5A) and eyebrow trichoscopy examination, including PIILIF in a beard specimen in one patient requesting beard-to-scalp hair transplantation (Figure 5B). This suggests that PIILIF may not merely be scalp-specific but rather part of a systemic condition affecting other non-scalp hair follicles. Notably, PIILIF-like features were seen in biopsies of hairy facial and body areas of patients with LPP and FFA.21 Conversely, isolated development of FD in the beard zone without scalp involvement has been reported.23 Some studies have reported PIILIF development in other isolated areas (perhaps ahead of future scalp disease). Findings of PIILIF in non-scalp zones of AKN and other primary cicatricial alopecias support the hypothesis of a unifying condition (PIILIF) for a myriad of scalp and body conditions manifesting as keratotic papules, atrophy, cicatricial alopecia or lichenoid histopathologies.24
PIILIF as a PCA Precursor and Proposed Mechanism
While our study supports the role of PIILIF in AKN and FD pathogenesis, several other studies also suggest its role in PCA in general. Multiple studies have found PIILIF-like features in NAS specimens of LPP, FFA, and central centrifugal cicatricial alopecia (CCCA).25–33,
During our study, all cases of dissecting cellulitis (DC) (n=2) and scalp discoid lupus erythematosus (DLE) (n=2) exhibited PIILIF in inactive lesions and NAS zones, suggesting the need for further studies regarding the potential role of PIILIF in DC and DLE. Finally, our finding of mast cell activity in all analyzed AKN, FD, and PIILIF specimens mirrors gene expression studies that found ubiquitous mast cell activity in LPP, FFA, and CCCA lesional and non-lesional biopsies.29 This suggests a central role of mast cells and their known profibrotic activities34,35 in the pathogenesis of PIILIF and PCAs. PIILIF may remain dormant, manifesting as a subclinical phenotype, often confused for seborrheic dermatitis. It may progress to classical PCA phenotypes depending on a range of influencing factors, which may include genetic predispositions,29,36 scalp or body location, age, sex, hormonal influence,37–40 neurologic factors,41 cytokines, mast cell activity,29,42 the ameliorating effect of therapeutic medications and procedures,6,43–45 trauma and other isomorphic or isotopic influences,46,47 or microbiome dysbiosis.48
Furthermore, we propose that in genetically predisposed individuals with PIILIF, various stimuli result in the loss of hair follicle immunity through the upregulation of Janus kinases and signal transducers and activators of transcription (JAK/STAT) and interferon-gamma activity.49–55 This results in scarring alopecia due to CD8-mediated cytotoxic attacks and hair follicle destruction.49,50,52 Fibrosis is accelerated by mast cell activity29,34,35,42 and transforming growth factor beta (TGF-β) upregulation, which, together with peroxisome proliferator-activated receptor-γ (PPAR-γ) dysfunction, causes a profibrotic epithelial-mesenchymal transition.51
Clinical Implications and Demographics
In our cohort, the diagnosis of AKN among four (10%) European-descended White men suggests that AKN may be more common in that demographic than previously reported.1–6 All four men were unaware of their AKN and had presented only with concerns of male patterned baldness; however, on examination, they had mild scattered erythematous papules in their nape areas (Figure 6), some of which showed characteristics of AKN and others of PIILIF on histology. All four attributed the minor nape irritation to “dandruff”, which they treated with anti-dandruff shampoos. The failure to recognize the presence of AKN in White men is likely due to its mild presentation and the simultaneous presence of PIILIF involving the entire scalp, which is often confused for seborrheic dermatitis. Therefore, some patients can subclinically manifest PIILIF without symptoms because of inadvertent treatments or genetics or be misdiagnosed as seborrheic dermatitis. These patients may respond partially to anti-dandruff therapies, such as ketoconazole, whose anti-inflammatory or retinoic acid agonistic activity may be beneficial.56,57
 |
Figure 6 A European-descended White male showing scattered erythematous papules (Red arrows) in the nape area found to be AKN upon histologic analysis. Abbreviation: AKN, acne keloidalis nuchae. |
Finally, our finding of nearly equal AKN incidence in Hispanics (41%) and men of African descent (49%) supports that AKN commonly affects men of color, including Hispanic, Middle Eastern, and Asian demographics.4
Overall, our findings suggest that apart from therapies directed locally at AKN lesions, an emphasis on early diagnosis and expanded treatment approaches directed at inflammatory scalp-wide diseases (eg, steroids, tetracyclines, or biologics) are necessary for all patients. Therapeutic targets include PPAR-γ,58 AMP-activated protein kinase,59 JAK/STAT,49–55 TGF-β,60,61 and mast cell stabilizers29 However, secondary therapeutic measures that recognize peculiarities of specific diseases may be required, such as the use of antibiotics for FD,62 DHT blockers in FFA,20,21 and the avoidance of chemical and heat injuries in CCCA and shaving micro-traumas in AKN.3
Limitations
The study was limited by its retrospective nature. It was also performed in an AKN specialty clinic, which may skew the study population towards more advanced and treatment-resistant diseases. Since previous trichoscopy and histologic NAS studies of non-balding healthy patients showed no significant evidence of PIILIF,25,63,64, we did not believe a prospective control cohort was necessary to validate our findings.
Conclusion
We report the presence of PIILIF in NAS of all AKN and AKN-FD patients. This condition may be a precursor to AKN, FD, and possibly other PCAs. A potential PCA precursor may aid in early diagnosis, treatment, and improved prognosis. Thus, AKN management should focus on inflammatory, scalp-wide diseases. Further studies are needed to confirm the association between PIILIF and other PCAs and understand why PCAs may share a common pathogenic pathway.
Abbreviations
AKN, Acne keloidalis nuchae, AGA, Androgenic alopecia. DLE, Discoid lupus erythematosus. DC, Dissecting cellulitis. DHT, Dihydrotestosterone. FD, Folliculitis decalvans. FFA, Frontal fibrosing alopeciaJAK/STAT, Janus kinase, and signal transducers and activators of transcription. LPP, Lichen planopilaris. NAS, Normal-appearing-scalp. PCA, Primary cicatricial alopecia. PIILIF, Perifollicular infundibulo-isthmic lymphocytic infiltrate, and fibrosis. PPAR-Ɣ, peroxisome proliferator-activated receptor-gamma. TGF-β, Transforming growth factor- beta.
Data Sharing Statement
Source data is available on request.
Ethics and Consent
All procedures performed in studies involving human participants were in accordance with the Declaration of Helsinki (as was revised in 2013). Written informed consent was obtained from patients, including consent for publication and use of photographs. An institutional review board exemption was obtained from the Western Institutional Review Board (IRB) Copernicus Group, which has IRB authority over the site of the study and the source of all patient data – Dr. U Hair and Skin Clinic. The exemption was issued because the study uses deidentified data from procedures that were part of routine clinical care, with no plans to re-identify or contact the subjects.
Acknowledgments
We are particularly grateful to our patients and dermatology staff.
Funding
There is no funding to report.
Disclosure
Sanusi Umar owned shares and issued patents and patent applications in FineTouch Laboratories Inc. and Dr. U Devices Inc. at the time of this work. The other authors declare that they have no conflicts of interest in this work.
References
1. Dinehart SM, Herzberg AJ, Kerns BJ, Pollack SV. Acne keloidalis: a review. J Dermatol Surg Oncol. 1989;15(6):642–647. doi:10.1111/j.1524-4725.1989.tb03603.x
2. Herzberg AJ, Dinehart SM, Kerns BJ, Pollack SV. Acne keloidalis. Transverse microscopy, immunohistochemistry, and electron microscopy. Am J Dermatopathol. 1990;12(2):109–121. doi:10.1097/00000372-199004000-00001
3. Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483–489. doi:10.2147/CCID.S99225
4. Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14(4):E61–E67.
5. Dinehart SM, Tanner L, Mallory SB, Herzberg AJ. Acne keloidalis in women. Cutis. 1989;44(3):250–252.
6. Sperling LC, Homoky C, Pratt L, Sau P. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol. 2000;136(4):479. doi:10.1001/archderm.136.4.479
7. Cosman B, Wolff M. Acne keloidalis. Plast Reconstr Surg. 1972;50(1):25–30. doi:10.1097/00006534-197207000-00004
8. Halder RM. Pseudofolliculitis barbae and related disorders. Dermatol Clin. 1988;6(3):407–412. doi:10.1016/S0733-8635(18)30652-1
9. Mikiel D, Polańska A, Żaba R, Adamski Z, Dańczak-Pazdrowska A. Usefulness of high-frequency ultrasonography in the assessment of alopecia areata - comparison of ultrasound images with trichoscopic images. Postepy Dermatol Alergol. 2022;39:132–140. doi:10.5114/ada.2020.102641
10. Sahu VK, Datta A, Sarkar T, Gayen T, Chatterjee G. Role of trichoscopy in evaluation of alopecia areata: a study in a tertiary care referral centre in the Eastern India. Indian J Dermatol. 2022;67(2):127–132. doi:10.4103/ijd.ijd_577_21
11. Karnik P, Tekeste Z, McCormick TS, et al. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J Invest Dermatol. 2009;129(5):1243–1257. doi:10.1038/jid.2008.369
12. Olsen EA, Bergfeld WF, Cotsarelis G, et al; Workshop on cicatricial alopecia. Summary of North American Hair Research Society (NAHRS)-sponsored workshop on cicatricial alopecia, Duke University Medical Center, 2001. J Am Acad Dermatol. 2003;48(1):103–110. doi:10.1067/mjd.2003.68
13. East-Innis ADC, Stylianou K, Paolino A, Ho JD. Acne keloidalis nuchae: risk factors and associated disorders - a retrospective study. Int J Dermatol. 2017;56:828–832. doi:10.1111/ijd.13678
14. Doche I, Coelho EQ, Quaresma MV, da Matta Rivitti-Machado MC. Acne keloidalis nuchae and folliculitis decalvans: same process affecting the follicle or coexisting diseases? A retrospective study. Int J Dermatol. 2019;58(10):e200–e203. doi:10.1111/ijd.14565
15. Doche I, Hordinsky MK, Valente NS, et al. Evidence for lymphocytic inflammation in non-lesional scalp of folliculitis decalvans: an observational study of 25 patients. J Eur Acad Dermatol Venereol. 2022;36(2):e109–e111. doi:10.1111/jdv.17649
16. Yip L, Barrett TH, Harries MJ. Folliculitis decalvans and lichen planopilaris phenotypic spectrum: a case series of biphasic clinical presentation and theories on pathogenesis. Clin Exp Dermatol. 2020;45(1):63–72. doi:10.1111/ced.13989
17. Egger A, Stojadinovic O, Miteva M. Folliculitis decalvans and lichen planopilaris phenotypic spectrum-a series of 7 new cases with focus on histopathology. Am J Dermatopathol. 2020;42(3):173–177. doi:10.1097/DAD.0000000000001595
18. Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical, histological and therapeutic findings. Br J Dermatol. 1999;140:328–333. doi:10.1046/j.1365-2133.1999.02675.x
19. Ceruti JM, Leirós GJ, Balañá ME. Androgens and androgen receptor action in skin and hair follicles. Mol Cell Endocrinol. 2018;465:122–133.
20. Pindado-Ortega C, Saceda-Corralo D, Moreno-Arrones ÓM, et al. Effectiveness of dutasteride in a large series of patients with frontal fibrosing alopecia in real clinical practice. J Am Acad Dermatol. 2021;84:1285–1294. doi:10.1016/j.jaad.2020.09.093
21. Vañó-Galván S, Saceda-Corralo D, Alonso-Castro L, Urech M, Espada J. Antiandrogenic drugs, a therapeutic option for frontal fibrosing alopecia patients. J Am Acad Dermatol. 2016;74(4):e77. Erratum in: J Am Acad Dermatol 2018;78:431. doi:10.1016/j.jaad.2015.11.047
22. Chew AL, Bashir SJ, Wain EM, Fenton DA, Stefanato CM. Expanding the spectrum of frontal fibrosing alopecia: a unifying concept. J Am Acad Dermatol. 2010;63(4):653–660. doi:10.1016/j.jaad.2009.09.020
23. Senatore S, Maglie R, Maio V, Montefusco F, Antiga E. Folliculitis decalvans with exclusive beard involvement. Indian J Dermatol Venereol Leprol. 2021;87:569–571. doi:10.25259/IJDVL_694_20
24. Turegano MM, Sperling LC. Lichenoid folliculitis: a unifying concept. J Cutan Pathol. 2017;44(7):647–654. doi:10.1111/cup.12938
25. Doche I, Romiti R, Hordinsky MK, Valente NS. ”Normal-appearing” scalp areas are also affected in lichen planopilaris and frontal fibrosing alopecia: an observational histopathologic study of 40 patients. Exp Dermatol. 2020;29:278–281. doi:10.1111/exd.13834
26. Porriño-Bustamante ML, Pinedo-Moraleda FJ, Fernández-Flores Á, Montero-Vílchez T, Fernández-Pugnaire MA, Arias-Santiago S. Frontal fibrosing alopecia: a histopathological comparison of the frontal hairline with normal-appearing scalp. J Clin Med. 2022;11(14):4121. doi:10.3390/jcm11144121
27. Felix K, De Souza B, Portilla N, et al. Dermatoscopic evaluation of central centrifugal cicatricial alopecia beyond the vertex scalp. JAMA Dermatol. 2020;156(8):916–918. doi:10.1001/jamadermatol.2020.1287
28. Mirmirani P, Willey A, Headington JT, Stenn K, McCalmont TH, Price VH. Primary cicatricial alopecia: histopathologic findings do not distinguish clinical variants. J Am Acad Dermatol. 2005;52(4):637–643. doi:10.1016/j.jaad.2004.07.069
29. Wang EHC, Monga I, Sallee BN, et al. Primary cicatricial alopecias are characterized by dysregulation of shared gene expression pathways. PNAS Nexus. 2022;1(3):c111. doi:10.1093/pnasnexus/pgac111
30. Saceda-Corralo D, Desai K, Pindado-Ortega C, Moreno-Arrones OM, Vañó-Galván S, Miteva M. Histological evidence for epidermal and dermal atrophy of the alopecic band in treatment-naïve patients with frontal fibrosing alopecia. J Eur Acad Dermatol Venereol. 2021;35:e47–e49. doi:10.1111/jdv.16792
31. Pindado‐Ortega C, Perna C, Saceda‐Corralo D, Fernández‐Nieto D, Jaén‐Olasolo P, Vañó‐Galván S. Frontal fibrosing alopecia: histopathological, immunohistochemical and hormonal study of clinically unaffected scalp areas. J Eur Acad Dermatol Venereol. 2020;34(2):e84–e85. doi:10.1111/jdv.15977
32. Sperling LLC, Skelton CHG, Smith CKJ, Sau CP, Friedman K. Follicular degeneration syndrome in men. Arch Dermatol. 1994;130:763–769. doi:10.1001/archderm.1994.01690060093012
33. Jordan CS, Chapman C, Kolivras A, Roberts JL, Thompson NB, Thompson CT. Clinicopathologic and immunophenotypic characterization of lichen planopilaris and central centrifugal cicatricial alopecia: a comparative study of 51 cases. J Cutan Pathol. 2020;47(2):128–134. doi:10.1111/cup.13592
34. Conti P, Caraffa A, Mastrangelo F, et al. Critical role of inflammatory mast cell in fibrosis: potential therapeutic effect of IL −37. Cell Prolif. 2018;51(5):e12475. doi:10.1111/cpr.12475
35. Strattan E, Palaniyandi S, Kumari R, et al. Mast cells are mediators of fibrosis and effector cell recruitment in dermal chronic graft-vs.-host disease. Front Immunol. 2019;10:2470. doi:10.3389/fimmu.2019.02470
36. Dlova NC, Jordaan FH, Sarig O, Sprecher E. Autosomal dominant inheritance of central centrifugal cicatricial alopecia in black South Africans. J Am Acad Dermatol. 2014;70(4):679–82.e1. doi:10.1016/j.jaad.2013.11.035
37. Ranasinghe GC, Piliang MP, Bergfeld WF. Prevalence of hormonal and endocrine dysfunction in patients with lichen planopilaris (LPP): a retrospective data analysis of 168 patients. J Am Acad Dermatol. 2017;76(2):314–320. doi:10.1016/j.jaad.2016.05.038
38. Jerjen R, Pinczewski J, Sinclair R, Bhoyrul B. Clinicopathological characteristics and treatment outcomes of fibrosing alopecia in a pattern distribution: a retrospective cohort study. J Eur Acad Dermatol Venereol. 2021;35(12):2440–2447. doi:10.1111/jdv.17604
39. Lobato-Berezo A, March-Rodríguez A, Deza G, Bertolín-Colilla M, Pujol RM. Frontal fibrosing alopecia after antiandrogen hormonal therapy in a male patient. J Eur Acad Dermatol Venereol. 2018;32(7):e291–e292. doi:10.1111/jdv.14825
40. Panchaprateep R, Ruxrungtham P, Chancheewa B, Asawanonda P. Clinical characteristics, trichoscopy, histopathology and treatment outcomes of frontal fibrosing alopecia in an Asian population: a retro-prospective cohort study. J Dermatol. 2020;47(11):1301–1311. doi:10.1111/1346-8138.15517
41. Peters EM, Liotiri S, Bodo E, et al. Probing the effects of stress mediators on the human hair follicle: substance P holds central position. Am J Pathol. 2007;171(6):1872–1886. doi:10.2353/ajpath.2007.061206
42. George AO, Akanji AO, Nduka EU, Olasode JB, Odusan O. Clinical, biochemical and morphologic features of acne keloidalis in a black population. Int J Dermatol. 1993;32(10):714–716. doi:10.1111/j.1365-4362.1993.tb02739.x
43. Azurdia RM, Graham RM, Weismann K, Guerin DM, Parslew R. Acne keloidalis in Caucasian patients on cyclosporin following organ transplantation. Br J Dermatol. 2000;143(2):465–466. doi:10.1046/j.1365-2133.2000.03694.x
44. Carnero L, Silvestre JF, Guijarro J, Albares MP, Botella R. Nuchal acne keloidalis associated with cyclosporin. Br J Dermatol. 2001;144(2):4290430. doi:10.1046/j.1365-2133.2001.04049.x
45. Grunwald MH, Ben‐Dor D, Livni E, Halevy S. Acne keloidalis‐like lesions on the scalp associated with antiepileptic drugs. Int J Dermatol. 1990;29(8):559–561. doi:10.1111/j.1365-4362.1990.tb03468.x
46. Alahmari L, Almesned R, Alhumidi A, Alkhalifah A. Lichen planopilaris with Koebner phenomenon. JAAD Case Rep. 2018;4(8):848–850. doi:10.1016/j.jdcr.2018.05.009
47. Yadav S, Kumar R, Sharma A, Saikia UN, Dogra S. Isomorphic phenomenon in discoid lupus erythematosus with review of reported cases. Rheumatol Int. 2013;33(6):1651–1652. doi:10.1007/s00296-011-2289-9
48. Constantinou A, Polak-Witka K, Tomazou M, et al. Dysbiosis and enhanced beta-defensin production in hair follicles of patients with lichen planopilaris and frontal fibrosing alopecia. Biomedicines. 2021;9(3):266. doi:10.3390/biomedicines9030266
49. Yang CC, Khanna T, Sallee B, Christiano AM, Bordone LA. Tofacitinib for the treatment of lichen planopilaris: a case series. Dermatol Ther. 2018;31(6):e12656. doi:10.1111/dth.12656
50. Imanishi H, Ansell DM, Chéret J, et al. Epithelial-to-mesenchymal stem cell transition in a human organ: lessons from lichen planopilaris. J Invest Dermatol. 2018;138(3):511–519. doi:10.1016/j.jid.2017.09.047
51. Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1(15):e89776. doi:10.1172/jci.insight.89776
52. Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle’s epithelial stem cell niche. J Pathol. 2013;231(2):236–247. doi:10.1002/path.4233
53. Liu LY, Craiglow BG, Dai F, King BA. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76(1):22–28. doi:10.1016/j.jaad.2016.09.007
54. Mackay-Wiggan J, Jabbari A, Nguyen N, et al. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight. 2016;1(15):e89790. doi:10.1172/jci.insight.89790
55. Alves de Medeiros AK, Speeckaert R, Desmet E, Van Gele M, De Schepper S, Lambert J. JAK3 as an emerging target for topical treatment of inflammatory skin diseases. PLoS One. 2016;11(10):e0164080. doi:10.1371/journal.pone.0164080
56. Vanden Bossche H, Willemsens G, Janssen PA. Cytochrome-P-450-dependent metabolism of retinoic acid in rat skin microsomes: inhibition by ketoconazole. Skin Pharmacol. 1988;1(3):176–185. doi:10.1159/000210771
57. Van Wauwe JP, Coene MC, Goossens J, Van Nijen G, Cools W, Lauwers W. Ketoconazole inhibits the in vitro and in vivo metabolism of all-trans-retinoic acid. J Pharmacol Exp Ther. 1988;245:718–722.
58. Mirmirani P, Karnik P. Lichen planopilaris treated with a peroxisome proliferator-activated receptor gamma agonist. Arch Dermatol. 2009;145:1363–1366. doi:10.1001/archdermatol.2009.283
59. Araoye EF, Thomas JAL, Aguh CU. Hair regrowth in 2 patients with recalcitrant central centrifugal cicatricial alopecia after use of topical metformin. JAAD Case Rep. 2020;6(2):106–108. doi:10.1016/j.jdcr.2019.12.008
60. Umar S, Kan P, Carter MJ, Shitabata P. Treatment-refractory central centrifugal cicatricial alopecia responsive to a novel botanical treatment. Clin Cosmet Investig Dermatol. 2022;15:609–619. doi:10.2147/CCID.S358618
61. Umar S, Kan P, Carter MJ, Shitabata P, Novosilska M. Lichen planopilaris responsive to a novel phytoactive botanical treatment: a case series. Dermatol Ther. 2022;12(7):1697–1710. doi:10.1007/s13555-022-00749-3
62. Rambhia PH, Conic RRZ, Murad A, Atanaskova-Mesinkovska N, Piliang M, Bergfeld W. Updates in therapeutics for folliculitis decalvans: a systematic review with evidence-based analysis. J Am Acad Dermatol. 2019;80(3):794–801.e1. doi:10.1016/j.jaad.2018.07.050
63. Nirmal B, Somiah S, Sacchidanand SA, Biligi DS, Palo S. Evaluation of perifollicular inflammation of donor area during hair transplantation in androgenetic alopecia and its comparison with controls. Int J Trichology. 2013;5(2):73–76. doi:10.4103/0974-7753.122963
64. Sueki H, Stoudemayer T, Kligman AM, Murphy GF. Quantitative and ultrastructural analysis of inflammatory infiltrates in male pattern alopecia. Acta Derm Venereol. 1999;79(5):347–350. doi:10.1080/000155599750010238
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.