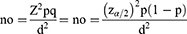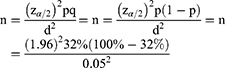Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Unsuppressed Viral Load Level in Public Health Facilities: Nonvirological Predictors among Adult Antiretroviral Therapy Users in Southwestern Ethiopia
Authors Waju B, Dube L, Ahmed M , Assefa SS
Received 15 February 2021
Accepted for publication 14 April 2021
Published 14 May 2021 Volume 2021:13 Pages 513—526
DOI https://doi.org/10.2147/HIV.S304653
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Birhanu Waju,1 Lamessa Dube,2 Muktar Ahmed,2,3 Semira Shimeles Assefa4
1ICAP Ethiopia HIV Prevention, Care and Treatment Program, Addis Ababa, Ethiopia; 2Jimma University, Department of Epidemiology, Jimma, Ethiopia; 3Australian Centre for Precision Health, Adelaide, SA, Australia; 4Department of Biomedical Sciences, Jimma University, Jimma, Ethiopia
Correspondence: Semira Shimeles Assefa Tel +251 96-040-2329
Email [email protected]
Background: Unsuppressed viral load in patients on antiretroviral (ARV) therapy occurs when treatment fails to suppress a patient’s viral load, and is associated with decreased survival and increased HIV transmission. Identifying the level of unsuppressed viral load with its associated factors has benefits in controlling transmission and reducing burden. Therefore, this study aimed to assess unsuppressed viral load (> 1,000 copies/mL) and associated factors among HIV patients taking first-line antiretroviral treatment at public health facilities in Jimma, Ethiopia.
Methods: A facility-based cross-sectional study was conducted on 669 patients on first-line ARV therapy (at least 6 months) in public health facilities in Jimma. Sociodemographic, treatment, clinical, immunological, and viral load data were extracted from medical records, entered into EpiData 3.1, and analyzed with SPSS 20. Multivariate logistic regression analysis was performed to identify factors independently associated with viral nonsuppression, considering a 95% CI with P< 0.05 statistically significant.
Results: Among the participants, 258 (38.6%) were aged 25– 34 years. Median age was 35 years. Prevalence of unsuppressed viral load was 20.3%. Risk of unsuppressed viral loads was 91% lower among ARV therapy patients who had been taking ARV therapy < 2 years (AOR 0.09, 95% CI 0.01– 0.83), lower baseline BMI (AOR 4.44, 95% CI 1.56– 12.64), lower baseline CD4 (AOR 2.76, 95% CI 1.45– 5.29), poor adherence to ARV therapy medication (AOR 3.19, 95% CI 1.29– 7.89), and immunological failure (AOR 4.26, 95% CI 2.56– 7.09) were the independent predictors of unsuppressed viral load.
Conclusion: This study revealed that there is a high level of virological failure among adult HIV patients, and confirms the need to develop close follow-up strategies of targeted interventions for patients in care who are at high risk of unsuppressed viral load.
Keywords: human immunodeficiency virus, HIV, antiretroviral therapy, viral load suppression
Introduction
Globally, HIV has been a great catastrophe of public health. New infections are still challenging, due to prevention services not being provided with sufficient intensity on an adequate scale and not addressed to the people who need them the most.1 According to 2018 global report, in 2017 nearly 36.9 million individuals were living with the virus, of whom 27.7 million (75%) already knew that they were HIV-positive and 21.7 million (59%) accessing the treatment. Africa is the most influenced continent, and it is estimated to have a burden of over two-thirds of the world’s total viral infections. In Ethiopia, the national HIV prevalence is 1.16%.3 In 2017, 722,248 Ethiopians were living with HIV, of whom 527,700 (73%) knew that they were HIV-positive status and 420,000 (71%) people on (ARV) treatment.2
World Health Organization (WHO) clinical staging, immunological (CD4 T-cell count), and monitoring of routine viral load suppression are methods used to monitor treatment outcomes. Immunological and clinical monitoring has lower positive predictive value and poor sensitivity for identifying treatment failure compared to viral suppression. The main reason for suggesting the need for viral load monitoring as the preferred method compared to clinical and immunological monitoring is to provide earlier and more accurate signs of treatment failure and so move patients on to second-line drugs, which thus reduces the accumulation of drug-resistance mutations and improves clinical outcomes.4 Moreover, at the individual patient level, continued viral suppression can stop the appearance of drug-resistance mutations and reduce the occurrence of failure seen clinically. Those patients who succeed in achieving virological suppression early using first-line ARV regimens would stay longer in such regimens have a reduced risk of acquiring infections that occur opportunistically and face a lower mortality rate.5 Therefore, use of regular viral load testing to monitor treatment response is the gold standard, and has been part of WHO treatment guidelines since 2013.4
Many studies have demonstrated that lower HIV viral suppression appears together with a wide range of factors in different settings; however, the level and cause of the problem differ from country to country, eg, the nonsuppression rate of viral load in South Africa is 15%,4 Swaziland 16%,6 Uganda 29%,7 Cambodia 23.2%,8 Zimbabwe 14%,9 and Los Angeles 27%.10 In these studies, including sociodemographic and psychological factors, previous treatment failure, long periods on ARV therapy, low baseline CD4, ARV regimen, poor absorption of ARVs, poor adherence to treatment, comorbidities, drug resistance, drug toxicity, substance abuse, weak social support networks, sexually transmitted infections (STIs), and awareness of the benefits of viral suppression were negatively associated with viral load suppression on ARV therapy.13
In Ethiopia before 2016, as per WHO criteria, treatment outcomes of patients with HIV were monitored clinically and immunologically using CD4 T-cell counts. This approach is considered a poor predictors of HIV-treatment failure, resulting in very late recognition of virological nonsuppression and inappropriate treatment switching to second-line treatment options.14 The 2018 national HIV-care and -treatment guidelines of Ethiopia recommended routine and targeted viral loads to monitor ARV therapy–patients’ treatment outcomes and to standardize the quality of HIV services. Based on this, the viral load testing has been applied since 2016.15
The WHO defines viral suppression as a viral load <1,000 copies/mL of HIV1 RNA after a minimum of 6 months from initiation of ARV therapy with adherence support. Clinical failure in adults and adolescents is defined as the presence of new or recurrent clinical conditions showing severe immunodeficiency WHO clinical stage 4 after 6 months of treatment.4 Unsuppressed viral load may occur due to numerous risk factors, including sociodemographic and psychological variables, poor adherence to treatment, previous treatment failure, comorbidities, poor absorption of ARVs, drug toxicity, and substance abuse leading to poor adherence, STIs, and lack of knowledge or awareness of the benefits of viral suppression.6,19–24 There are, however, other factors, such as sex, age, low baseline CD4, advanced HIV, long periods on ARVs, and ARV regimen, that are correlated with treatment failure.21
There have been few studies done on predictors of nonviral suppression to determine ARV-therapy outcomes among patients on first-line ARV therapy in Ethiopia. Therefore, this study was designed to evaluate the extent of viral suppression and factors independently related to unsuppressed viral load among HIV patients on first-line ARV therapy at public health facilities (HFs) in Jimma,southwest Ethiopia.
Methods
Study Setting
The current study was done in Jimma, in Oromia state 353 km southwest of the national capital — Addis Ababa. Based on 2017 population projections, Jimma’s population is 194,139. There are six public HFs, four of which provide chronic HIV/AIDS-care and -treatment services. This study was carried out at Shenen Gibe Hospital, Jimma Medical Center, Jimma Health Center, and Higher 2 Health Center from March 1 to 20, 2019.
Jimma Medical Center (formerly Jimma University Specialized Hospital) is the only teaching and referral hospital overseen by the Federal Ministry of Education. It is viral load–testing facility for southwest regions, and provides testing for all catchment ARV-therapy providers for regional HFs. Since March 2016. Shenen Gibe Hospital, Jimma Health Center, and Higher2 Health Center have been administered by the Oromia Regional Health Bureau, and they provide HIV/ARV-therapy services in Jimma. They refer viral load samples to Jimma Medical Center for testing.
Study Design
This was a cross-sectional study.
Source and Study Population
All adult HIV patients on ARV therapy at public HFs in Jimma were considered the source population. Those on first-line ARV therapy were the study population.
Eligibility Criteria
Adult (age ≥15 years) HIV-positivepatients who had been on first-line ARV therapy for at least 6 months and had had a first viral load test from March 2016 to February 2019 were included. ARV-therapy clients transferred to other HFs, lost to follow-up, and those who had restarted their medication or whose data were incomplete were not included.
Sampling Technique and Sample-Size Determination
Cochran’s sample size–calculation formula was applied to determine the sample size of our study.
A 95% confidence level with an error of 5% was preferred (40):
Based on the 2017 UNAIDS 90-90-90 framework for Ethiopia,r 32% viral suppression was taken as a baseline to calculate sample size to represent our study population:2
The sample size required was 334.4. However, to improve study power,thiswas multiplied by two, for a total sample for data collection of 669 records,
where Z2 is the abscissa of the normal curve that cuts off an area at the tails (1–α equals the desired confidence level — 95%), n the sample size, pthe estimated proportion of an attribute present in the population, dthe desired level of precision, and q1–p(40).
Sampling Techniques
Medical records of those attending ARV-therapy clinics at public HFs were used. Records meeting the inclusion criteria were selected for the study sample using simple random sampling. Records eventually included in the analysis were selected randomly using a Microsoft Excel randomizer. Once random numbers had been generated, the records in the Excel sheet were assigned serial numbers, which guided selection. The records selected were evaluated for completeness. If a record were missing information or incomplete, it was substituted with the succeeding randomly selected record until the desired sample size had been obtained.
To determine sample size for each HF, numbers of viral load tests during from March 2016 to February 2019 at each HF were reviewed. Then, the sample size for each HF was determined on the basis of number of tests done. Final samples were 462 for Jimma Medical Center, 64 for Shenen Gibe Hospital, 134 for Jimma Health Center, and nine for Higher 2 Health Center. (Table 1).
 |
Table 1 Sampling distribution of study participants |
Data-Collection Procedures
Data were collected using a structured data-abstraction tool customized from Federal Ministry of ARV-therapy patient-intake forms, follow-up charts, and registers. A retrospective review of routinely collected HIV information and viral load test data for patients from March 1 to 20, 2019 was done. Data were collected by four ARV therapy–trained data clerks working at ARV therapy clinics. Four supervisors and a principal investigator monitored all activity on a daily basis throughout.
Data collection included basic patient information, such as sociodemographic characteristics of ARV-therapy patients, clinical and treatment characteristics, laboratory results, information, and comorbidities: STIs, TB infection, and medically diagnosedincommunicable disease.
Data Quality Management
Before data collection, data collectors and supervisors got a 1-day orientation on the contents of tools, how the data-collection process need to be and the general aims of the study with the principal investigator. Data clerks and ARV-therapy providers from HFs were recruited as data collectors and supervisors. Completeness, consistency, missing data and outliers checked. Regular supervision of supervisors and data collectors was conducted by the principal investigator to maintain data quality.
Data Processing
Data were checked, coded, and entered into EpiData EntryClient 3.1. Then, data were cleaned and statistical analysis doneSPSS 20.0. In data presentation, means, percentages, and frequencies are used as descriptive statistics to present demographic, clinical, and ARV treatment–related characteristics of participants.
Statistical Analysis
We performed a sequential statistical analysis considering predictors and outcome variables. First, candidate variables were selected using simple logistic regression, and those withP<0.25 were selected. Next,multiple logistic analysis was doneusing the backward likelihood ratioto identify final independent factors of nonviral suppression.
The statistical model fitted with predictors was evaluated for goodnessoffit using the Hosmer–Lemeshow test. No evidence was seen of lack of fit(P=0.298). Variables with statistically significant associations with unsuppressed viral load were declared predictors based onAORs with 95% Cls and P<0.05.
Results
Sociodemographic Characteristics
Of the 669 patients, 258 (38.6%) were aged 25–34 years. Median age was 35 years. Females accounted for 68.2% of participants. A majority (560, 93.7%) were urban dwellers. More than half (389 (58%) were married, 171 (25.6%) housewives, and 128 (19.1%) unemployed. In sum, 295 (44.1%) had received primary education, followed by 204 (30.5%) with secondary education(Table 2).
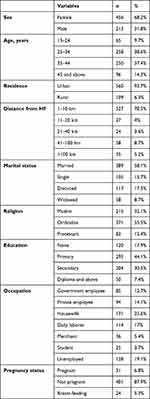 |
Table 2 Baseline sociodemographic characteristics of study participants on first-line ART (n=669) |
Relevant Clinical Characteristics
A total of 660 (98.7%) patients had current WHO clinical stage I and nine (1.3%) had clinical stage II and above . A majority (544, 81.3%) of ARV-therapy patients had BMI >18.5 kg/m2, 61 (9.1%) were moderately malnourished (BMI 16–18.5 kg/m2), and and 19 (2.8%) severely malnourished (BMI <16 kg/m2) at baseline.
Most patients (439, 65.6%) had CD4 >250 cells/mm3, 165 (24.7%) had CD4 >100–250 cells/mm3, and 65 (9.7%) had CD4 ≤100 cells/mm3 at baseline. In total, 525 (78.5%) patients had Hb >10 g/dL and 144 (21.5%) ≤10 g/dL at baseline, 102 (15.2%) had had immunological failure in the year prior to viral load testing being conducted. Likewise, 40 (6%) ofARV patients had developed STIs and 35 (5.2%) TB infection after initiation of ARV therapy (Table 3).
 |
Table 3 Clinical characteristics of adult HIV-positive patients on first-line ART (n=669) |
Treatment Characteristics
More than half the study population (358, 53.5%) were on the the 1e regimen (efavirenz–lamivudine–tenofovir [3TC–Efv–TDF]) as a fixeddose combination, 171 (25.6%) on 1c (azidothymidine–3TC–nevirapine (Nvp), 82 (12.2%) on 1f (3TC–Nvp–TDF, and58 (8.7%) on 1d (AZT–3TC–Efv).
More than half (359, 53.2%) the patients had been on ARV therapy for 6–10 years,g ARV therapy 145 (21.7%) 3–5 years, 132 (19.7%) >11 years, and 36 (5.4%) <2 years. Median treatment duration was 3 years.
In sum, 174 (26%) had not disclosed their HIV status. A majority (643,96.1%) had good adherence to their medication. Most (456, 68.2%) of ARV therapy clients got drug refillson the scheduled date of appointment, whereas 213 (31.8%) got their refills late on multiple occasions (Table 4).
 |
Table 4 Treatment characteristics of adult HIV-positive patients on first-line ART (n=669) |
Viral Load Suppression
Of the 669 study participants, 533 (79.7%) had achieved viral load suppression (≤1,000 copies/mL). However, 136 (20.3%) had unsuppressed viral loads (>1,000 copies/mL) after ≥6 months’ ARV therapy.
Of the 136 ARV clients with unsuppressed viral load, 36% were male and 64% female. Regarding regimen, 41.2% were on 1e, 30% on 1c, 19.8% on 1f, and 8.8% on 1d. Unsuppressed viral load among patients with current WHO clinical stage 1 was 97%, h 22.8% had been on ARV therapy for 3–5 years, 50% for 6–10 years, and 26.5% >11 years, 61.7% had baseline BMI >18.5 kg/m2, 30.9% 16–18.5 kg/m2,and 7.4% <16 kg/m2(Table 5).
 |
 |
 |
Table 5 viral load suppression among HIV patients on first-line ART(n=669) |
Predictors of Unsuppressed Viral Load
We ran a bivariate logistic regression analysis to determine candidate variables and clarify associations among independent variables and viral load–suppression status. Sex, age, marital status, disclosure of HIV status, education, ARV-therapy initiation, ARV-therapy regimen, baseline BMI, current WHO clinical stage, adherence to ARV-drug treatment, multiple late appointments in the last year, clinical failure in the year preceding viral load testing, immunological failure in the year preceding viral load testing, baseline CD4, baseline Hb, and TB infection after initiation of ARV therapy were identified as candidate variables (P<0.25) for subsequent analysis on multivariate logistic regression.
To discover the effects of the predictor variables on the viral load nonsuppression, multivariate logistic regression analysis was performed. ARV-therapy Initiation time, BMI, baseline CD4 count, adherence to ARV medication, and immunological failure had statistically significant associations with unsuppressed viral load after adjusting for other variables. Unsuppressed viral load was 91% less likely (AOR 0.09, 95% CI 0.01–0.83) among those on ARV therapy <2 years than those on therapy >3 years and 2.9 (95% CI 1.76–4.79) times as likely among those who were moderately malnourished (BMI 16–18.5 kg/m2). Viral load nonsuppression were 4.4 (95% CI 1.56–12.64) times as likely n those who had severe malnutrition (BMI <16kg/m2) at baseline than those with BMI >18.5 kg/m2. Unsuppressed viral load was 2.76 (95% CI 1.45–5.29)times as likely among those with baseline CD4 ≤100 cells/mm3 than those with <100 cells/mm3.TViral load nonsuppression was twice as likely (AOR 2.07, 95% CI CI 1.28–3.34) among those with baseline CD4 >100–250 cells/mm3 than those with baseline CD4 >250 cells/mm3, 3.2 (95% CI 1.29–7.89) times as likely among those with poor adherence to their medication than those with good adherence, and 4.26(95% CI 2.56, 7.09) times as likely among patients in those who had had immunological failure in the last years than those with no immunological failure (Table 6).
 |
Table 6 Predictors of unsuppressed viral load among adult ART users |
Discussion
We found that 20.3% of patients on first-line ARV therapy had a viral load>1,000 copies/mL, indicating that they had not achieved viral suppression after ≥6 months’ ARV therapy. Other studies have had comparable findings, eg, in Haiti (15%),5 Zimbabwe (18%),9 Cameroon (23.6%)22 and in Peru 24% (15%–24%).26 Also of note is when compared to the national target of HIV prevention and control, this figure is higher than that required to reach the 90-90-90 treatment targets. This is probably best explained by patients’ time on ARV therapy, lower baseline BMI measurement and CD4 count, poor adherence to medication, and low immunological failure, which increased the odds of unsuppressed viral load.
Unsuppressed viral load was 91% loweramong those on ARV therapy >2 years than those on ARVs >years. This finding is comparable to the Haiti study, where those that had been on ARV therapy for 2–3 years were all significantly less likely to achieve viral suppression.5 In contrast, similar studies conducted in South Africa, Kenya, and Cameroon, as duration of ARVtherapy increased, viral suppression decreased,5,8,22,29
The odds of unsuppressed viral load were higher CI among patients those with moderate malnutrition. Likewise, the odds of nonsuppression were higher among those who had severe malnutrition at baseline than those with BMI >18.5 kg/m2. This finding is similar to a study done in Uganda, where unsuppressed viral load was higher among HIV-infected adults with BMI ≤16–18.5 kg/m2 at baseline than those with BMI >18.5 kg/m2.19 In another study done in Tanzania, HIV patients with lower baseline BMI when starting ARV therapy were at significantly higher risk of high viral load and early mortality than those with baseline BMI of 18.5–22.9 kg/m2.
Unsuppressed viral load was 2.7 times more likely among those with baseline CD4 ≤100 cells/mm3, and the odds of nonsuppression among those with baseline CD4 >100–250 cells/mm3 were twice that of those with baseline CD4 >250 cells/mm3. This finding was comparable with studies done in Haiti, Swaziland, Vietnam, and Thailand, where HIV patients on ARV therapy with decreased baseline CD4 counts had higher odds of nonviral suppression than those with CD4 ≥500 cells/mm3 or greater.13,18,22,24 In contrast, a study conducted in Brazil found no difference between patients with baseline CD4 counts 350–499 and ≥500 cells/mm3.30
The odds of unsuppressed viral load among those with poor adherence to their medication were 3.2 timesthat of those with good adherence. This is supported by other studies in similar settings, where poor adherence to ARV medication and missed doses on a daily basis were more likely to result in non-viral suppression than good adherence.9,13,14,23,25,27,28 Nonadherence is associated with non-viral suppression, and adherence to treatment is essential in ensuring viral suppression among patients on ARV therapy.
The odds of unsuppressed viral load were higher in those who had had immunological failure in the last year than those who had not. This is comparable to other findings in the literature, where immunological failure in HIV patients with CD4 count <100 cells/mm3 were more likely to have nonviral suppression than those with >250 cells/mm3.5,29,30
The results of this study are consistent with others in the literature and can help health-care workers in public HFs to identify factors that can affect viral suppression among patients on first-line ARV therapy and support the expansion of improved viral load–monitoring interventions. Targeted efforts aimed at improving treatment response through distinguishing patients at risk of virological failure will allow early adherence interventions and help shifts to second- or third-line therapy. These findings are also important for policy-making and reviewing guidelines of HIV management that could avert possible virological failure.
Limitations
This study is not without limitations. First, we reviewed records of patients with viral load test results, which may have resulted in underestimation of the actual proportion of patients on ARV therapy with unsuppressed viral loads. In addition, such adherence as on-time drug pickups, pill counts, and other factors that could affect adherence, such as alcohol and khat consumption, mental health status, and psychosocial factors, (depression and stigma) were not included in the medical records.
Conclusion
We found that there was low viral suppression (79.7%) compared to the UNAIDS target of 90%. The key independent factors for viral nonsuppression were duration on ARV therapy (<2 years), low BMI,low baseline CD4 count, poor adherence to ARV medication, and immunological failure. These results reinforce the need to develop strategies in regard to these predictors to maximize adherence, improve nutritional assessment and clinical management of patients with high viral load, and, more commitment to sustaining treatment outcomes, and closer follow-up of focused interventions for patients on ARVtreatment who are at higher risk of unsuppressed viral loads.
Abbreviations
Hb, hemoglobin; ART, ARV therapy.
Ethics Approval
Formal permission letters and institutional ethics clearance were secured from the institutional review board of Jimma University to conduct this study and from relevant local administrative bodies and public health facilities. Jimma University The IRB waived the requirement to obtain individual-level informed consent, since the study involved secondary analysis of existing data collected as part of routine care, and no additional data were needed that required informed consent. This study adhered to the Declaration of Helsinki, and all data were deidentified. We further ensured proper safeguards were in place to prevent accidental disclosure of sensitive data about study participants.
Acknowledgments
We are so thankful to the study participants and data collectors. Also, we would like to thank Jimma University Medical Center, Shenen Gibe Hospital, Jimma Health Center, and higher 2 Health Center for facilitating all research activities during the study period.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, agreed to submit to the current journal, gave final approval to the version to be published, and agree to be accountable for all aspects of the work.
Funding
This research was not funded by anybody or any institution.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Stover J, Bollinger L, Izazola JA, Loures L, DeLay P, Ghys PD; Fast Track modeling working Group. What is required to end the AIDS epidemic as a public health threat by 2030? The cost and impact of the fast-track approach. PLoS One. 2016;11(5):e0154893.
2. Huerga H, Van Cutsem G, Farhat JB, et al. Progress towards the UNAIDS 90-90-90 goals by age and gender in a rural area of KwaZulu-Natal, South Africa: a household-based community cross-sectional survey. BMC Public Health. 2018;18(1):303. doi:10.1186/s12889-018-5208-0
3. Ehnri F. HIV Related Estimates and Projections for Ethiopia. Addis Ababa: FMoH; 2012:6–14.
4. Nelson LJ, Beusenberg M, Habiyambere V, et al. Adoption of national recommendations related to use of antiretroviral therapy before and shortly following the launch of the 2013 WHO consolidated guidelines. Aids. 2014;28:S217–S224. doi:10.1097/QAD.0000000000000239
5. Louis FJ, Buteau J, François K, et al. Virologic outcome among patients receiving antiretroviral therapy at five hospitals in Haiti. PLoS One. 2018;13(1):e0192077.
6. Joseph Davey D, Abrahams Z, Feinberg M, et al. Factors associated with recent unsuppressed viral load in HIV-1-infected patients in care on first-line antiretroviral therapy in South Africa. Int J STD AIDS. 2018;29(6):603–610. doi:10.1177/0956462417748859
7. Uzande C, Edwards JK, Owiti PO, Maravanyika AT, Mashizha S, Mafaune PT. Are you suppressed? Viral load test coverage for people living with HIV in Mutare District, Manicaland Province, Zimbabwe, 2015–2017. BioRxiv. 2018;324350.
8. Chhim K, Mburu G, Tuot S, et al. Factors associated with viral non-suppression among adolescents living with HIV in Cambodia: a cross-sectional study. AIDS Res Ther. 2018;15(1):20. doi:10.1186/s12981-018-0205-z
9. Jobanputra K, Parker LA, Azih C, et al. Factors associated with virological failure and suppression after enhanced adherence counselling, in children, adolescents and adults on antiretroviral therapy for HIV in Swaziland. PLoS One. 2015;10(2):2. doi:10.1371/journal.pone.0116144
10. Sayles JN, Rurangirwa J, Kim M, Kinsler J, Oruga R, Janson M. Operationalizing treatment as prevention in Los Angeles County: antiretroviral therapy use and factors associated with unsuppressed viral load in the ryan white system of care. AIDS Patient Care STDS. 2012;26(8):463–470. doi:10.1089/apc.2012.0097
11. World Health Organization. HIV Drug Resistance Report 2017; 2017.
12. Charles M, Leger PD, Severe P, et al. Virologic, clinical and immunologic responses following failure of first-line antiretroviral therapy in Haiti. J Int AIDS Soc. 2012;15(2):17375. doi:10.7448/IAS.15.2.17375
13. Das M, Chu PL, Santos G-M, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5(6):6. doi:10.1371/journal.pone.0011068
14. Hailemariam S, Bune GT, Ayele HT. Malnutrition: prevalence and its associated factors in people living with HIV/AIDS, in Dilla University Referral Hospital. Arch Public Health. 2013;71(1):13. doi:10.1186/0778-7367-71-13
15. Hull MW, Montaner J. Antiretroviral therapy: a key component of a comprehensive HIV prevention strategy. Curr HIV/AIDS Rep. 2011;8(2):85–93. doi:10.1007/s11904-011-0076-6
16. Gagnon M, Guta A. HIV viral load: a concept analysis and critique. Res Theory Nurs Pract. 2014;28(3):204–227. doi:10.1891/1541-6577.28.3.204
17. Izudi J, Alioni S, Kerukadho E, Ndungutse D. Virological failure reduced with HIV-serostatus disclosure, extra baseline weight and rising CD4 cells among HIV-positive adults in Northwestern Uganda. BMC Infect Dis. 2016;16(1):614. doi:10.1186/s12879-016-1952-x
18. Mattson CL, Freedman M, Fagan JL, et al. Sexual risk behaviour and viral suppression among HIV-infected adults receiving medical care in the United States. AIDS. 2014;28(8):1203. doi:10.1097/QAD.0000000000000273
19. Council A. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents;2011.
20. Boullé C, Guichet E, Kouanfack C, et al. Virologic failure and human immunodeficiency virus drug resistance in rural Cameroon with regard to the UNAIDS 90-90-90treatment targets. Open Forum Infect Dis. 2016;3(4):. doi:10.1093/ofid/ofw233
21. Centers for Disease Control and Prevention. Evidence of HIV Treatment and Viral Suppression in Preventing the Sexual Transmission of HIV; 2018.
22. Flynn AG, Anguzu G, Mubiru F, et al. Socioeconomic position and ten-year survival and virologic outcomes in a Ugandan HIV cohort receiving antiretroviral therapy. PLoS One. 2017;12(12):12. doi:10.1371/journal.pone.0189055
23. Chao C, Tang B, Hurley L, et al. Risk factors for short-term virologic outcomes among HIV-infected patients undergoing regimen switch of combination antiretroviral therapy. AIDS Res Hum Retroviruses. 2012;28(12):1630–1636. doi:10.1089/aid.2012.0005
24. Jorge AR, Jorge PB, Elsa GL, et al. Risk factors associated with virologic failure in HIV-infected patients receiving antiretroviral therapy at a public hospital in Peru. Rev Chilena Infectol. 2013;30(1):42.
25. Anude CJ, Eze E, Onyegbutulem HC, et al. Immuno-virologic outcomes and immuno-virologic discordance among adults alive and on anti-retroviral therapy at 12 months in Nigeria. BMC Infect Dis. 2013;13(1):113. doi:10.1186/1471-2334-13-113
26. Crabtree-Ramírez B, Villasís-Keever A, Galindo-Fraga A, Del Río C, Sierra-Madero J. Effectiveness of highly active antiretroviral therapy (ART) among HIV-infected patients in Mexico. AIDS Res Hum Retroviruses. 2010;26(4):373–378. doi:10.1089/aid.2009.0077
27. Meireles MV, Pascom AR; Duantiretroviral therapy e EC. Factors associated with early virological response in HIV-infected individuals starting antiretroviral therapy in Brazil (2014–2015): results from a large HIV surveillance cohort. J Acquir Immune Defic Syndr. 2018;78(4):e19. doi:10.1097/QAI.0000000000001684
28. Sabaté E, Sabaté E, editors. Adherence to Long-Term Therapies: Evidence for Action. World Health Organization; 2003.
29. Hailu GG, Hagos DG, Hagos AK, Wasihun AG, Dejene TA. Virological and immunological failure of ART and associated risk factors among adults and adolescents in the Tigray region of Northern Ethiopia. PLoS One. 2018;13(5). doi:10.1371/journal.pone.0196259
30. Sang RK, Miruka FO. Factors associated with virologic failure amongst adults on antiretroviral therapy in Nyanza Region, Kenya. IOSR J Dent Med Sci. 2016;15(7):108–121. doi:10.9790/0853-15076108121
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.