Back to Journals » International Medical Case Reports Journal » Volume 17
Unilateral Biportal Endoscopy for the Resection of Thoracic Intradural Extramedullary Tumors: Technique Case Report and Literature Review
Authors Peng W, Zhuang Y, Cui W, Chen W, Chu R, Sun Z, Zhang S
Received 11 October 2023
Accepted for publication 28 March 2024
Published 9 April 2024 Volume 2024:17 Pages 301—309
DOI https://doi.org/10.2147/IMCRJ.S444226
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Vinay Kumar
Wei Peng, Yin Zhuang, Wei Cui, Wenjin Chen, Rupeng Chu, Zhenzhong Sun, Shujun Zhang
Department of Spine Surgery, Wuxi Ninth People’s Hospital Affiliated to Soochow University, Wuxi, Jiangsu, People’s Republic of China
Correspondence: Shujun Zhang, Department of Spine Surgery, Wuxi Ninth People’s Hospital Affiliated to Soochow University, No. 999 Liangqing Road, Binhu District, Wuxi, Jiangsu, People’s Republic of China, Tel +86-13861741839, Email [email protected]
Abstract: This study describes a patient with an intradural extramedullary (IDEM) tumor removed entirely using the unilateral biportal endoscopic technique (UBE), achieving satisfactory clinical outcomes. A 60-year-old woman had a diagnosis of meningioma with sensations and motor dysfunction in the lower extremities and perineum and gait disturbances for three years, which has worsened over the last month. Preoperative imaging data showed a sizeable IDEM tumor at the T10 level, significantly compressing the thoracic spinal cord to the right side, with 80% intraspinal encroachment. The IDEM tumor was removed entirely by UBE surgery. To the best of our knowledge, this study may be the first to report the application of UBE techniques for IDEM tumor treatment. In this case, UBE provides a magnified and clear surgical field, greater maneuverability, and a less invasive surgical procedure. The procedure objectives were pathological confirmation, spinal cord decompression, and complete tumor removal; all were met. The patient was satisfied with her dramatically improved clinical symptoms. UBE may be an alternative surgical treatment option for benign IDEM tumors presenting with symptomatic, especially the non-giant lateral and posterior tumors.
Keywords: unilateral biportal endoscopy, endoscopic spine surgery, intradural extramedullary, tumor, meningioma
Introduction
Spinal intradural extramedullary (IDEM) tumors mainly stem from cellular components of the spinal cord, end filaments, nerve roots, and meninges, with 80% being schwannomas and meningiomas.1,2 Clinical signs and symptoms are mainly related to the spinal cord and nerve compression, most manifest as myelopathy, radiculopathy, and sphincter disorders below the tumor segment. Conventional open surgery was often performed for these lesions, which involved a long incision, the division of bilateral paraspinal muscles, and the resection of both laminae with an additional facetectomy in ventral tumors, or sometimes it required an extension of the bone window into the neural foramen.3 This approach destroys most of the structures of the posterior column, resulting in inferior spinal stability and possible spinal deformity.4,5 Since the development of microscopic techniques, hemilaminectomy has become increasingly popular. This technique removes the IDEM tumors, only separating the affected side’s paraspinal muscles and removing the vertebral arch. Therefore, most of the posterior column structures and the articular process were preserved, resulting in the spinal stability being minimally impaired. Pure endoscopy has recently been reported to be an effective treatment for IDEM tumors, achieving positive results.6–8
Unilateral biportal endoscopy (UBE) is a novel technique of minimally invasive spinal surgery (MISS) that allows free hand manipulation of instruments with a magnified clear view, which has been widely applied in spinal degenerative diseases,9–11 extradural lesions and tumors12,13 in recent years. UBE is more flexible, convenient, has minimal tissue damage, less blood loss, and causes less postoperative pain than conventional surgeries and uniaxial endoscopic approaches. Two studies conducted by Kim et al and Wang et al have reported that extradural tumors have been successfully biopsied and removed using the UBE technique, improving dramatic symptoms.12,13 However, the UBE technique for IDEM tumor had limited reports.
In this paper, we present a case of an IDEM tumor that was removed entirely by UBE and express our experiences and lessons learned.
Materials and Methods
Case Presentation
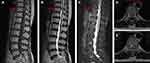
The patient was a 60-year-old woman who presented with gait disturbances, numbness and weakness in the lower extremities, lumbar and abdominal girdling sensations, and numbness in the perineum. Symptoms were more severe on the left side than the right, lasting for 3 years and worsening for 1 month. A physical examination revealed increased muscle tone in the lower extremities and ankle hyporeflexia. The patient’s history included hypertension and hyperlipidemia. Preoperative magnetic resonance imaging (MRI) showed a sizeable intradural tumor at the T10 level with an intact envelope and well-defined borders, significantly compressing the thoracic spinal cord to the right side, with 80% intraspinal encroachment (Figure 1). The tumor showed hypo-intensity on the T1 and T2-weighted images and obvious homogenous enhancement on the T1 fat suppression contrast-enhanced image. Meningiomas were initially suspected to be the cause of this IDEM tumor. The patient vehemently opposed conventional open surgeries and agreed to a unilateral biportal endoscopic approach. The preoperative modified Japanese Orthopaedic Association (mJOA) score was 11. The study was conducted by the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Wuxi Ninth People’s Hospital Affiliated to Soochow University. Informed consent was obtained from the patients, who provided written informed consent for the publication of this article.
Procedure
Procedure Position and Instruments
Under general anesthesia, the patient was placed in a prone position on a radiolucent frame for posterior surgery. Surgical drapes were placed aseptically from the upper thoracic spine to the lower thoracic spine in a watertight manner. An endoscope with a 30-degree angle, a high-definition imaging system, an irrigation system with 3000 cc of sodium chloride, and a standard laminectomy set were used to perform thoracic spinal decompression and tumor removal. The surgical approach was selected on the left side of the body, which was determined by the relative position of the tumor concerning the spinal cord. Two 10 mL syringe needles were used to confirm the T10 pathological level with preoperative C-arm fluoroscopy. The viewing portal was placed cranially, and the working portal was located caudally.
Unilateral Approach and Bilateral Decompression
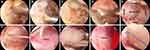
To enlarge the working and viewing portals, a sequential dilator was used to split the paraspinal muscle with minimal damage to the multifidus of the thoracic region. The bleeding control and soft tissue detachment were performed under arthroscopic guidance with a bipolar radiofrequency instrument, and a preliminary workspace was established (Figure 2A). The T10 translaminar approach was used to access the tumor center during the procedure. To ensure precision, a 1.5mm Kirschner needle was utilized to verify the T10 pathological level with the help of intraoperative C-arm fluoroscopy, as shown in Figure 2B. Once the spinous process was exposed, the T10 and most T9 lamina were dissected sequentially. A high-speed diamond burr and Kerrison rongeurs along the inferior border of the upper lamina and the medial part of the inferior articular process to expose the cranially superior and inferior attachment of the ligamentum flavum (LF) and then totally removed (Figure 2C and D). The partial inferior lamina was also removed until reaching the lower edge of the tumor border (Figure 2E). The location of the tumor determined the range of laminotomy. As a result of removing the majority of T10 and 1/3 of T9 lamina, an oval opening of 5.8 cm was created in the bone.
Tumor Removal
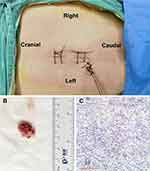
To determine the range of incisions needed, we assessed the upper and lower edges of the tumor. Using a nerve root stripper, we lightly palpated the dural sac to estimate the tumor extent. Then, under endoscopic guidance, a sharp blade was used to gently tease the dural sac with an approximately 2.5 cm-long incision, after which the tumor was exposed (Figure 2F). Next, a dissecting plane along the tumor envelope was meticulously created between the tumor and the nerve tissue (Figure 2G). Then, the IDEM tumor was carefully dissected along the dissection plane until entirely detached from the nerve tissues. In this case, the tumor had three tight adhesions to the nerve tissue, which were gently peeled off with a nerve root stripper. While dissecting the tumor, it broke into three fragments due to its brittle texture. Lastly, the tumor was removed piecemeal (Figure 2H). After confirmation of complete tumor resection, endoscopic advancement of the dural sac for exploration confirmed clean removal of the mass, and the herniated nerve tissue was retracted into the dural sac (Figure 2I). Finally, 6–0 PROLENE interrupted the dural sac suturing (Figure 2J). Endoscopic suturing of the dural sac is a challenging procedure, and the aim is to prevent scar tissue from invading the dural sac, which could result in neurological symptoms. The skin wounds were closed, and a drainage tube was inserted (Figure 3A). Its size was about 1.5*2.0 cm (Figure 3B).
Results
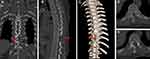
The patient’s neurological deficits, including sensory and motor weakness, were improved after UBE surgery. The drainage tube was successfully removed at 8 days postoperatively, and the total hospital stay following surgery was 10 days. The IDEM tumor was identified as a meningioma based on pathological examination of surgical specimens (Figure 3C). Figure 4 of the postoperative CT imaging shows that the lamina was hemi-laminotomized while the spinous process and the facet joint were preserved. Postoperative MRI revealed that the spinal canal was sufficiently decompressed following the complete removal of the IDEM tumor (Figure 5). During the following three months, the postoperative mJOA score was up to 15, and no recurrence of symptoms and no severe complications were observed.
Discussion
To the best of our knowledge, this study may be the first to report the application of UBE techniques to treat IDEM tumors. The procedure objectives were pathological confirmation, spinal cord decompression, and complete tumor removal; all were met. The patient was satisfied with her dramatically improved clinical symptoms.
IDEM tumors are primarily benign schwannomas and meningiomas that can be surgically cured by complete tumor resection. During the evolution of spine surgery, open approaches are gradually replaced with limited bone removal techniques, such as hemilaminectomy and MISS, to prevent the potentially destabilizing dissection of osseoligamentous structures and muscles.1,2,4,14–18 By applying microscopic techniques, hemilaminectomy can significantly reduce surgical injury, intraoperative bleeding, operation time, and postoperative hospitalization compared to conventional open surgery.19–22 A further advantage of this procedure is that it allows for better preservation of the posterior ligamentous complex and the articular eminence of the vertebral body.19,21,22 Therefore, the spinal column’s original physiological and mechanical properties are maintained, resulting in satisfactory clinical outcomes and spinal stability even without an internal fixation device.16,21 Moreover, the annulus structure of the spinal canal is less disrupted, thereby reducing the path for scar tissue invasion into the spinal canal, which may reduce the risk of medically induced spinal stenosis. However, hemilaminectomy has limitations due to the limited exposure range and bone window, resulting in a limited maneuvering space that requires extensive surgical expertise.23 Consequently, the scope of adaptation is limited, and the procedure is more suitable for cases with unilateral lesions.
Endoscopic technology has become widely used in spinal surgery since its development and widespread application. Various studies have demonstrated the safety and effectiveness of minimally invasive approaches using hemilaminectomy with the help of an endoscope.7,14,23 An endoscope-assisted micro neurosurgical technique has been used for the first time to remove multilevel IDEM tumors in the lumbar region by Burtcher et al.24 Subsequently, Barami et al reported that an endoscope-assisted posterior approach was used in two cases to resect ventral IDEM tumors.25 Parihar et al was the only other major series on “pure endoscopic” surgery to use the “Destandau” (convergent) port for treating IDEM lesions up to 41 mm in diameter.26 The surgical approach of endoscopic-assisted spinal surgery was very safe and did not cause damage to essential tissue structures. Furthermore, endoscopy’s panoramic illumination and visualization offer definite advantages over microscopy when dissecting tumors from adjacent neural and vascular structures, requiring even smaller bony and dural exposures.27,28 Therefore, complementary to microscopy technology, endoscopy employs a multi-angled endoscope to directly visualize and remove the lesion in the bone window that cannot be observed and manipulated under the microscope. However, because of its inherent viewing channel coaxial with the working channel, uniaxial endoscopy has the disadvantages of inconvenient manipulation and limited surgical vision, leading to limitations in bilateral decompression.
UBE is a novel technique operated through a percutaneous endoscope, which has been widely used for treating degenerative spinal diseases in recent years.29 Compared to uniaxial endoscopy, UBE offers a more transparent and magnified surgical field due to continuous monitoring of a high-definition arthroscop.30 Studies have also shown that flexible and unrestricted working tubes improve maneuverability, convenience, and iatrogenic injury reduction.31 The indications of UBE surgery are increasingly extensive, including lumbar spinal stenosis, lumbar interbody fusion, gaseous lumbar pseudocysts, lumbar disc herniate, and intraspinal extradural tumors.12,13,29,32–34 In the present study, we have successfully performed UBE on an IDEM tumor and obtained satisfactory results.
Several concerns must be considered when treating IDEM tumors with the UBE technique. To begin with, what kinds of IDEM tumors are suitable for treatment with UBE? Due to the posterior approach, UBE is particularly suitable for treating tumors in the spinal cord’s dorsal or anterolateral part, provided the tumor does not extend into both extraforaminal regions. In this case, the IDEM tumor was in the spinal canal’s anterolateral sides, and it did not extend beyond a single segment or into the intervertebral space, which allowed us to remove the tumor with a minimal retraction of the spinal cord and nerve roots. UBE is not the first treatment option when a tumor is in the ventral or anterior region, and the spinal cord surrounds it in the cervical or thoracic spine, requiring spinal cord retraction. The preoperative MRI images indicated that the envelope of our tumor was intact, and postoperative pathology identified it as meningioma. It has been noted that IDEM tumors treated with endoscopy have been primarily benign so far; a primary malignant tumor has not been reported. For long segments of the tumor, Dhandapani et al have reported that a longer schwannoma up to 6.8 cm was excised under pure endoscopy through smaller bony fenestration using the “sliding delivery” technique.3 However, based on our experience, microscopy surpasses endoscopy in providing an overall view than endoscopy. Nevertheless, it is vital to acknowledge the presence of blind spots within microscopy’s visual field; in contrast, endoscopy offers a better local field of view. Thereby minimizing potential harm to the posterior column’s structural integrity with the aid of endoscopy.
Next, how to design incisions? Design incisions are intended to facilitate intraoperative manipulation, minimize spinal cord stretching, and reduce the risk of aggravating spinal cord compression during the procedure. In our case, the preoperative MRI images have shown the intradural tumor was located at the anterolateral and crossed the midline of the spinal cord, depressing the spinal cord to the right side. Thus, we selected the left side to increase the convenience of manipulation and avoid stretching the spinal cord. If the tumor did not cross the midline of the spinal cord, the incision was made on the ipsilateral side of the tumor. An appropriate extent of the incision should be determined based on preoperative imaging data, and the working channel should be capable of exposing the free end of the tumor easily, if necessary, increasing the number of incisions.
Moreover, points to note for intraoperative operations. Firstly, it is necessary to grind out enough bone to gain sufficient maneuvering space. Moreover, in the context of intracapsular resection of a tumor, the direction of force throughout the process should be avoided by stretching or exerting pressure on the spinal cord as much as possible, which may result in further injury to the spinal cord. Furthermore, despite the narrow operation field of the hemilaminectomy approach, the operation space can provide adequate hemostasis. It is mainly since intradural tumors are mostly benign and not supported by abnormally large blood vessels. Therefore, the position of the blood-supplying arteries of the spinal cord is stable if there is no vascular malformation, and it is relatively easy to protect these arteries during surgery. In our case, no injury to the blood-supplying arteries of the spinal cord has occurred, even if there were some arterial hemorrhages.
En bloc resection is recommended for intradural tumors as it reduces the possibility of recurrence. The texture of schwannoma tumors makes it possible to remove them en bloc because they are less likely to fragment when removed. In contrast, piecemeal removal is more common for meningiomas, primarily due to their brittle texture and tendency to fragment upon removal. In this case, the IDEM tumor was a meningioma dissected from the spinal cord in fragments, and there were no signs of recurrence after three follow-up visits. When the dura incisions are greater than 1 cm long, sutures should be applied to close the dural sac continuously or intermittently with non-invasive sutures (6–0). The dura suture was designed to prevent scar tissue from invading the dural sac, which could result in neurological symptoms.
In addition, it is essential to recognize that UBE for IDME tumors involves a steep learning curve. Surgeons must be proficient in performing endoscopic posterior approaches from the cervical spine to the lumbar spine. Although UBE for IDME benign tumors has achieved positive results in our cases and has desirable characteristics, this technique should be considered in select cases. In the case of large tumors with apparent neurological deficits, microendoscopic surgery may be an appropriate option.
Data Sharing Statement
The data set generated during this study is available from the corresponding author (SJZ) upon justified request.
Ethics Approval
Ethical approval was obtained from the ethics committee of Wuxi Ninth People’s Hospital Affiliated to Soochow University. All procedures were performed in accordance with the ethical standards of the Ethics Committee of the hospital, and under the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to Participate
All persons gave their informed consent for their inclusion in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from the General Project of Wuxi Municipal Health Commission in 2020 (grant number M202002) and the Science and Technology Bureau of Wuxi (NO. K20221063 and Y20222029).
Disclosure
The authors declare no competing interests.
References
1. Afathi M, Peltier E, Adetchessi T, Graillon T, Dufour H, Fuentes S. Minimally invasive transmuscular approach for the treatment of benign intradural extramedullary spinal cord tumours: technical note and results. Neurochirurgie. 2015;61(5):333–338. doi:10.1016/j.neuchi.2015.05.001
2. Haji FA, Cenic A, Crevier L, Murty N, Reddy K. Minimally invasive approach for the resection of spinal neoplasm. Spine. 2011;36(15):E1018–26. doi:10.1097/BRS.0b013e31820019f9
3. Dhandapani S, Karthigeyan M. “Microendoscopic” versus “pure endoscopic” surgery for spinal intradural mass lesions: a comparative study and review. Spine J. 2018;18(9):1592–1602. doi:10.1016/j.spinee.2018.02.002
4. McGirt MJ, Garces-Ambrossi GL, Parker SL, et al. Short-term progressive spinal deformity following laminoplasty versus laminectomy for resection of intradural spinal tumors: analysis of 238 patients. Neurosurgery. 2010;66(5):1005–1012. doi:10.1227/01.NEU.0000367721.73220.C9
5. Dhandapani M, Dhandapani S, Agarwal M, Mahapatra AK. Pain perception following different neurosurgical procedures: a quantitative prospective study. Contemp Nurse. 2016;52(4):477–485. doi:10.1080/10376178.2016.1222240
6. Caballero-Garcia J, Linares-Benavides YJ, Leitao ULS, Aparicio-Garcia C, Lopez-Sanchez M. Minimally invasive removal of extra- and intradural spinal tumors using full endoscopic visualization. Global Spine J. 2022;12(1):121–129. doi:10.1177/2192568220948806
7. Mobbs RJ, Maharaj MM, Phan K, Rao PJ. Unilateral hemilaminectomy for intradural lesions. Orthop Surg. 2015;7(3):244–249. doi:10.1111/os.12184
8. Senturk S, Unsal UU. Percutaneous full-endoscopic removal of lumbar intradural extramedullary tumor via translaminar approach. World Neurosurg. 2019;125:146–149. doi:10.1016/j.wneu.2019.01.206
9. Pranata R, Lim MA, Vania R, July J. Biportal endoscopic spinal surgery versus microscopic decompression for lumbar spinal stenosis: a systematic review and meta-analysis. World Neurosurg. 2020;138:e450–e458. doi:10.1016/j.wneu.2020.02.151
10. Heo DH, Lee DC, Park CK. Comparative analysis of three types of minimally invasive decompressive surgery for lumbar central stenosis: biportal endoscopy, uniportal endoscopy, and microsurgery. Neurosurg Focus. 2019;46(5):E9. doi:10.3171/2019.2.FOCUS197
11. Heo DH, Son SK, Eum JH, Park CK. Fully endoscopic lumbar interbody fusion using a percutaneous unilateral biportal endoscopic technique: technical note and preliminary clinical results. Neurosurg Focus. 2017;43(2):E8. doi:10.3171/2017.5.FOCUS17146
12. Kim SK, Bendardaf R, Ali M, Kim HA, Heo EJ, Lee SC. Unilateral biportal endoscopic tumor removal and percutaneous stabilization for extradural tumors: technical case report and literature review. Front Surg. 2022;9:863931. doi:10.3389/fsurg.2022.863931
13. Wang T, Yu H, Zhao SB, et al. Complete removal of intraspinal extradural mass with unilateral biportal endoscopy. Front Surg. 2022;9:1033856. doi:10.3389/fsurg.2022.1033856
14. Turel MK, D’Souza WP, Rajshekhar V. Hemilaminectomy approach for intradural extramedullary spinal tumors: an analysis of 164 patients. Neurosurg Focus. 2015;39(2):E9. doi:10.3171/2015.5.FOCUS15170
15. Tola S, De Angelis M, Bistazzoni S, Chiaramonte C, Esposito V, Paolini S. Hemilaminectomy for spinal meningioma: a case series of 20 patients with a focus on ventral- and ventrolateral lesions. Clin Neurol Neurosurg. 2016;148:35–41. doi:10.1016/j.clineuro.2016.06.015
16. Pompili A, Caroli F, Crispo F, et al. Unilateral laminectomy approach for the removal of spinal meningiomas and schwannomas: impact on pain, spinal stability, and neurologic results. World Neurosurg. 2016;85:282–291. doi:10.1016/j.wneu.2015.09.099
17. Gandhi RH, German JW. Minimally invasive approach for the treatment of intradural spinal pathology. Neurosurg Focus. 2013;35(2):E5. doi:10.3171/2013.5.FOCUS13163
18. Yasargil MG, Tranmer BI, Adamson TE, Roth P. Unilateral partial hemi-laminectomy for the removal of extra- and intramedullary tumours and AVMs. Adv Tech Stand Neurosurg. 1991;18:113–132. doi:10.1007/978-3-7091-6697-0_3
19. Raygor KP, Than KD, Chou D, Mummaneni PV. Comparison of minimally invasive transspinous and open approaches for thoracolumbar intradural-extramedullary spinal tumors. Neurosurg Focus. 2015;39(2):E12. doi:10.3171/2015.5.FOCUS15187
20. Wong AP, Lall RR, Dahdaleh NS, et al. Comparison of open and minimally invasive surgery for intradural-extramedullary spine tumors. Neurosurg Focus. 2015;39(2):E11. doi:10.3171/2015.5.FOCUS15129
21. Zong S, Zeng G, Du L, Fang Y, Gao T, Zhao J. Treatment results in the different surgery of intradural extramedullary tumor of 122 cases. PLoS One. 2014;9(11):e111495. doi:10.1371/journal.pone.0111495
22. Koch-Wiewrodt D, Wagner W, Perneczky A. Unilateral multilevel interlaminar fenestration instead of laminectomy or hemilaminectomy: an alternative surgical approach to intraspinal space-occupying lesions. Technical note. J Neurosurg Spine. 2007;6(5):485–492. doi:10.3171/spi.2007.6.5.485
23. Chern JJ, Gordon AS, Naftel RP, Tubbs RS, Oakes WJ, Wellons JC. Intradural spinal endoscopy in children. J Neurosurg Pediatr. 2011;8(1):107–111. doi:10.3171/2011.4.PEDS10533
24. Burtscher J, Felber S, Twerdy K, Langmayr JJ. Endoscope-assisted interlaminar removal of an ependymoma of the cauda equina. Minim Invasive Neurosurg. 2002;45(1):41–44. doi:10.1055/s-2002-23579
25. Barami K, Dagnew E. Endoscope-assisted posterior approach for the resection of ventral intradural spinal cord tumors: report of two cases. Minim Invasive Neurosurg. 2007;50(6):370–373. doi:10.1055/s-2007-993203
26. Parihar VS, Yadav N, Yadav YR, Ratre S, Bajaj J, Kher Y. Endoscopic management of spinal intradural extramedullary tumors. J Neurol Surg a Cent Eur Neurosurg. 2017;78(3):219–226. doi:10.1055/s-0036-1594014
27. Dhandapani S, Negm HM, Cohen S, Anand VK, Schwartz TH. Endonasal endoscopic transsphenoidal resection of tuberculum sella meningioma with anterior cerebral artery encasement. Cureus. 2015;7(8):e311. doi:10.7759/cureus.311
28. Dhandapani S, Singh H, Negm HM, et al. Endonasal endoscopic reoperation for residual or recurrent craniopharyngiomas. J Neurosurg. 2017;126(2):418–430. doi:10.3171/2016.1.JNS152238
29. Hwa Eum J, Hwa Heo D, Son SK, Park CK. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: a technical note and preliminary clinical results. J Neurosurg Spine. 2016;24(4):602–607. doi:10.3171/2015.7.SPINE15304
30. Park JH, Jun SG, Jung JT, Lee SJ. Posterior percutaneous endoscopic cervical foraminotomy and diskectomy with unilateral biportal endoscopy. Orthopedics. 2017;40(5):e779–e783. doi:10.3928/01477447-20170531-02
31. Merter A, Karaeminogullari O, Shibayama M. Comparison of radiation exposure among 3 different endoscopic diskectomy techniques for lumbar disk herniation. World Neurosurg. 2020;139:e572–e579. doi:10.1016/j.wneu.2020.04.079
32. Soliman HM. Irrigation endoscopic decompressive laminotomy. A new endoscopic approach for spinal stenosis decompression. Spine J. 2015;15(10):2282–2289. doi:10.1016/j.spinee.2015.07.009
33. Deng Y, Yang M, Xia C, Chen Y, Xie Z. Unilateral biportal endoscopic decompression for symptomatic thoracic ossification of the ligamentum flavum: a case control study. Int Orthop. 2022;46(9):2071–2080. doi:10.1007/s00264-022-05484-0
34. Zhu C, Cheng W, Wang D, Pan H, Zhang W. A helpful third portal for unilateral biportal endoscopic decompression in patients with cervical spondylotic myelopathy: a technical note. World Neurosurg. 2022;161:75–81. doi:10.1016/j.wneu.2022.02.021
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.