Back to Journals » Clinical Interventions in Aging » Volume 18
Unilateral Bi/Multi-Portal Endoscopy for the Treatment of Complicated Lumbar Degenerative Diseases with Utilization of Uniaxial Spinal Endoscope, Instead of Arthroscope: Technique Note and Clinical Results
Authors Yang L, Zhou L, Wang G, Qiu M , Liang F, Jia C, Xu W, Fu Q, Yang L, Ba G
Received 16 May 2023
Accepted for publication 28 July 2023
Published 9 August 2023 Volume 2023:18 Pages 1295—1308
DOI https://doi.org/10.2147/CIA.S417462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Liyu Yang,1,* Long Zhou,1,* Guanqi Wang,2 Min Qiu,1 Feng Liang,1 Changqing Jia,1 Weibing Xu,3 Qin Fu,1 Liqing Yang,1 Gen Ba1
1Department of Orthopedic, Shengjing Hospital of China Medical University, Shenyang, Liaoning, People’s Republic of China; 2Rehabilitation Center, Shengjing Hospital of China Medical University, Shenyang, Liaoning, People’s Republic of China; 3Spinal Surgery, Dalian Central Hospital Affiliated to Dalian Medical University, Dalian, Liaoning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Gen Ba, Department of Orthopedic, Shengjing Hospital of China Medical University, Sanhao Street 36, He Ping District, Shenyang, Liaoning Province, People’s Republic of China, 110004, Tel +86 18940259116, Email [email protected]
Objective: This article aims to discuss a novel surgical strategy, referred to as unilateral bi/multi-portal endoscopy (UME), which used a uniaxial spinal endoscope instead of an arthroscope in the traditional unilateral biportal endoscopy (UBE) surgical procedure in our study of the treatment of complicated lumbar degenerative diseases.
Methods: This retrospective study included 42 patients diagnosed with high-migrated lumbar disc herniation and bilateral spinal stenosis who underwent UME surgery from January 2021 to December 2021. Patients included 20 men and 22 women, with an average age of 55.97± 14.92 years. The average follow-up period was 13.19 months. The demographic data, operation time (min), and complications were recorded and analyzed. The visual analogue scale (VAS), Oswestry Disability Index (ODI) scores were used to evaluate the surgical outcomes. Three-dimensional CT scans and MRI were conducted to evaluate the radiographic improvement.
Results: A total of 26 patients were diagnosed with lumbar disc herniation and 16 with lumbar spinal stenosis. All 42 patients underwent UME surgery and achieved satisfactory outcomes. The operation time was 154.46± 46.09 min. The average follow-up time was 13.19± 1.33 months. The preoperative back pain (VAS-Back) and the last follow-up VAS-Back were 3.84± 1.00 and 0.70± 0.46, respectively (P < 0.05). The preoperative leg pain (VAS-Leg) and the last follow-up VAS-Leg were 6.46± 1.08 and 1.03± 0.64, respectively (P < 0.05). Significant differences existed between preoperative ODI scores (58.70± 11.22%) and the last follow-up ODI scores (9.24± 3.04%; P< 0.05). All patients achieved significant pain relief and functional improvement after the surgery. No severe complications occurred, except for two cases of postoperative dysesthesia and one case suffered from vertebral compression fractures induced by a postoperative accidental injury. Symptoms of numbness disappeared within one week with treatment using dexamethasone and neurotrophic drugs. The vertebral fracture case recovered with percutaneous kyphoplasty treatment.
Conclusion: This study suggests that UME is a promising treatment strategy for high-migrated disc herniation and bilateral spinal stenosis.
Keywords: unilateral bi/multi-portal endoscopy, lumbar degenerative diseases, high-migrated disc herniation, bilateral spinal stenosis, ligamentum flavum, spinal endoscope
Introduction
Lumbar degenerative disease is a common clinical disease that plagues public health, particularly for those over 60 years of age.1 Surgical intervention is often recommended for patients with severe or progressive nerve injury, chronic pain, or ineffective non-surgical treatment. Open surgery is the classical procedure for the treatment of lumbar degenerative diseases. However, the considerable iatrogenic trauma associated with surgery, particularly intervertebral fusion combined with pedicle screw internal fixation, exerts a strong negative effect on anatomical tissues such as muscles and ligaments.2 Although the immediate postoperative outcome is typically good, concerns exist of long-term adjacent disc reprotrusion, high risk of infection and hemorrhage, severe back pain, secondary stenosis of the spinal canal, and subsidence of the fusion cage.3,4
Percutaneous spinal endoscopy under aqueous medium for discectomy and spinal canal decompression, such as percutaneous endoscopic transforaminal discectomy (PETD) and percutaneous endoscopic interlaminar discectomy (PEID) have gradually replaced the traditional open laminectomy with discectomy in recent years.5,6 Percutaneous spinal endoscopy has certain advantages, like direct surgical vision, reduced trauma, and less hemorrhage. Comparable with open surgery, spinal endoscopy is well suited for the rapid postoperative recovery of elderly patients and possesses the same postoperative outcomes.7,8
With the development of single-channel uniaxial spinal endoscopy, surgeons have increasingly discovered significant defects involving the coaxiality of the visual field and working channel. In the clinical applications of endoscopic technology, the disadvantages of single-channel spinal endoscopic technology are gradual exposure during treatment of migrated or calcified disc herniation, central lumbar spinal canal stenosis, unilateral or bilateral lateral recess, nerve root canal stenosis with root symptoms, and assisted endoscopic interbody fusion.9 These include the lack of a three-dimensional visual field, the narrow visual angle, and mirror space occupation during the operation. Due to the diameter limitation of the pipe, the required specialized surgical equipment cannot be used. In addition, a very high rate exists of damage to surgical instruments, and the cost of replacing specialized equipment is relatively high. Moreover, the efficient removal of bone and ligamentum flavum is low, compared to open surgery.10
Unilateral biportal endoscopy (UBE) was discovered to overcome the above difficulties in 2017 by Heo.11 UBE technique establishes percutaneous observation and operation channels via two small incisions on the same side. Endoscopes are inserted into the observation channel to directly view the surgical field. In addition, operation tools are inserted into the operation channel for multiple surgical operations both within and around the spinal canal. The dual-channel separation feature distinguishes this technology from coaxial endoscopy. It possesses the advantages of a clear and wide field of vision, flexible and convenient operation, and simple requirements for surgical instruments.12
Previous UBE technology typically employed arthroscopy within the observation channel; however, it was only used for observation, not for instruments. As part of this study, we were the first to employ a novel surgical strategy using the uniaxial spinal endoscope as an observation portal. During observation, we could also minimally manipulate endoscopic operation under coaxial endoscopy using the observation pipe. Specifically, we could reach places that are generally difficult to reach with open surgical instruments and conduct meticulous operation around nerve tissue, which greatly benefited our unilateral bi/multi-portal endoscopy (UME). This study aims to introduce this novel procedure and approach to treating patients with herniated lumbar discs and lumbar spinal stenosis.
Materials and Methods
Between January and December 2021, 42 patients were selected for analysis. Among them, 26 patients had confirmed prolapsed LDH diagnosis, and 16 had lumbar spinal stenosis. According to the onset segments, 26 cases involved L4–5 segments, 5 involved L3–4, 9 involved L5–S1 segments separately, and 2 involved L2–3 (Table 1). These patients received either uniaxial spinal endoscopic discectomy or uniaxial spinal endoscopic laminectomy to correct bilateral decompression via unilateral laminotomy employing the percutaneous biportal spinal endoscopic approach. All patients clinically presented with unilateral lower limb radiculopathy, the result of migrated or huge prolapsed herniated disc or central canal spinal stenosis with intermittent neurologic claudication, and no response to six weeks of conservative therapy. The patients with migrated disc were evaluated by MRI, which was positioned at the level of Grade 1, 2 (Rostral high, from beyond the superior margin of the upper pedicle to 3 mm below the inferior margin of the upper pedicle) and 5, 6 (caudal high, from the middle of the lower pedicle to the inferior margin of the lower pedicle or beyond the inferior margin of the lower pedicle).13 Huge prolapsed herniated disc were diagnosed by the Michigan State University (MSU) classification with the grade of 2–3 (more than 50% of the distance from the non-herniated posterior aspect of the disc to the intra-facet line).14 Lumbar spinal stenosis were graded at the level of C and D (no rootlets can be recognized, the dural sac demonstrating a homogeneous gray signal with no CSF signal visible, with less or no epidural fat posteriorly) according to the Schizas’ classification by preoperative MRI.15 The midsagittal canal diameter (CD) and axial central canal area (CCA) on preoperative and postoperative MRI were calculated to evaluate the decompression of spinal canal.16 Presurgical computed tomography (CT) and lumbar dynamic flexion–extension X-rays were performed for all 42 patients to evaluate the existence of calcified disc herniation and lumbar instability. Excluded from the analysis were patients with diffuse calcified disc herniations over two segments, segmental instability, degenerative or isthmic spondylolisthesis, or who were complicated by lumbar infection, tumor, tuberculosis, or chronic disc herniations following prior surgery. UME was performed by the same surgeon. Instruments such as basic spine instruments and a 30° 3.75-mm internal diameter spinal endoscope system (SPINENDOS®, Germany) were used during the operation. In addition, we employed a grinding and drilling system (Zi Rui® Technology, Guizhou, China) under endoscopic guidance to improve laminotomy efficacy.
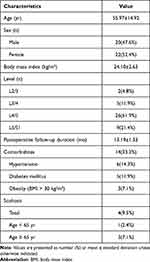 |
Table 1 Patient Demographic and Clinical Characteristics |
Surgical Procedure
All patients received the UME under general anesthesia. A typical patient was placed on a Jackson table or radiolucent Wilson frame in the prone position to reduce abdominal pressure. We used two syringe needles to simulate the puncture approach under fluoroscopic guidance to verify the pathological level and establish two entry points. The exact target point was the junction between the lower lamina margin and 1–1.5 cm lateral to the midline, as established based on the patient’s stature and presurgical clinical manifestations. However, the puncture point was not fixed, and the target point was altered according to the pathological lesion. The target point was usually located at the inner edge of the ipsilateral pedicle for the ipsilateral lateral recess and migrated disc, and it was removed to the midline of the pedicle for the bilateral decompression in the sagittal plane. If the lumbar disc had migrated caudally, the puncture target point was moved moderately 0.5–1cm to the caudal side. Generally, the first entry point for the scopic portal was generated 1 cm lateral to the midline and superior to the mid-intervertebral disc line, near the lower lamina margin. Then, approximately 2–3cm to the caudal side, the working portal was generated 1 cm lateral to the midline and inferior to the mid-intervertebral disc line, near the medial margin of the inferior vertebral body pedicle. Meanwhile, the entrance of the spinal endoscope into the scopic portal was altered into the working portal, according to the angle of decompression and degree of convenience. The dominant hand of the surgeon was usually reserved for the working portal and the nondominant hand for the scopic portal. The working portal and scopic portal could be exchanged for difficult operations (Figure 1).
A 7-mm skin incision and fascia (scopic portal) was generated vertical to the target point. Serial dilators were then inserted one by one to the surface of the laminar bone. Next, a second 1-cm incision was generated for the working portal via transverse insertion. Before the endoscopic procedure, we reaffirmed using X-rays that the dilators of the two portals touched and crossed above the laminar bone. A 7.0-mm-diameter cannula was placed inside the scopic portal to place and maintain the spinal endoscope (Figure 2). A waterproof surgical drape was applied via two flushing pipelines attached to the spinal endoscope. This ensured a clear view before the nerve tissue–related procedure. The first step was to move the muscle and fat surrounding the laminar and interlaminar space to generate a preliminary working space. Subsequently, radio frequency (RF) probes were used via the working cannulas to contract the connective tissue on the laminar bone surface and remove any soft tissue remnants to create the triangulation space between lamina, spinous process (SP), and muscle. Once the SP transitioned to the laminar junction and the lamina was confirmed, a partial hemilaminotomy was carried out with a waterproof electric drill. First, we ground off the insertion point of the upper laminar ligamentum flavum. Then, we removed a portion of the upper laminar lower edge to expand the decompression range. Next, the upper part of the lower lamina was ground to ensure that the distal end of the migrated disc, the traversing nerve root, and the insertion point of the lower laminar ligamentum flavum were exposed. The ligamentum flavum was preserved until the removal of the laminar bone to serve as a protective barrier against the irrigation of the flushing fluid. A blunt nerve dissector, similar to the dura elevator, was used to confirm the space between the ligament flavum and dura. On confirmation of a lack of adhesion between the ligamentum flavum and dura, the laminectomy punch was inserted within the working channel to perform an ipsilateral flavectomy to expose and decompress the dura and ipsilateral traversing nerve root (Figure 1).
During intraspinal venous plexus bleeding correction, RF ablation under a spinal endoscope was used within the scopic cannulas, and the proliferative vascular fiber adhesion tissue around the intervertebral disc was accurately separated before removing the prolapsed disc. Subsequently, an annular incision was made using the spinal RF probe. Disc particles were gradually extracted following gentle retraction. The amount of intervertebral disc resection performed depended on the looseness of the disc during the operation (Figure 3).
For those patients who required decompression to correct spinal canal stenosis, the ipsilateral lamina bone was removed using a grinding drill. An ipsilateral partial laminar and medial facet resection, along with decompression, were then performed using conventional instruments. These steps preceded the undercutting and drilling of the contralateral lamina and base of the SP to reserve adequate space for placing the endoscope and securing it to the contralateral side. The entrance of the nerve root canal and the lateral recess were carefully expanded using an endoscopic Kerrison punch or grinding drill. We performed contralateral laminotomy and lateral recess decompression in patients with severe bony spinal stenosis or contralateral spinal stenosis using an endoscopic Kerrison punch. Spinal endoscopic RF probes aided in bleeding management around the contralateral traversing nerve root. Lastly, we systematically evaluated the shoulder and axillar areas around the contralateral nerve root for any compression caused by a hyperplastic prominent articular process and residual ligamentum flavum.
Statistical Analysis
All data analyses were conducted with SPSS software (version 16; SPSS, Chicago, Illinois, USA). Data are expressed as means ± standard deviations. Patient variables were assessed via the paired t-test. P < 0.05 was set as the significance standard.
Results
Follow-Up
All 42 patients had successful UME surgery. All were subsequently followed up for a duration of at least 12 months (time points: one month, three months, six months and one year after surgery), with an average follow-up of 13.19±1.33 months.
General Results
This study included 20 male and 22 female patients who underwent UME surgery at our hospital. The average patient age was 55.97±14.92 years (ranging from 30 to 77 years). Among them, 26 patients were diagnosed with LDH, and the remaining 16 patients were diagnosed with lumbar stenosis. Surgery was done in 26 cases at L4–5, nine cases at L5–S1, five cases at L3–4, and 2 cases at L2–3. The mean UME surgical duration was 115±35.43 minutes and general postoperative hospital stays was 5.98±0.86 days (Table 2).
 |
Table 2 Variables Related to Surgery |
Radiographic Improvement
Three months after surgery, the lumbar postoperative MRI image did not reveal any nerve compression. In LDH patients, sufficient protruded disc tissue was removed, and in lumbar stenotic cases, adequate thickened ligamentum flavum and hyperplastic facet joints were removed. Adequate space existed around the nerve, as evidenced by MRI and three-dimensional CT scans. The midsagittal canal diameter (CD) and axial central canal area (CCA) on MRI were enlarged after 3 months in the postoperative MRI (Table 3). The patients experienced marked pain relief after surgery, and the postsurgical MRI (three months) exhibited excellent decompression (Figures 4 and 5).
 |
Table 3 Evaluation of Spinal Canal Decompression |
Visual Analogue Scale
All UME-operated patients reported marked pain relief at every follow-up, relative to the presurgical pain scale (P < 0.05). The lower back VAS was reduced from a preoperative 3.84±1.00 to a postoperative 1.97±0.54 (one month), 1.65±0.48 (three months), 1.27±0.55 (six months) and 0.7±0.46 (one year). Additionally, the leg VAS was reduced from a preoperative 6.46±1.08 to a postoperative 2.14±0.58 (one month), 1.59±0.49 (three months), 1.38±0.48 (six months) and 1.03±0.46 (one year).
Oswestry Disability Index
The ODI scores after UME surgery improved significantly from a preoperative 58.7±11.22% to a postoperative 28.57±6.74% (1 month), 17.22±4.70% (3 months), 11.76±3.56% (6 months) and 9.24±3.04 (one year, P<0.05). These results suggest a marked functional enhancement after UME surgery (Table 4).
 |
Table 4 Preoperative and Postoperative Functional Evaluation |
Discussion
Open surgery, a standard effective treatment for lumbar degenerative diseases, elicits an adverse effect on paraspinal muscles and intraspinous ligaments, which eventually causes lumbar instability, postoperative lumbago, and muscle atrophy.17 Any bone removal also compromises stability at the surgical location. Eventually, the removal of excessive bone and ligament compels surgeons to perform unnecessary spinal interbody fusion operations.18 Intervertebral fusion is known to generate stress on adjoining segments, thereby displacing the center of rotation of the lumbar spine. This promotes the recurrence of adjacent disc herniation and secondary spinal canal stenosis.19
Unilateral biportal endoscopy (UBE) was a novel surgical procedure to solve the problems caused by open surgery. De Antoni was the first to employ two independent channels via arthroscopy during lumbar surgery in 1996.20 The introduction of independent channels for observation and operation greatly improves the flexibility and efficiency of surgery. In 2013, Soliman demonstrated the resection of a prolapsed nucleus pulposus using a dual-channel endoscopy while treating lumbar disc herniation (LDH). At the 12-month follow-up, 95% of patients showed satisfactory outcomes.21 Hwa Eum et al22 applied percutaneous dual-channel endoscopic decompression technology to correct lumbar spinal stenosis in 2016. They considered dual-channel endoscopic technology to be similar to microscopic decompression technology and to provide good surgical vision. In their study, the central area of the spinal canal and bilateral lateral recess areas were also effectively reduced via unilateral laminectomy while avoiding bilateral incisions and reducing surgical trauma.
In general, uniportal endoscopy is used to treat patients with slight disc herniation and mild unilateral or central canal stenosis. However, from the perspective of clinical practice, large central disc herniation in cranial segments (L2–3 and L3–4) and discs with long prolapse distance in L4–5 and L5–S1 segments are often difficult to operate using a single-channel spinal endoscopy. More importantly, nerve injury, dural sac avulsion, and inadequate decompression occur on occasion during immature uniportal endoscopic spine surgery. Certain patients (5.1%) experience dural sac tears in fully endoscopic lumbar discectomy,23 whereas no severe nerve injury occurred in patients with LDH treated by UME surgery in our study. We thought the disc removal gave the benefits of an enlarged spinal canal and a safer, convenient visual field. A shorter time and less space were needed to stretch the disc, and the risk of nerve injury was decreased. UBE offers advantages of both open and endoscopic spinal surgeries. It enhances both translaminar endoscopic decompression and microscopic unilateral laminectomy.24 At the same time, the use of continuous pressure saline irrigation significantly reduces endoscopy-related bleeding. Moreover, the contralateral dural sac sinks slightly under the pressure of the irrigation fluid. This allows the endoscope to reach the contralateral traversing nerve root.25 Due to the use of independent channels dedicated to distinct surgical instruments during traditional UBE surgery, the operation space of each surgical instrument rarely conflicts with others during the operation. Another advantage of UBE surgery is that open surgical instruments can be inserted into the working portal during the operation. Therefore, UBE also greatly enhances the clarity and efficiency of lamina and ligamentum flavum removal during the operation.
When comparing postoperative imaging measurements, namely, the degree of dural sac decompression and expansion, the uniportal spinal endoscopy offered the least satisfactory decompression, whereas the UBE surgery achieved complete decompression, similar to the outcome of open surgery. Additionally, the mean facetectomy angle was markedly reduced in patients who received biportal endoscopy-guided surgery, as opposed to patients who received microscopy-guided surgery, which indicates that the former retained more bone structures to maintain the stability of the lumbar spine.26 Moreover, in contrast to open surgery, UBE utilizes a smaller bone resection area, thereby facilitating more facet preservation. On the one hand, less bone resection enables decompression of the neural canal without pedicle screw internal fixation. During contralateral decompression UBE surgery, the scope and working instrument must surpass the lamina to reach the contralateral ligamentum flavum, in what is called the over-The-top technique.12 On the other hand, more facet preservation ensures the stability of surgical segments following the operation. Kim and Choi showed that no obvious slippage or lumbar instability occurred two years after UBE surgery to correct lumbar spondylolisthesis.27 In addition, Lin et al28 demonstrated no difference between complications related to UBE and conventional interventions.
When conventional UBE surgery utilizes arthroscopy as a viewing tool, it cannot employ minimally invasive instruments, adopted under spinal endoscopy, in the observation portal. This may increase the risk of potential nerve injury, such as a dura tear or nerve root injury while separating and clearing the peripheral nerve structures within the spinal cannulas, as presented in a recent study comparing UBE and PELD for discectomy in the treatment of LDH.29 Therefore, we explored this novel surgical procedure, using the uniaxial spinal endoscope to replace the arthroscope. Fortunately, no dura tear or nerve root injury has occurred during UME surgery in our study to date. Just as the traditional UBE surgical technique can effectively remove the lamina and ligamentum flavum, UME can safely and rapidly distinguish peripheral nerve tissue in the coaxial direction of the vision field, residual ligamentum flavum, and fibrous ligament tissue. On removal of the lamina and ligament with open surgical instruments, they can easily be discovered and resected according to the decompression need. This is the primary advantage of spinal endoscopy. At the same time, RF hemostasis, under spinal endoscopy, is more accurate. In turn, this reduces the thermal effect during operation, lowers turbid water irrigation, and enhances the clarity of the visual field. More importantly, the instruments utilized in UME are small and endoscopic. UME can protect the dura and nerve root by rotating the smooth working pipe and stretching the disc as soon as possible, unlike during the use of the nerve root retractor in traditional UBE surgery. Tearing and injury of the dura and nerve root can be avoided to some extent. Further, we used endoscopic forceps to stretch the disc under the monitoring of the spinal endoscope. Less space is required for instruments during UME surgery, which decreases iatrogenic injury caused by the entrance of open surgical instruments used in traditional UBE surgery.
This surgical procedure significantly improves the convenience of the procedure by allowing seamless conversion to a minimally invasive dual-channel spinal endoscopic procedure after a uniaxial spinal endoscopic operation that requires open surgical instruments (Kerrison forceps, ball mill drills, etc.), such as removal of bone from the lamina and spinous root and thicker ligamentum flavum. At the same time, the operator does not need to change special surgical instruments during the operation, which makes it more friendly and easier for beginners to get started. More, the use of this procedure allows the surgeon to increase discrimination in patients with complicated spinal degeneration and less precise anatomic landmarks. By using a uniaxial spinal endoscope first, the operator can more accurately and easily identify the starting point of the procedure and avoid serious surgical errors, especially in areas that require more delicate manipulation, such as hemostasis near nerve roots and bony decompression of nerve root canal, as well as in patients with more specific surgical procedures such as thinness of the ligamentum flavum and neurodevelopmental variation. The use of this procedure also facilitates dual-channel spinal endoscopy for beginners, who can first use a uniaxial spinal endoscope to establish the operating environment in the smaller gap between the vertebral plate and the erector spinae muscle for initial cleanup and position confirmation, which can reduce subsequent unnecessary and excessive muscle damage and bone loss.
Another major advantage of unilateral bi/multi-portal endoscopy (UME) is the protection of neurological safety, especially during the removal of large migrated intervertebral discs. In conventional biportal endoscopic spine surgery, the nerve root hook is often supported by an assistant for nerve nudging and protection while the operator holds the arthroscope with one hand for visualization, and the migrated and prolapsed disc may largely crush the nerve root or cause the nerve root hook to shift, resulting in irreversible mechanical damage to the nerve tissue. Although the free disc is removed intact, the postoperative presentation of such patients is characterized by persistent sensory abnormalities such as numbness and soreness as well as non-recovery and slow recovery of muscle strength loss. Our new surgical application of uniaxial spinal endoscopy circumvents this problem by using a uniaxial endoscope instead of an arthroscope, allowing the operator to view the operative field while protecting the nerve roots with the rounded lingual lobe of the working sleeve, encapsulating the protruding free disc in the sleeve. The nerve tissue is completely blocked outside the sleeve, and as long as the sleeve is continuously pressed against the fibrous ring hole, there is no risk of injury to the nerve root, increasing the intraoperative stability of the operator and the safety of the nerve tissue.
In some patients who undergo lumbar decompression using the classic dual-channel arthroscopic approach, there are often difficulties in decompressing the nerve roots on the contralateral side of the operation. Because of the wide and fixed angles of the classical biportal endoscopic instruments, it is difficult to treat the hyperplastic bone and hemorrhages in some of the more difficult angles, especially the removal of the ligamentum flavum at the contralateral cephalic end and the decompression of nerve root canal from dorsal to the caudal and the hemostasis of peri-neural root vascular bleeding. Also, during the use of the original UBE conventional instrumentation, a larger space may be required, which requires the removal of more bone from the vertebral plates as well as the spinous roots, and these coarse instruments may cause mechanical compression damage to the nerve tissue in the small space in order to achieve surgical decompression goals and exact hemostasis. With our innovative unilateral bi/multi-portal endoscopy, the procedure can be simplified by precisely placing the retractable radiofrequency electrode at the bleeding point under the single-channel spine endoscope to reduce local heat dissipation and avoid burns to the nerve tissue. The Kerrison forceps utilized in another working portal can be used to precisely decompress hyperplastic bone and ligamentum flavum, which are more difficult to remove for uniaxial spinal endoscopy. After removal of lamina, uniaxial endoscope can be flexibly moved in the scopic portal. The operator can also bluntly dislodge and release the nerve roots with a canal sleeve to ensure adequate decompression under the premise of safety and preserve more bone, reduce unnecessary postoperative lumbar instability, and reduce the recurrence of lumbar disc herniation and medically induced spinal stenosis in adjacent segments.
Compared with the previous unilateral biportal endoscopy using an arthroscope as the primary lens, our new procedure has a significantly lower incidence of postoperative complications (see Table 2) and no significant postoperative intracanal hematoma or infection, which, from another perspective, suggests that the new procedure reduces excessive injury and stripping of the spinal canal and lamina muscles, and reduces the formation of ineffective physiological cavities.
However, it is worth noting that among our 42 patients, there were also four patients with postoperative related complications. One of them was a patient with a herniated disc at L5-S1 combined with a dilated dural sac, who had a transient headache and sensory numbness in the perineal area immediately after surgery, which we considered to be caused by the residual saline in the spinal canal after endoscopic surgery. The headache and the symptoms related to the cauda equina occurred because of the high epidural pressure in this patient due to the special dural dilatation. In the other patient with bilateral decompression, even though we used uniaxial endoscopic instruments to stop the bleeding of the vessels near the contralateral nerve root canal, the nerve tissue was still irritated, causing transient postoperative numbness, which had completely resolved one month after surgery. This patient’s case suggests that more care should be taken to protect the nerve tissue and that a more prudent surgical approach, such as our new uniaxial spinal endoscopic procedure, should be used.
Limitations
Our research encountered certain limitations. This was designed as a retrospective study introducing a new technology. The sample size was relatively small, with no control group. In the future, a randomized controlled trial involving a large sample population is warranted to illustrate the effects of surgery and compare it with other corresponding endoscopic surgeries in particular. In addition, the duration of our postoperative follow-up, on average 13 months, was short. With the continued development of UME technology and the increase in the number of patients receiving UME surgery, more long-term and comprehensive follow-up records can be achieved in our future clinical study, which may better demonstrate the safety and efficacy of UME technology in treating patients with lumbar degenerative disease.
Conclusion
UME combines advantages of both open and endoscopic surgeries. It provides the same satisfactory postoperative outcomes. UME has great potential to become an alternative intervention to microscopic laminotomy. More studies are necessary to elucidate the true benefits of UME over other approaches.
Abbreviations
UME, unilateral bi/multi-portal endoscopy; UBE, unilateral biportal endoscopy; VAS, visual analog scale; ODI, Oswestry disability index; CT, computed tomography; MRI, magnetic resonance imaging; PETD, percutaneous endoscopic transforaminal discectomy; PEID, percutaneous endoscopic interlaminar discectomy; CD, canal diameter; CCA, central canal area.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Approval and Informed Consent
This study was approved by the ethics committee of Shengjing Hospital of China Medical University (2022PS164J), and written informed consent was obtained from participants prior to data collection. This study was conducted in accordance with the Declaration of Helsinki.
Compliance with Ethical Standards
The authors received consent from the patient to participate in the submission.
Funding
This work was supported by grants from the Liaoning Natural Science Foundation (No.2020-MS-04); The Guiding Plan of Scientific Research Foundation of Liaoning Province (No. 2019-ZD-0761); National Key R&D program of China (No.2019YFC0121400). The funders had no role in the organization of the research, in the decision to publish the research data, in the writing of the article content, or interpretation of results, analysis or data collection.
Disclosure
The authors report no conflicts of interest.
References
1. Mobbs RJ, Li J, Sivabalan P, et al. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine. 2014;21:179–186. doi:10.3171/2014.4.SPINE13420
2. Iguchi T, Kurihara A, Nakayama J, et al. Minimum 10-year outcome of decompressive laminectomy for degenerative lumbar spinal stenosis. Spine. 2000;25:1754–1759. doi:10.1097/00007632-200007150-00003
3. Kim JY, Ryu DS, Paik HK, et al. Paraspinal muscle, facet joint, and disc problems: risk factors for adjacent segment degeneration after lumbar fusion. Spine. 2016;16:867–875. doi:10.1016/j.spinee.2016.03.010
4. Hu ZJ, Fang XQ, Zhou ZJ, et al. Effect and possible mechanism of muscle-splitting approach on multifidus muscle injury and atrophy after posterior lumbar spine surgery. J Bone Joint Surg Am. 2013; 2013:95.
5. Kim M, Kim H, Adsul NM, et al. Evolution of spinal endoscopic surgery. Neurospine. 2019;16:6–14. doi:10.14245/ns.1836322.161
6. Choi KC, Shim HK, Hwang JS, et al. Comparison of surgical invasiveness between microdiscectomy and 3 different endoscopic discectomy techniques for lumbar disc herniation. World Neurosurg. 2018;116:e750–e758. doi:10.1016/j.wneu.2018.05.085
7. Bresnahan LE, Smith JS, Ogden AT, et al. Assessment of paraspinal muscle cross-sectional area after lumbar decompression: minimally invasive versus open approaches. Clin Spine Surg. 2017;30:E162–E168. doi:10.1097/BSD.0000000000000038
8. Qin R, Liu B, Hao J, et al. Percutaneous endoscopic lumbar discectomy versus posterior open lumbar microdiscectomy for the treatment of symptomatic lumbar disc herniation: a systemic review and meta-analysis. World Neurosurg. 2018;120:352–362. doi:10.1016/j.wneu.2018.08.236
9. Ahn Y, Oh HK, Kim H, et al. Percutaneous endoscopic lumbar foraminotomy: an advanced surgical technique and clinical outcomes. Neurosurgery. 2014;75:
10. Ruetten S, Komp M, Merk H, et al. Full⁃endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine. 2008;33:
11. Heo DH, Son SK, Eum JH, et al. Fully endoscopic lumbar interbody fusion using a percutaneous unilateral biportal endoscopic technique: technical note and preliminary clinical results. Neurosurg Focus. 2017;43:E8. doi:10.3171/2017.5.FOCUS17146
12. Kim J-E, Choi D-J. Unilateral biportal endoscopic decompression by 30° endoscopy in lumbar spinal stenosis: technical note and preliminary report. J Orthop. 2018;15:366–371. doi:10.1016/j.jor.2018.01.039
13. Ahn Y, Jeong TS, Lim T, Jeon JY. Grading system for migrated lumbar disc herniation on sagittal magnetic resonance imaging: an agreement study. Neuroradiology. 2018;60:101–107. doi:10.1007/s00234-017-1943-7
14. Mysliwiec LW, Cholewicki J, Winkelpleck MD, Eis GP. MSU classification for herniated lumbar discs on MRI: toward developing objective criteria for surgical selection. Eur Spine J. 2010;19:1087–1093. doi:10.1007/s00586-009-1274-4
15. Schizas C, Theumann N, Burn A, et al. Qualitative grading of severity of lumbar spinal stenosis based on the morphology of the dural sac on magnetic resonance images. Spine. 2010;35:1919–1924. doi:10.1097/BRS.0b013e3181d359bd
16. Li J, Li H, Zhang N, et al. Radiographic and clinical outcome of lateral lumbar interbody fusion for extreme lumbar spinal stenosis of Schizas grade D: a retrospective study. BMC Musculoskelet Disord. 2020;21:259. doi:10.1186/s12891-020-03282-6
17. Fu CJ, Chen WC, Lu ML, Cheng CH, Niu CC. Comparison of paraspinal muscle degeneration and decompression effect between conventional open and minimal invasive approaches for posterior lumbar spine surgery. Sci Rep. 2020;10:14635. doi:10.1038/s41598-020-71515-8
18. Costa F, Sassi M, Cardia A, et al. Degenerative lumbar spinal stenosis: analysis of results in a series of 374 patients treated with unilateral laminotomy for bilateral microdecompression. J Neurosurg Spine. 2007;7:579–586. doi:10.3171/SPI-07/12/579
19. Leone A, Guglielmi G, Cassar-Pullicino VN, Bonomo L. Lumbar intervertebral instability: a review. Radiology. 2007;245:62–77. doi:10.1148/radiol.2451051359
20. De Antoni DJ, Claro ML, Poehling GG, et al. Translaminar lumbar epidural endoscopy: anatomy, technique, and indications. Arthroscopy. 1996;12:
21. Soliman HM. Irrigation endoscopic discectomy: a novel percutaneous approach for lumbar disc prolapse. Eur Spine J. 2013;22:
22. Hwa Eum J, Hwa Heo D, Son SK, et al. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: a technical note and preliminary clinical results. J Neurosurg Spine. 2016;24:
23. Örükoğlu AG, Göker B, Tahta A, et al. Fully endoscopic interlaminar and transforaminal lumbar discectomy: analysis of 47 complications encountered in a series of 835 patients. Neurocirugia. 2017;28:235–241. doi:10.1016/j.neucir.2017.03.003
24. Kim HS, Choi SH, Shim DM, et al. Advantages of new endoscopic Unilateral Laminectomy for Bilateral Decompression (ULBD) over conventional microscopic ULBD. Clin Orthop Surg. 2020;12:330–336. doi:10.4055/cios19136
25. Heo DH, Lee N, Park CW, et al. Endoscopic unilateral laminotomy with bilateral discectomy using biportal endoscopic approach: technical report and preliminary clinical results. World Neurosurg. 2020;137:31–37. doi:10.1016/j.wneu.2020.01.190
26. Heo DH, Lee DC, Park CK. Comparative analysis of three types of minimally invasive decompressive surgery for lumbar central stenosis: biportal endoscopy, uniportal endoscopy, and microsurgery. Neurosurg Focus. 2019;46:E9. doi:10.3171/2019.2.FOCUS197
27. Kim JE, Choi DJ. Clinical and radiological outcomes of unilateral biportal endoscopic decompression by 30 arthroscopy in lumbar spinal stenosis: minimum 2-year follow-up. Clin Orthop Surg. 2018;10:328–336. doi:10.4055/cios.2018.10.3.328
28. Lin GX, Huang P, Kotheeranurak V, et al. A systematic review of unilateral biportal endoscopic spinal surgery: preliminary clinical results and complications. World Neurosurg. 2019;125:425–432. doi:10.1016/j.wneu.2019.02.038
29. Jiang HW, Chen CD, Zhan BS, et al. Unilateral biportal endoscopic discectomy versus percutaneous endoscopic lumbar discectomy in the treatment of lumbar disc herniation: a retrospective study. J Orthop Surg Res. 2022;17:30. doi:10.1186/s13018-022-02929-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





