Back to Journals » OncoTargets and Therapy » Volume 15
Undifferentiated Large Cell Carcinoma of the Thymus Associated with Plasma-Cell Type Castleman Disease-Like Reaction: A Case Series
Authors Han L, Wang EH, Wang L
Received 27 December 2021
Accepted for publication 4 March 2022
Published 10 March 2022 Volume 2022:15 Pages 237—242
DOI https://doi.org/10.2147/OTT.S356043
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Arseniy Yuzhalin
Lihua Han,1 En-Hua Wang,2 Liang Wang2
1The Central Laboratory of Morphology, Shenyang Medical College, Shenyang, People’s Republic of China; 2Department of Pathology, First Affiliated Hospital and College of Basic Medical Sciences, China Medical University, Shenyang, People’s Republic of China
Correspondence: Lihua Han, The Central Laboratory of Morphology, Shenyang Medical College, Shenyang, People’s Republic of China, Email [email protected]
Abstract: Undifferentiated large cell carcinoma of the thymus with a Castleman disease (CD)-like reaction is a thymic carcinoma accompanied by an inflammatory reaction closely resembling the morphological features of CD. This disease is extremely rare and distinctive, only five cases have been documented in a single report, and all five cases were associated with a reaction like the hyaline vascular type CD. For the first time, we report two cases of undifferentiated large cell carcinoma of the thymus with a plasma cell type CD-like reaction. The two cases presented similar histological findings and immunoprofiles. Undifferentiated large cells were arranged in nests and cords within hyperplastic follicles, mimicking pseudogerminal centers. Abundant plasma cells were distributed in the interfollicular areas. The tumor cells were positive for CK-pan and BRG1 staining but negative for CD5, CD117, CK5/6, p63, p40, and EBER. Therefore, the diagnoses of squamous cell carcinoma, lymphoepithelial carcinoma, or micronodular thymic carcinoma with lymphoid hyperplasia were excluded. Even though the carcinoma cells showed high-grade nuclear pleomorphism with prominent nucleoli, these two cases presented indolent clinical courses, which were consistent with the previous report.
Keywords: Castleman disease-like reaction, immunohistochemistry, prognosis, thymic carcinoma, undifferentiated large cell carcinoma
Introduction
Undifferentiated large cell carcinoma of the thymus with a Castleman disease-like reaction is extremely rare and is categorized as thymic carcinoma, not otherwise specified (NOS). With a unique high-grade histological morphology, its clinical course is remarkably indolent.1 To date, only one study has reported this rare malignancy of the thymus.2 In contrast to previously reported cases of Castleman disease of the hyaline vascular type (HVCD) -like reaction, the two cases in current study were undifferentiated large cell carcinoma with a plasma cell type CD-like reaction, which has never been described.
Case Presentation
Case 1
A 35-year-old man presented with fatigue and blepharoptosis. The patient had no history of malignancy or chronic inflammatory disease, and no overuse of alcohol or tobacco. Chest computed tomography (CT) revealed a 3.5-cm solid mass with a clear boundary in the anterior-superior mediastinum. A total thymectomy was performed without additional therapy. With no further treatment, the patient survived without evidence of recurrence for 72 months.
Case 2
A 60-year-old man with no history of tobacco or alcohol, was found to have an enlarged lymph node, 3 cm in diameter, in the right supraclavicular area with no other abnormalities. A lymph node resection was performed and analysis of the excised lymph mass revealed carcinoma. However, no primary carcinoma was found during imaging studies such as CT, magnetic resonance imaging (MRI), and ultrasound scanning; therefore, the patient refuse to undergo further treatment. The patient’s mediastinal lymph nodes were slightly enlarged during follow-up examinations, and no other lesions were found on subsequent checkups. The patient survived for 58 months after the initial diagnosis.
Pathological Findings
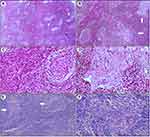
The microscopic features of both cases were similar. In Case #1, low magnification analysis showed numerous proliferative lymphoid follicular-like patterns that were varied in size and contained hyperplastic germinal centers. Within certain follicles, undifferentiated large tumor cells were arranged in nests or cords in the middle and surrounded by reactive lymphocytes (Figure 1A). This pattern closely mimicked the pseudo-germinal centers (Figure 1B). At high magnification, most of the interfollicular areas were filled with plasma cells (Figure 1C). The tumor cells in the pseudo-germinal center were pleomorphic with prominent nucleoli. A small number of lymphocytes were evident in the epithelial cords (Figure 1D). Similar patterns were observed in Case #2 (Figure 1E and F).
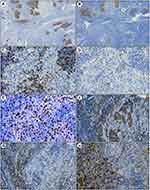
Immunohistochemistry of both cases revealed that, lymphoid follicles contained prominent CD21-positive follicular dendritic cells (Figure 2A). Cancer cells mimicking the pseudo-germinal center were positively stained by CK-pan (Figure 2B) and BRG1, but negatively for CD5, CD117, CD1α, p40, p63, PAX8, NUT, and CK5/6 (Figure 2C and D). Thus, the diagnoses of micronodular thymic carcinoma with lymphoid hyperplasia and lymphoepithelial carcinoma were excluded. Markers for other tumors such as CK7, TTF-1, synaptophysin, chromogranin A, SALL4, AFP, and OCT3/4, were also negative. In these tumor cells, the Ki-67 index was approximately 90% (Figure 2E) and p53 staining was widespread and strong. The lymphocytes in the follicles and surrounding tumor islands were positive for CD20 (Figure 2F) and negative for Bcl-2. Perifollicular lymphocytes were positive for CD3 (Figure 2G), and abundant plasma cells in the interfollicular area were positive for CD138 (Figure 2H). TdT-positive immature T cells were not found within the tumor tissues, indicating there were no immature lymphocytes. In situ hybridization for EBV showed negative results.
In both cases, the diagnoses were rendered as undifferentiated large cell carcinoma of the thymus associated with a plasma-cell type CD-like reaction.
Discussion
Undifferentiated large cell carcinoma with a CD-like reaction, an extremely rare finding, is currently classified as thymic carcinoma NOS. Previously, only five cases were documented by Nonaka et al.2 All these cases were undifferentiated large cell carcinomas with HVCD-like reactions. Hyalinized vessels and onion-skin-like lymphocytic distribution around the germinal center were relatively easy to recognize. However, cases with plasma cells-type Castleman disease-like reactions have never been reported, and the diagnosis is more challenging. Pathologists should be cautious of undifferentiated cancer cells that account for only 10% of the whole tissue on the slide and surrounded by a large amount of lymphoid tissue, as they could easily be misdiagnosed as lymphoma or CD. Pleomorphism and evident atypia of cancer cells with immunoreaction to CK-pan can provide hints for diagnosis.
It is thought that undifferentiated large cell carcinoma with a CD-like reaction is the primary malignancy of the thymus. However, in case 2, the initial lesion was found in the right supraclavicular fossa, and during the postoperative follow-up, the patient’s lymph nodes in the anterior mediastinum were found to be slightly enlarged without other abnormalities. Interestingly, Nonaka et al reported a similar case2 in which a thymic neoplasm was also found 16 years after the initial detection of metastases in two distant lymph nodes. In Case #2, it was unknown whether the primary site was the thymus or ectopic thymic tissue in the right supraclavicular fossa. CD5, CD117, and PAX8 are useful markers for carcinomas arising from the thymus, even in ectopic sites.3,4 However, based on Nonaka’s report2 and our study, undifferentiated large cell carcinoma with a CD-like reaction appeared to lose the expression of CD5, CD117 and PAX8, which might be due to the undifferentiated state of the tumor cells.
Undifferentiated large cell carcinoma associated with a CD-like reaction is characterized by undifferentiated tumor cells arranged in nests or cords, surrounded by abundant lymphoid stroma. Using this information, metastatic cancer should be excluded from the differential diagnosis. In the current study, both patients underwent careful imaging examinations, and long-term follow-up revealed no other primary lesions. Moreover, biomarkers used to indicate possible origins of metastases yielded negative staining. In addition to metastatic cancer, lymphocyte-rich Hodgkin’s lymphoma and mediastinal large B-cell lymphoma were further excluded due to negative staining for CD 15, CD30, and EBER.
Undifferentiated large cell carcinoma associated with CD-like reactions needs to be distinguished from lymphoepithelial carcinoma and micronodular thymic carcinoma with lymphoid hyperplasia.1,2 The former has more pronounced lymphoepithelial lesions and demonstrates squamous cell differentiation (positive for CK5/6, p63, and P40).5 Moreover, lymphoepithelial carcinoma has higher rates of Epstein-Barr virus infection than other types of thymic epithelial tumors. In micronodular thymic carcinoma with lymphoid hyperplasia, the lesion lacks a CD-like architecture, and the cancer cells are positive for CD5 and CD117 staining.6–10
Unlike undifferentiated large cell carcinoma occurring at any other sites, undifferentiated large cell carcinoma of the thymus associated with a CD-like reaction has a strikingly indolent clinical presentation.1,2 Therefore, a correct diagnosis is imperative for clinical management. This CD-like reaction is most likely a morphological manifestation driven by the host immune response against tumor cells, and this particular morphological change appears to be a reasonable explanation for the relatively good prognosis of these two patients, similar to the previous report.2
Data Sharing Statement
The datasets supporting the conclusions of this article are included within the article.
Ethical Approval and Consent to Participate
Ethical approval for this study was obtained from the institutional ethic review boards of the First Affiliated Hospital of China Medical University.
Patient Consent for Publication
Informed consents were obtained from the patients for publication. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the Natural Science Foundation of Liaoning to Liang Wang (2021-MS-160).
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article.
References
1. Board WCOTE. Thoracic Tumours.
2. Nonaka D, Rodriguez J, Rollo JL, et al. Undifferentiated large cell carcinoma of the thymus associated with Castleman disease-like reaction: a distinctive type of thymic neoplasm characterized by an indolent behavior. Am J Surg Pathol. 2005;29(4):490–495. doi:10.1097/01.pas.0000155148.45423.b5
3. Kunc M, Kamieniecki A, Walczak G, et al. Intrasalivary thymic carcinoma: a case report and literature review. Head Neck Pathol. 2021. doi:10.1007/s12105-021-01394-6
4. Weissferdt A, Moran CA. Pax8 expression in thymic epithelial neoplasms: an immunohistochemical analysis. Am J Surg Pathol. 2011;35(9):1305–1310. doi:10.1097/PAS.0b013e3182260735
5. Ose N, Kawagishi S, Funaki S, et al. Thymic lymphoepithelial carcinoma associated with Epstein-Barr virus: experiences and literature review. Cancers. 2021;13(19):4794. doi:10.3390/cancers13194794
6. Weissferdt A, Moran CA. Micronodular thymic carcinoma with lymphoid hyperplasia: a clinicopathological and immunohistochemical study of five cases. Mod Pathol. 2012;25(7):993–999. doi:10.1038/modpathol.2012.40
7. Thomas de Montpreville V, Mansuet-Lupo A, Le Naoures C, et al. Micronodular thymic carcinoma with lymphoid hyperplasia: relevance of immunohistochemistry with a small panel of antibodies for diagnosis-a RYTHMIC study. Virchows Arch. 2021;479(4):741–746. doi:10.1007/s00428-021-03044-2
8. Mneimneh WS, Gökmen-Polar Y, Kesler KA, et al. Micronodular thymic neoplasms: case series and literature review with emphasis on the spectrum of differentiation. Mod Pathol. 2015;28(11):1415–1427. doi:10.1038/modpathol.2015.104
9. Wang B, Li K, Song Q-K, et al. Micronodular thymic tumor with lymphoid stroma: a case report and review of the literature. World J Clin Cases. 2019;7(23):4063–4074. doi:10.12998/wjcc.v7.i23.4063
10. Tateyama H, Saito Y, Fujii Y, et al. The spectrum of micronodular thymic epithelial tumours with lymphoid B-cell hyperplasia. HistoPathology. 2001;38(6):519–527. doi:10.1046/j.1365-2559.2001.01133.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.