Back to Journals » Drug Design, Development and Therapy » Volume 18
Understanding Sorafenib-Induced Cardiovascular Toxicity: Mechanisms and Treatment Implications
Authors Li J, Zhang L, Ge T, Liu J, Wang C, Yu Q
Received 25 October 2023
Accepted for publication 9 March 2024
Published 18 March 2024 Volume 2024:18 Pages 829—843
DOI https://doi.org/10.2147/DDDT.S443107
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Jue Li,1,* Lusha Zhang,2,* Teng Ge,2 Jiping Liu,1 Chuan Wang,1 Qi Yu1,2
1Engineering Research Center of Brain Health Industry of Chinese Medicine, Key Laboratory of Pharmacodynamics and Material Basis of Chinese Medicine of Shaanxi Administration of Traditional Chinese Medicine, Pharmacology of Chinese medicine, Shaanxi University of Chinese Medicine, Xianyang, 712046, People’s Republic of China; 2Shaanxi Key Laboratory of Ischemic Cardiovascular Diseases and Institute of Basic and Translational Medicine, Xi’an Medical University, Xi’an, 710021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qi Yu; Chuan Wang, Email [email protected]; [email protected]
Abstract: Tyrosine kinase inhibitors (TKIs) have been recognized as crucial agents for treating various tumors, and one of their key targets is the intracellular site of the vascular endothelial growth factor receptor (VEGFR). While TKIs have demonstrated their effectiveness in solid tumor patients and increased life expectancy, they can also lead to adverse cardiovascular effects including hypertension, thromboembolism, cardiac ischemia, and left ventricular dysfunction. Among the TKIs, sorafenib was the first approved agent and it exerts anti-tumor effects on hepatocellular carcinoma (HCC) and renal cell carcinoma (RCC) by inhibiting angiogenesis and tumor cell proliferation through targeting VEGFR and RAF. Unfortunately, the adverse cardiovascular effects caused by sorafenib not only affect solid tumor patients but also limit its application in curing other diseases. This review explores the mechanisms underlying sorafenib-induced cardiovascular adverse effects, including endothelial dysfunction, mitochondrial dysfunction, endoplasmic reticulum stress, dysregulated autophagy, and ferroptosis. It also discusses potential treatment strategies, such as antioxidants and renin-angiotensin system inhibitors, and highlights the association between sorafenib-induced hypertension and treatment efficacy in cancer patients. Furthermore, emerging research suggests a link between sorafenib-induced glycolysis, drug resistance, and cardiovascular toxicity, necessitating further investigation. Overall, understanding these mechanisms is crucial for optimizing sorafenib therapy and minimizing cardiovascular risks in cancer patients.
Keywords: sorafenib, vascular endothelial growth factor receptor, hypertension, cardiovascular toxicity, cancer
Introduction
Sorafenib, the first FDA-approved inhibitor of the vascular endothelial growth factor (VEGF) pathway, was initially developed for the treatment of renal cell carcinoma (RCC). Since then, it has been approved for use in hepatocellular carcinoma (HCC), acute myeloid leukemia, sclerofibromatosis, and metastatic melanoma.1 However, as its clinical applications have expanded, reports of drug resistance and side effects among patients receiving sorafenib treatment have become increasingly common.2
Notably, when these side effects occur in the cardiovascular system, patients often experience hypertension, thrombosis, and cardiac toxicity.3 The adverse cardiovascular effects caused by sorafenib may be attributed to the complex interaction between the drug and vasculature. Since sorafenib can block various targets in the VEGF pathway, inhibiting this pathway may disrupt the homeostasis of the vasculature and lead to dysfunction in the cardiovascular system. Therefore, elucidating the underlying mechanism behind sorafenib-induced adverse cardiovascular effects is necessary to prevent these side effects and drug resistance.
Sorafenib is an Inhibitor of Multiple Kinases Including VEGFR Family Members
Origin, Structure, and Pharmacology of Sorafenib
The VEGF pathway plays a crucial role in promoting angiogenesis during tumor progression, making it an attractive target for therapeutic interventions aimed at suppressing tumor angiogenesis. Consistent with this strategy, numerous inhibitors and neutralizing antibodies have been developed to block VEGFs, VEGF receptors, and their tyrosine kinases. One such inhibitor is sorafenib, also known as Nexavar or sorafenib tosylate, which has been shown to inhibit various targets within the VEGF pathway.4 Interestingly, sorafenib was originally discovered during a biochemical analysis conducted to evaluate the structure-activity relationship of inhibitor precursors targeting C-RAF kinase, a gene associated with rapid fibrosarcoma growth. It is a diaryl urea compound with a unique dual-action mechanism on both Raf kinase and vascular endothelial growth factor receptors (VEGFRs).5 Bayer Pharmaceuticals performed high-throughput screening from a pool of 20,000 compounds to identify a potent Raf1 kinase inhibitor. Ultimately, 3-thienyl urea (BAY43-9006), later named sorafenib, was selected for further preclinical development.6 Sorafenib is a member of the class of phenylureas, with urea in which one of the nitrogens is substituted by a 4-chloro-3-trifluorophenyl group while the other is substituted by a phenyl group, which in turn is substituted at the para position by a [2-(methylcarbamoyl) pyridin-4-yl] oxy group. In 2000, sorafenib entered Phase I clinical trials and subsequently gained approval from the FDA in 2005 as a first-line treatment for advanced cancer.6 Despite its initial development as a raf1 inhibitor, sorafenib has demonstrated inhibitory effects on other targets, including B-raf, VEGFR1/2/3, platelet-derived growth factor receptor-β (PDGFRβ), fibroblast growth factor receptor 1 (FGFR1), c-Kit, Flt-3 and RET. This complex pharmacological profile may contribute to the occurrence of adverse effects and drug resistance in patients.5
Sorafenib, when taken orally, generally exhibits a relative bioavailability of 38–49%. It reaches its maximum plasma concentration (Cmax) in approximately 3 hours. According to a study, sorafenib appears to have an apparent volume of distribution of 213L.7 In vitro testing has shown that sorafenib binds to plasma proteins at a rate of 99.5%. The liver plays a significant role in the metabolism of sorafenib, primarily through cytochrome P450 3A4 (CYP3A4) and glucuronidation via uridine diphosphate glucuronyltransferase 1A9 (UGT1A9). In the gastrointestinal tract, bacterial glucuronidase enzymes may hydrolyze glucuronide conjugates, leading to the reabsorption of unconjugated drug.8 Metabolites of sorafenib have been identified in plasma, feces, and urine. In plasma, five metabolites were detected, while three were found in feces and two in urine. Notably, the N-oxide metabolite demonstrates a similar potency to sorafenib itself. Within 14 days of oral administration, approximately 96% of the administered dose was recovered. Of this amount, 77% was excreted in feces (with 51% being unchanged sorafenib), and the remaining 19% was excreted as glucuronidated metabolites in urine. Sorafenib has an elimination half-life ranging from 25 to 48 hours. Oral sorafenib, approved by the FDA as a multi-target kinase inhibitor, is widely used as the first-line treatment for RCC and HCC, serving as a cornerstone in the treatment of these cancers.9 Moreover, sorafenib’s efficacy as a broad-acting anti-tumor agent has led to its investigation in clinical trials for new indications and treatment modalities, such as acute myeloid leukemia, desmoid tumors and metastatic melanoma.10 Furthermore, a recent animal study have demonstrated that low doses of sorafenib can effectively improve non-alcoholic steatohepatitis (NASH), indicating that the induction of mitochondrial uncoupling and activation of the AMPK pathway are the primary mechanisms.
Targets of Sorafenib Involving with Cardiovascular System
Sorafenib suppresses cellular proliferation and angiogenesis but promotes apoptosis,11 whereby it inhibit kinase pathways, including cell surface tyrosine kinase receptors such as ckit, FMS-like tyrosine kinase (FLT-3), VEGFR and platelet-derived growth factor receptor-beta (PDGFR), as well as downstream intracellular serine/threonine kinases such as Raf-1 and extracellular signal-regulated kinase (ERK).12 Given that sorafenib has such complex influences, its adverse effects may be attributed to the inhibition of various targets. Notable, two main pathways are involved in the pharmacological action of sorafenib: Firstly, targeting Raf-1, B-Raf, and Ras/Raf/MEK/ERK signaling pathways contribute to the inhibition of tumor cell proliferation;13 secondly, targeting receptor-type tyrosine-protein kinase FLT3 (FLT-3), c-kit, VEGFR-2, VEGFR-3, PDGFR, and VEGF/VEGFR signaling pathways contribute to the suppression of angiogenesis.14,15 These molecular targets play crucial roles in maintaining vascular homeostasis, and their inhibition by sorafenib disrupts normal cellular functions, leading to cardiovascular toxicity. Specifically, sorafenib inhibits Raf-1, a kinase involved in hypertrophy and cardiomyocyte response to pressure, thereby disrupting vascular contractility and promoting cardiomyocyte apoptosis.16 Raf-1 is also reported to promotes cardiomyocyte survival through a MEK/ERK–independent mechanism.17 Raf-1 in vascular smooth muscle cells (VSMCs) can regulate vascular contractility through regulation of calcium sensitization,18 and the Raf-1–MEK1/2–ERK1/2 MAPK signaling pathway is also involved in VSMC proliferation and neointimal hyperplasia.19 In addition to normal growth and development, the VEGF/VEGFR pathway is vital for physiological responses and homeostasis in cardiovascular system.20 Sorafenib targets VEGFRs, crucial for endothelial cell survival, proliferation, and migration. Inhibition of the VEGF/VEGFR pathway not only suppresses angiogenesis but also disrupts vascular integrity, leading to endothelial dysfunction and increased risk of thrombotic events.21 All vasculatures express the VEGF/VEGFR, which is significantly upregulated when stress or injury occurs.22 In endothelial cells (ECs), VEGF stimulates the production of nitric oxide (NO) and prostaglandin I2 (PGI2) by interacting with VEGFR.23 Therefore, it is a key pathway for EC survival, proliferation and migration, further contributing to vasodilation and the prevention of blood cells adherence.24 Inhibiting the VEGF/VEGF pathway not only suppresses angiogenesis, but also disrupts vascular integrity and the normal interactions between EC and other cells.25 In view of sorafenib therapy associating with myocardial ischemia,26,27 and some case reports revealing that long-term sorafenib application causes coronary stenosis and atherosclerosis in carotid artery, inhibiting the VEGF/VEGFR pathway by sorafenib may contribute to such adverse effects.28 Moreover, sorafenib-induced inhibition of PDGFR and FGFR1 can affect VSMCs, contributing to vascular remodeling and neointimal hyperplasia.9 Additionally, a new study also suggests that sorafenib can affect cardiac metabolism, suggesting that sorafenib cardiotoxicity is related to its deleterious effects on specific cardiac metabolic pathways.29 The dysregulation of these molecular targets by sorafenib ultimately results in adverse cardiovascular effects such as hypertension, myocardial ischemia, decreased left ventricular ejection fraction (LVEF), and congestive heart failure. Overall, the broad spectrum of molecular targets of sorafenib in the cardiovascular system underscores the complexity of its cardiotoxic effects, highlighting the need for a comprehensive understanding of its mechanisms to develop targeted therapeutic strategies and mitigate cardiovascular risks in patients undergoing sorafenib treatment.
Sorafenib and Its Cardiovascular Toxicity
Hypertension Events
Hypertension is the most common cardiovascular side effect when HCC and RCC patients subjected to sorafenib treatment (Table 1).8,9 As early as three weeks into sorafenib treatment, blood pressure increases can persist for 18 weeks before settling down. In different groups of patients, mean systolic and diastolic pressures increase by 16% and 11%, respectively. Sorafenib has been shown to increase the risk of all grade hypertension compared to controls.30,31 Sorafenib was found to cause hypertension in 5% of patients with advanced HCC (all grades), with 2% of cases of grade 3 severity.32 All grades of hypertension occurred in 78 patients (17%) compared with 5 patients (1%) who received placebo in a randomized, double-blind, placebo-controlled Phase 3 trial of sorafenib in patients with advanced RCC.26 Akutsu et al found that 58% of patients treated with sorafenib developed grade II or higher hypertension as a consequence of taking the drug.33 Among patients with sorafenib-induced hypertension, 19.1% had new-onset hypertension, according to a systematic review and meta-analysis.9 In an observational study of TKI-treated patients with tumors (gastrointestinal mesenchymal tumors, HCC, RCC), the incidence of new or exacerbated hypertension mediated by sorafenib was 27%-59%.34–36 The incidence of severe hypertension caused by sorafenib was highest (29.03%) in a meta-analysis of 68,077 patients.37 Such phenomenon was proved by another meta-analysis of 20,494 patients.38 In the pathophysiology, inhibition of VEGFRs disrupts endothelial function and promotes vasoconstriction, contributing to elevated blood pressure; sorafenib-mediated inhibition of PDGFR-beta and FGFR may lead to vascular remodeling and increased vascular resistance.9 It is important to recognize and manage hypertension in patients taking sorafenib, as poorly controlled hypertension is considered a major risk factor for cardiovascular disease (CVD) incidence and mortality, including stroke, coronary heart disease, peripheral arterial disease, and heart failure.31 To manage sorafenib-induced hypertension effectively, a multifaceted approach is necessary.39 First-line interventions typically involve antihypertensive medications such as calcium channel blockers, angiotensin-converting enzyme inhibitors (ACEi), or angiotensin receptor blockers. Furthermore, close monitoring of blood pressure is essential throughout treatment, with prompt adjustments to medication regimens as needed (Table 2). Moreover, in cases of severe or refractory hypertension, dose reduction or temporary interruption of sorafenib therapy may be necessary (Table 2). Collaboration between oncologists, cardiologists, and other healthcare providers is vital to optimize blood pressure control while ensuring continued efficacy of cancer treatment.
 |
Table 1 Sorafenib and Its Cardiovascular Toxicity |
 |
Table 2 Summary of in Mechanisms, Monitoring and Management in Sorafenib-Induced Cardiac Adverse Events |
Other Cardiovascular Toxic Effects
In addition to hypertension, sorafenib can also cause a series of sever cardiovascular events,27,49–51 which include myocardial ischemia, decreased LVEF, congestive heart failure, and coronary artery spasm (Table 1).5,40,52,53 In the CARDIO-SOR study, patients with advanced HCC had a relative incidence of acute coronary syndrome (ACS) and heart failure (HF) of 28% and 18%, respectively.34 Another study showed that 22 patients (4.9%) in the sorafenib group suffered an ischemic or infarct event, compared to 2 patients (0.4%) in the placebo group in a randomized, double-blind, placebo-controlled phase 3 trial of sorafenib for advanced RCC (Table 1).26 Spasms in the coronary artery play a crucial role in the pathogenesis of cardiac ischemia (Table 1). Some trials have shown that sorafenib also caused coronary artery spasm and myocardial infarction, usually manifested as chest pain and abnormal electrocardiographic ST-T changes (Table 1).42,43 Cardiovascular toxicity developed in 50 of 73 patients with RCC who received sorafenib (68%), including 15 of 73 patients (21%) when hypertension was excluded.34,54 From January 2008 to June 2019, a study at Osaka University Hospital showed that sorafenib was associated with impairment of left ventricular diastolic function in patients with preserved LVEF, particularly in those with risk factors for heart failure with preserved ejection fraction (HfpEF).41 There is evidence that sorafenib (30–40 mg/kg/day) induces myocyte necrosis, even in the absence of cardiac injury, and that it dramatically increases mortality when administered to patients with myocardial infarction.40 Vasculopathy associated with sorafenib can follow a fulminant course in young patients who do not otherwise have cardiovascular problems.55 Several studies have suggested that sorafenib can lead to QT prolongation, which is a disturbance in the heart’s electrical system that can potentially lead to serious arrhythmias and sudden cardiac death (Table 1).48,56 However, the exact mechanism by which sorafenib causes QT prolongation is not fully understood. It may involve inhibition of ion channels involved in cardiac repolarization, such as the human ether-a-go-go-related gene (hERG) potassium channel57 (Table 2).
Hemorrhage is another typical complication of VEGFR-targeted drugs, with sorafenib associated with a higher risk of bleeding than controls in a meta-analysis (Table 1).30 Sorafenib causes abnormal coagulation in patients, leading to thrombotic events,44 especially arterial thrombosis,45 which requires more studies to elucidate the underlying mechanism.58,59 As a molecular targeted agent, sorafenib inhibits bone marrow growth and reduces platelet production, resulting in an increased risk of bleeding.46 According to a meta-analysis, there was a 2.2% risk of grade 3 or higher bleeding among 2109 oncology patients taking sorafenib (95% CI: 1.3 ~ 3.6).47 RCC patients were significantly more likely to experience any form of bleeding in sorafenib trials than non-RCC patients.47 A major problem with sorafenib is cardiac damage, but it is manageable with careful cardio monitoring and appropriate treatment if detected at the earliest signs (Table 2). Therefore, it is important for physicians to be aware of the cardiotoxic effects of sorafenib and to understand the biological mechanisms, monitoring and management (Table 2). This will allow them to diagnose cardiotoxicity at an early stage and avoid jeopardizing the overall success of treatment.10
Mechanisms of Sorafenib-Induced Cardiovascular Toxicity
Endothelial Damage and NO Inhibition
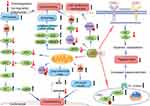
Endothelial dysfunction refers to impaired functioning of the endothelium, the inner lining of blood vessels, which plays a crucial role in regulating vascular tone, blood flow, and maintaining vascular homeostasis.60 Sorafenib inhibits pathways like VEGF and RAF kinases, crucial for ECs proliferation and function.21 This disruption leads to impaired angiogenesis and dysregulated nitric oxide signaling, resulting in increased vascular tone, hypertension, and thrombotic events. The mechanisms of sorafenib-caused cardiovascular toxicity is not fully understood. Most available studies suggest that sorafenib-induced hypertension is associated with the inhibition of VEGF in cardiac tissue.61 According to Quintanilha et al, there is a common intronic single nucleotide polymorphism (SNP) in PIK3R5 that increases the risk of grade 2 hypertension among patients taking VEGF inhibitors, including sorafenib.62 NO is essential for maintaining normal endothelial cell function, vascular homeostasis and vascular neovascularization. ECs normally produce NO and prostaglandins (PGI2), which act on guanylate cyclase, producing cyclic guanosine monophosphate (cGMP).63 Sorafenib targets VEGFR, which reduces NO and PGI2 synthesis, causing vasoconstriction and leading to increased blood pressure (Figure 1).37 Our previous study found that sorafenib reduces the synthesis of NO by causing endothelial damage, which impairs vascular relaxation of ECs, thus leading to hypertension.64 Given that endothelial damage is a initial step for atherosclerotic progression, it is important to be aware of endothelial damage when managing sorafenib-induced myocardial ischemia and coronary artery spasm.
Endothelin-1 System
A G-protein-coupled receptor, the endothelin receptor (ETR) belongs to the endothelin receptor family, including endothelin type A (ETA) and enhancing type B (ETB).65 Unlike the ETA receptor, which is mainly located in the vascular smooth muscle, a majority of ETB receptors are located on the surface of the vascular ECs, where they are responsible for regulating vasodilation by NO-dependent manner.66 Whereas, when VSMCs express the ETB receptor, ET-1 ineracting with these receptors result in vasoconstriction, and such ETB receptors are called vasoconstrictive receptors.67 Our previous study found that in mesenteric arteries, sorafenib mediated the occurrence of hypertension and other adverse cardiovascular events by impairing endothelium-dependent vasodilation and enhancing ETB receptor-mediated vasoconstriction through JNK/MAPK pathway (Figure 1).64 In this study, sorafenib increased ETB expression in VSMCs and caused intense vasoconstriction. The level of blood pressure may have been increased by this novel mechanism in patients with sorafenib treatment.
Mitochondrial Dysfunction
Cells obtain most of their energy from the mitochondria through electron transport and oxidative phosphorylation to create ATP. A drug that perturbs mitochondrial metabolism or homeostasis may cause myocardial disorders. Because cardiac muscle is highly dependent on aerobic metabolism, drugs that interfere with this metabolism can damage them. There are multiple mitochondrial processes that can be inhibited by various drugs, causing mitochondrial toxicity, such as biogenesis, substrate oxidation and oxidative phosphorylation.44,68–70 Mitochondrial dysfunction plays a crucial role in sorafenib-induced cardiovascular toxicity, contributing to myocardial disorders through various mechanisms. Cells rely heavily on mitochondria for energy production, primarily through electron transport and oxidative phosphorylation to generate ATP. Disruption of mitochondrial metabolism or homeostasis by drugs can lead to cardiac muscle damage due to its high dependence on aerobic metabolism. By activating reactive oxygen species (ROS), Kawabata et al discovered that stanniocalcin 1 (STC1) downregulation causes cardiotoxicity in response to sorafenib.71 Sorafenib disrupts mitochondrial cristae in rats by acting as a mitochondrial uncoupler and complex V inhibitor.72 Previous studies have shown that sorafenib causes cardiotoxicity by enhancing ROS to inhibit mitochondrial complex III, opening of the mitochondrial permeability transition pore (mPTP) and over-activating CaMKII, further inducing disruption of Ca2+ homoeostasis and cardiac damage.73 In isolated rat cardiac fibers and H9c2, sorafenib-induced mitochondrial toxicity may be ascribed to impaired ETS enzyme complex function, leading to mitochondrial ROS accumulation and apoptosis.74 Pentraxin 3 (PTX3) levels are often elevated in HCC patients, and it has been shown that inhibition of PTX3 improves left ventricular dysfunction in animal models.62 Sorafenib treatment increases PTX3 expression, thereby resulting in reduced extracellular signal-regulated kinase (ERK) 1/2 expression that affects cardiomyocyte contraction, while also activating c-Jun N-terminal kinase (JNK) downstream pathways to disrupt mitochondrial respiration and trigger apoptosis (Figure 1).12 Additionally, some studies also suggests that sorafenib-induced mitochondrial toxicity can be evoked via regulating ERK/STC1/ROS signal pathway, ATF4/SLCTALL/GSH/ROS signal pathway, mTOR/TFEB/MFN2/MAM/Ca2+/CaMKII signal pathway (Figure 1).75 In summary, the multifaceted effects of sorafenib on mitochondrial function underscore its potential for inducing cardiovascular toxicity, highlighting the importance of understanding and mitigating these adverse effects in clinical settings.
Endoplasmic Reticulum Stress
The endoplasmic reticulum (ER) is a cellular component that plays a vital role in the synthesis, folding, maturation and post-translation modification of secretory and transmembrane proteins. The proper functioning of ER is essential for achieving and maintaining intracellular homeostasis and overall wellness.76 When the ER environment is disrupted, it can lead to the accumulation of unfolded or misfolded proteins, resulting in endoplasmic reticulum stress (ERS). Faced with ERS, cells initiate a survival mechanism known as the unfolded protein response (UPR) to manage the stress. Over the past few years, there has been a growing body of evidence suggesting that ERS is linked to the development of ischemic heart disease, atherosclerosis, cardiac hypertrophy, hypertension, cardiomyopathy, heart failure, and arrhythmia.77 According to a new study, sorafenib has been found to cause ERS in rat primary cardiomyocytes, resulting in cardiotoxicity by increasing the expression of pro-inflammatory factors and cardiac fetal genes.78 In addition to cardiomyocytes, sorafenib-induced ERS may involve with other adverse cardiovascular effects, but more studies are needed to address this mechanism.
Autophagy
Autophagy is a highly conserved biological process that plays a crucial role in both physiological and pathological conditions. Its primary function is to maintain cellular homeostasis in response to stress stimuli such as nutrient scarcity, energy depletion, and disruptions in the redox state. This process facilitates the removal of damaged or long-lived organelles, misfolded proteins, aggregated proteins, and intracellular pathogens. Consequently, the regulation of autophagy by nutrient availability depends on several key cellular energy sensors, including mTORC1, AKT/PKB, AMPK, and PRKA/PKA.79 It has been frequently reported that autophagy is involved in sorafenib resistance in HCC.80,81 Indeed, sorafenib can activate both caspase s 9 and 3 via inhibition of the chaperone GRP78, causing an endoplasmic reticulum stress response that increases the expression of Beclin1 and ATG5, resulting in autophagosome formation.82 It is well known that autophagy plays a dual role in cancer progression, which depends on the stages of tumor development. In HCC, sorafenib-induced autography can lead to sorafenib resistance. However, unlike HCC, Liang et al report that sorafenib inhibits the basal autophagy activity of cardiomyocytes, indicating that impairments in autophagy and mitochondrial dynamics are involved in sorafenib-induced cardiomyocyte apoptosis.83 Nevertheless, given the extensive involvement of autophagy in various CVDs, it is essential to carefully examine the effects of sorafenib-induced autophagy on conditions such as hypertension, myocardial ischemia, decreased LVEF, congestive heart failure, and coronary artery spasm.84
Ferroptosis and Other Programmed Cell Deaths
Ferroptosis is an iron-dependent cell death characterized by the accumulation of lipid hydroperoxides.85 In this process, ferroptosis-inducing factors can directly or indirectly affect glutathione peroxidase through different pathways, resulting in the damage of the antioxidant defense dependent on glutathione (GSH) and the accumulation of toxic lipid ROS in cell, ultimately leading to oxidative cell death.86 Of note, ferroptosis involves with various CVDs, including cardiomyopathy, myocardial infarction, ischemia/reperfusion injury, and heart failure.87 Jiang et al report that ferroptosis of cardiomyocytes is an important cause of sorafenib- related cardiotoxicity, suggesting that activating transcription factor 4 (ATF4) is a key regulator to promote cardiomyocytes survival by up-regulation of SLC7A11 and suppression of ferroptosis.10 Another study reveals that activating transcription factor 3 (ATF3)-mediated ferroptosis is one of the key mechanisms leading to sorafenib-induced cardiotoxicity.88 Moreover, a recent study has reported that overexpression of mitofusin-2 (MFN2) alleviates sorafenib-induced cardiomyocyte necroptosis via the mitochondria-associated ER membrane (MAM)-calmodulin-dependent protein kinase II delta (CaMKIIδ) pathway in vitro and in vivo.89 Sorafenib also causes apoptosis of cardiac- and bone-derived c-kit+ stem cells, thereby reducing endogenous cardiac repair capacity.40 According to these novel studies, sorafenib has the ability to trigger different types of programmed cell death, which may contribute to the potential mechanisms for its cardiotoxicity.
Taken together, given sorafenib as a multi-target inhibitor, it acts through complex mechanisms that contribute to sorafenib-induced cardiovascular toxicity. In order to reveal these mechanisms, it is crucial to comprehend the interactions among the implicated pathways. Additionally, it is essential to differentiate between tissue types, as sorafenib’s effects can vary significantly across different tissues (Figure 1).
Treatment of Sorafenib-Induced Cardiovascular Toxicity
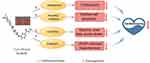
Based on the mechanism of sorafenib, combination of drugs can alleviate its toxic and side effects (Figure 2). Hesperetin is a flavanone, that is mainly found in citruses, such as lemons, limes, tangerines and other fruits. According to a recent study, the inhibition of TLR4/NLRP3 signaling pathway by hesperetin mitigates sorafenib-induced cardiotoxicity.90 Additionally, network simulations suggest that antioxidant N-acetyl cysteine (NAC) may protect cardiomyocytes against sorafenib-induced apoptosis.91 Co-administration of losartan with sorafenib significantly reduced levels of glycine, urea and some fatty acids, and almost prevented sorafenib-induced damage in cardiac tissue.92 In clinical trials of anti-VEGF therapy in cancer, drugs that inhibit the renin-angiotensin system (RAS) have proven most effective, especially ACEi.93 As an ACEi, captopril can attenuate sorafenib-induced hypertension.94 Wang et al found that adaptation of human iPSC-derived cardiomyocytes to explore sorafenib could reduce acute cardiotoxicity via metabolic reprogramming.50 Hence, efforts to find agents, including traditional herbal medicines, natural compounds and chemical medicine, that can counter sorafenib’s negative effects are essential. These endeavors will help reduce the toxicity and side effects of sorafenib and improve its clinical application.
Remaining Question: Does it Exist a Link Between Drug Resistance and Sorafenib-Induced Cardiovascular Toxicity?
Interestingly, despite sorafenib being capable of easily causing hypertension, such side effect also shows an association with the drug’s effectiveness in sorafenib-treated patients. A meta-analysis reveals that patients with RCC have a significantly higher incidence of hypertension, and the occurrence of hypertension may be associated with improved prognosis.9 Furthermore, another study finds that hypertension within 2 weeks of initiation of therapy may be a predictor of the anti-cancer efficacy of sorafenib in HCC patients.33 Such predictive value of sorafenib-induced hypertension is also found in metastatic renal cell cancer (mRCC) patients.95 Based on these data, the early stage of mild hypertension is likely due to that vascular response to tumor tissue by sorafenib treatment. Despite this, if long-term intensive sorafenib is administrated, it needs to pay attention on patients with elevated blood pressure because there is the higher incidence of myocardial ischemia in these patients.27,33 Moreover, sorafenib-resistance HCC cells show different metabolic characteristics, such as hypoxia,96 increased expression of HIF-1α,97 as well as enhancement of aerobic glycolysis.98 Compared to these HCC cells, in vasculature, ECs and VSMCs prefer aerobic glycolysis even when these cells are exposed to sufficient oxygen. More importantly, the enhancement of glycolysis not only promotes proliferation and migration of ECs and VSMCs but also causes phenotypic transformation (a “contractile phenotype” to a “synthetic phenotype”) of VSMCs.99,100 Of note, glycolysis can promote dysfunction of ECs and inflammation in atherosclerosis.101 Our new study confirms this phenomenon, indicating that sorafenib indeed promotes glycolysis and the proliferation and migration of VSMCs.102
Moreover, some signaling pathways implicated in sorafenib resistance, such as the ERK103 and mTOR pathways,104 are also involved in the pathogenesis of sorafenib-induced cardiovascular toxicity. This suggests a potential overlap in the molecular mechanisms driving both drug resistance and cardiac damage. Mitochondrial dysfunction is a common feature in both drug-resistant cancer cells and cardiomyocytes exposed to sorafenib.105 Dysregulated mitochondrial metabolism and increased production of ROS contribute to both drug resistance and cardiovascular toxicity.106 Sorafenib-induced mitochondrial toxicity may further exacerbate drug resistance by promoting the survival of resistant cancer cells through mechanisms such as enhanced antioxidant defense and modulation of apoptotic pathways. Chronic inflammation is associated with both drug resistance and cardiovascular diseases.107 Sorafenib treatment can induce inflammation in the tumor microenvironment, leading to the recruitment of pro-inflammatory cells and the production of cytokines and chemokines.108 These inflammatory mediators may contribute to cardiovascular toxicity by promoting endothelial dysfunction, vascular inflammation, and myocardial injury. Moreover, inflammation-driven pathways implicated in sorafenib resistance, such as NF-κB signaling, may also play a role in the development of cardiovascular complications.109 The potential link between drug resistance and cardiovascular toxicity has important clinical implications for the management of cancer patients receiving sorafenib therapy. In summary, emerging evidence suggests a complex interplay between drug resistance and cardiovascular toxicity induced by sorafenib, highlighting the need for further research to elucidate shared mechanisms and develop effective therapeutic strategies to address these interconnected challenges in cancer therapy.
Limitations
While there have been numerous reviews exploring the relationship between VEGFR inhibitors and cardiac toxicity,110–112 the current review offers several unique contributions. Firstly, this article provides in-depth mechanistic insights into sorafenib-induced cardiovascular toxicity. Secondly, it discusses potential treatment strategies, the link between sorafenib-induced hypertension and treatment efficacy, and emerging research findings, such as the association between sorafenib-induced glycolysis and drug resistance. Overall, this article offers a more comprehensive understanding of sorafenib’s cardiovascular effects compared to existing reviews.
Although this review has provided valuable insights, it is important to acknowledge several limitations. Firstly, our understanding of the molecular mechanisms underlying sorafenib-induced cardiovascular toxicity remains incomplete. While we have discussed several potential pathways involved, the precise interactions between sorafenib and specific cellular targets in the cardiovascular system require studies to further elucidate. Secondly, the individual variability in patient response and susceptibility to cardiovascular side effects necessitates personalized treatment strategies, which may be challenging to implement in clinical practice. Finally, the potential interplay between sorafenib-induced cardiovascular toxicity and treatment efficacy, requires further investigation. While emerging evidence suggests a link between hypertension and improved cancer outcomes, the mechanisms underlying this association remain poorly understood. In order to optimize treatment, future studies should focus on defining these relationships and their impact.
Conclusion and Perspective
This review has elucidated several mechanisms underlying sorafenib-induced cardiovascular toxicity, including endothelial dysfunction, mitochondrial dysfunction, endoplasmic reticulum stress, dysregulated autophagy, and ferroptosis. To understand these mechanisms, it not only contributes to controlling cardiovascular adverse reactions but also helps broaden the drug’s application in other diseases. Several potential treatment strategies have been identified, including the use of antioxidants, renin-angiotensin system inhibitors, and metabolic reprogramming agents. These interventions hold promise for alleviating sorafenib-induced cardiovascular toxicity and improving patient outcomes. In conclusion, while sorafenib represents a significant advancement in cancer therapy, its cardiovascular toxicity poses clinical challenges. By unraveling the underlying mechanisms and implementing targeted interventions, clinicians can better manage cardiovascular side effects, optimize treatment strategies, and ultimately improve patient care in the era of precision oncology.
With increasing advancement of detection, diagnosis, and treatment, individuals with cancer are experiencing longer lifespans and their cancers could be managed as a chronic condition. By 2020, 89 small-molecule targeted antitumor drugs have been approved by the US FDA and the National Medical Products Administration (NMPA) of China.113 Among these targeted drugs, TKIs are designed to inhibit the corresponding kinases from playing its role of catalyzing phosphorylation. However, a raising challenge is that all TKIs can lead to cardiovascular adverse reactions, including some serious cardiovascular events caused by TKIs.114 As one of VEGFR- associated multi-targeted TKIs, sorafenib can block a broader range of targets. Such characteristic contributes to the intricate nature of the mechanisms involved in investigating its cardiovascular adverse effects. Consequently, comprehensive clinical studies are crucial to assess long-term cardiovascular outcomes in patients receiving sorafenib across different cancer types. Advanced experimental models can help elucidate molecular mechanisms, while personalized medicine approaches may optimize therapy and minimize risk. However, limitations include reliance on observational data, incomplete understanding of molecular mechanisms, uncertain management strategies, and the need for further research on potential links between cardiovascular toxicity and treatment efficacy. Despite these challenges, addressing these issues will enhance our understanding of sorafenib-induced cardiovascular toxicity and improve patient outcomes.
Acknowledgments
As part of the preparation of this manuscript, we would like to thank Figdraw (www.figdraw.com) for its assistance with drawing.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Youth Innovation Team of Shaanxi Universities (202056); National Natural Science Foundation of China (No. 81773795; No. 82374077); the Shaanxi Province Natural Science Foundation (No.2022JQ-822), Natural Science Research Project of Shaanxi Provincial Education Department (No. 22JS032; No. 21JP108).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Burchert A, Bug G, Fritz LV, et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN). J Clin Oncol. 2020;38(26):2993–3002. doi:10.1200/JCO.19.03345
2. Rimassa L, Danesi R, Pressiani T, Merle P. Management of adverse events associated with tyrosine kinase inhibitors: improving outcomes for patients with hepatocellular carcinoma. Cancer Treat Rev. 2019;77:20–28. doi:10.1016/j.ctrv.2019.05.004
3. Fu M, Guo J, Zhao Y, et al. Characteristics of Fall-Related Fractures in Older Adults with Cerebrovascular Disease: a Cross-Sectional Study. Clin Interv Aging. 2021;16:1337–1346. doi:10.2147/CIA.S316739
4. Weinberg RA. The molecular basis of oncogenes and tumor suppressor genes. Ann N Y Acad Sci. 1995;758:331–338. doi:10.1111/j.1749-6632.1995.tb24838.x
5. Escalante CP, Chang YC, Liao K, et al. Meta-analysis of cardiovascular toxicity risks in cancer patients on selected targeted agents. Support Care Cancer. 2016;24(9):4057–4074. doi:10.1007/s00520-016-3310-3
6. Smith RA, Barbosa J, Blum CL, et al. Discovery of heterocyclic ureas as a new class of raf kinase inhibitors: identification of a second generation lead by a combinatorial chemistry approach. Bioorg Med Chem Lett. 2001;11(20):2775–2778. doi:10.1016/s0960-894x(01)00571-6
7. Wang L, Chen M, Ran X, Tang H, Cao D. Sorafenib-Based Drug Delivery Systems: applications and Perspectives. Polymers (Basel). 2023;15(12). doi:10.3390/polym15122638
8. Baek Moller N, Budolfsen C, Grimm D, et al. Drug-Induced Hypertension Caused by Multikinase Inhibitors (Sorafenib, Sunitinib, Lenvatinib and Axitinib) in Renal Cell Carcinoma Treatment. Int J Mol Sci. 2019;20(19). doi:10.3390/ijms20194712
9. Li Y, Li S, Zhu Y, et al. Incidence and risk of sorafenib-induced hypertension: a systematic review and meta-analysis. J Clin Hypertens (Greenwich). 2014;16(3):177–185. doi:10.1111/jch.12273
10. Jiang H, Wang C, Zhang A, et al. ATF4 protects against sorafenib-induced cardiotoxicity by suppressing ferroptosis. Biomed Pharmacother. 2022;153:113280. doi:10.1016/j.biopha.2022.113280
11. Saha D, Ryan KR, Lakkaniga NR, et al. Targeting Rearranged during Transfection in Cancer: a Perspective on Small-Molecule Inhibitors and Their Clinical Development. J Med Chem. 2021;64(16):11747–11773. doi:10.1021/acs.jmedchem.0c02167
12. Chen YT, Masbuchin AN, Fang YH, et al. Pentraxin 3 regulates tyrosine kinase inhibitor-associated cardiomyocyte contraction and mitochondrial dysfunction via ERK/JNK signalling pathways. Biomed Pharmacother. 2023;157:113962. doi:10.1016/j.biopha.2022.113962
13. Humphreys BD, Atkins MB. Rapid development of hypertension by sorafenib: toxicity or target? Clin Cancer Res. 2009;15(19):5947–5949. doi:10.1158/1078-0432.CCR-09-1717
14. Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: a Report From the American Heart Association. Circulation. 2020;141(9):e139–e596. doi:10.1161/CIR.0000000000000757
15. Escudier B, Worden F, Kudo M. Sorafenib: key lessons from over 10 years of experience. Expert Rev Anticancer Ther. 2019;19(2):177–189. doi:10.1080/14737140.2019.1559058
16. Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69(2):223–240. doi:10.2165/00003495-200969020-00006
17. Yamaguchi O, Watanabe T, Nishida K, et al. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J Clin Invest. 2004;114(7):937–943. doi:10.1172/JCI20317
18. Sathishkumar K, Yallampalli U, Elkins R, Yallampalli C. Raf-1 kinase regulates smooth muscle contraction in the rat mesenteric arteries. J Vasc Res. 2010;47(5):384–398. doi:10.1159/000277726
19. Dong LH, Wen JK, Liu G, et al. Blockade of the Ras-extracellular signal-regulated kinase 1/2 pathway is involved in smooth muscle 22 alpha-mediated suppression of vascular smooth muscle cell proliferation and neointima hyperplasia. Arterioscler Thromb Vasc Biol. 2010;30(4):683–691. doi:10.1161/ATVBAHA.109.200501
20. Elaimy AL, Mercurio AM. Convergence of VEGF and YAP/TAZ signaling: implications for angiogenesis and cancer biology. Sci Signal. 2018;11(552). doi:10.1126/scisignal.aau1165
21. Li Y, Gao ZH, Qu XJ. The adverse effects of sorafenib in patients with advanced cancers. Basic Clin Pharmacol Toxicol. 2015;116(3):216–221. doi:10.1111/bcpt.12365
22. Zou J, Fei Q, Xiao H, et al. VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J Cell Physiol. 2019;234(10):17690–17703. doi:10.1002/jcp.28395
23. Naito H, Iba T, Takakura N. Mechanisms of new blood-vessel formation and proliferative heterogeneity of endothelial cells. Int Immunol. 2020;32(5):295–305. doi:10.1093/intimm/dxaa008
24. Chirkov YY, Nguyen TH, Horowitz JD. Impairment of Anti-Aggregatory Responses to Nitric Oxide and Prostacyclin: mechanisms and Clinical Implications in Cardiovascular Disease. Int J Mol Sci. 2022;23(3):1042. doi:10.3390/ijms23031042
25. Tian M, Chen K, Huang J, et al. Asiatic acid inhibits angiogenesis and vascular permeability through the VEGF/VEGFR2 signaling pathway to inhibit the growth and metastasis of breast cancer in mice. Phytother Res. 2021;35(11):6389–6400. doi:10.1002/ptr.7292
26. Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the Phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27(20):3312–3318. doi:10.1200/JCO.2008.19.5511
27. Totzeck M, Mincu RI, Mrotzek S, Schadendorf D, Rassaf T. Cardiovascular diseases in patients receiving small molecules with anti-vascular endothelial growth factor activity: a meta-analysis of approximately 29,000 cancer patients. Eur J Prev Cardiol. 2018;25(5):482–494. doi:10.1177/2047487318755193
28. Maraiki F, Aljubran A. Carotid and brachiocephalic arteries stenosis with long term use of sorafenib. Hematol Oncol Stem Cell Ther. 2014;7(1):53–55. doi:10.1016/j.hemonc.2013.06.005
29. Jensen BC, Parry TL, Huang W, et al. Effects of the kinase inhibitor sorafenib on heart, muscle, liver and plasma metabolism in vivo using non-targeted metabolomics analysis. Br J Pharmacol. 2017;174(24):4797–4811. doi:10.1111/bph.14062
30. Abdel-Rahman O, Fouad M. Risk of cardiovascular toxicities in patients with solid tumors treated with sorafenib: an updated systematic review and meta-analysis. Future Oncol. 2014;10(12):1981–1992. doi:10.2217/fon.14.42
31. Wu S, Chen JJ, Kudelka A, Lu J, Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9(2):117–123. doi:10.1016/S1470-2045(08)70003-2
32. Bellmunt J, Eisen T, Fishman M, Quinn D. Experience with sorafenib and adverse event management. Crit Rev Oncol Hematol. 2011;78(1):24–32. doi:10.1016/j.critrevonc.2010.03.006
33. Akutsu N, Sasaki S, Takagi H, et al. Development of hypertension within 2 weeks of initiation of sorafenib for advanced hepatocellular carcinoma is a predictor of efficacy. Int J Clin Oncol. 2015;20(1):105–110. doi:10.1007/s10147-014-0691-5
34. Carballo-Folgoso L, Alvarez-Velasco R, Lorca R, et al. Evaluation of cardiovascular events in patients with hepatocellular carcinoma treated with sorafenib in the clinical practice. The CARDIO-SOR study. Liver Int. 2021;41(9):2200–2211. doi:10.1111/liv.14941
35. Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1(1):72–78. doi:10.1016/j.jchf.2012.09.001
36. Fu Y, Wei X, Lin L, Xu W, Liang J. Adverse reactions of sorafenib, sunitinib, and imatinib in treating digestive system tumors. Thorac Cancer. 2018;9(5):542–547. doi:10.1111/1759-7714.12608
37. Santoni M, Conti A, Massari F, et al. Targeted therapy for solid tumors and risk of hypertension: a meta-analysis of 68077 patients from 93 phase III studies. Expert Rev Cardiovasc Ther. 2019;17(12):917–927. doi:10.1080/14779072.2019.1704626
38. Yang X, Pan X, Cheng X, Kuang Y, Cheng Y. Risk of Hypertension With Sorafenib Use in Patients With Cancer: a Meta-Analysis From 20,494 Patients. Am J Ther. 2017;24(1):e81–e101. doi:10.1097/MJT.0000000000000331
39. Brose MS, Frenette CT, Keefe SM, Stein SM. Management of sorafenib-related adverse events: a clinician’s perspective. Semin Oncol. 2014;41 Suppl 2:S1–S16. doi:10.1053/j.seminoncol.2014.01.001
40. Duran JM, Makarewich CA, Trappanese D, et al. Sorafenib cardiotoxicity increases mortality after myocardial infarction. Circ Res. 2014;114(11):1700–1712. doi:10.1161/CIRCRESAHA.114.303200
41. Yokoyama H, Shioyama W, Shintani T, et al. Vascular Endothelial Growth Factor Receptor Inhibitors Impair Left Ventricular Diastolic Functions. Int Heart J. 2021;62(6):1297–1304. doi:10.1536/ihj.21-307
42. Naib T, Steingart RM, Chen CL. Sorafenib-associated multivessel coronary artery vasospasm. Herz. 2011;36(4):348–351. doi:10.1007/s00059-011-3444-5
43. Arima Y, Oshima S, Noda K, et al. Sorafenib-induced acute myocardial infarction due to coronary artery spasm. J Cardiol. 2009;54(3):512–515. doi:10.1016/j.jjcc.2009.03.009
44. Touyz RM, Herrmann SMS, Herrmann J. Vascular toxicities with VEGF inhibitor therapies-focus on hypertension and arterial thrombotic events. J Am Soc Hypertens. 2018;12(6):409–425. doi:10.1016/j.jash.2018.03.008
45. Elice F, Rodeghiero F, Falanga A, Rickles FR. Thrombosis associated with angiogenesis inhibitors. Best Pract Res Clin Haematol. 2009;22(1):115–128. doi:10.1016/j.beha.2009.01.001
46. Das A, Mahapatra S, Bandyopadhyay D, et al. Bleeding with vascular endothelial growth factor tyrosine kinase inhibitor: a network meta-analysis. Crit Rev Oncol Hematol. 2021;157:103186. doi:10.1016/j.critrevonc.2020.103186
47. Je Y, Schutz FA, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009;10(10):967–974. doi:10.1016/S1470-2045(09)70222-0
48. Kloth JS, Pagani A, Verboom MC, et al. Incidence and relevance of QTc-interval prolongation caused by tyrosine kinase inhibitors. Br J Cancer. 2015;112(6):1011–1016. doi:10.1038/bjc.2015.82
49. Jang S, Zheng C, Tsai HT, et al. Cardiovascular toxicity after antiangiogenic therapy in persons older than 65 years with advanced renal cell carcinoma. Cancer. 2016;122(1):124–130. doi:10.1002/cncr.29728
50. Wang H, Sheehan RP, Palmer AC, et al. Adaptation of Human iPSC-Derived Cardiomyocytes to Tyrosine Kinase Inhibitors Reduces Acute Cardiotoxicity via Metabolic Reprogramming. Cell Syst. 2019;8(5):412–426 e417. doi:10.1016/j.cels.2019.03.009
51. Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis Oncol. 2018;2:13. doi:10.1038/s41698-018-0056-z
52. Mercurio V, Pirozzi F, Lazzarini E, et al. Models of Heart Failure Based on the Cardiotoxicity of Anticancer Drugs. J Card Fail. 2016;22(6):449–458. doi:10.1016/j.cardfail.2016.04.008
53. Tomita Y, Naito S, Sassa N, et al. Sunitinib Versus Sorafenib as Initial Targeted Therapy for mCC-RCC With Favorable/Intermediate Risk: multicenter Randomized Trial CROSS-J-RCC. Clin Genitourin Cancer. 2020;18(4):e374–e385. doi:10.1016/j.clgc.2020.01.001
54. Wu C, Shemisa K. Sorafenib-Associated Heart Failure Complicated by Cardiogenic Shock after Treatment of Advanced Stage Hepatocellular Carcinoma: a Clinical Case Discussion. Case Rep Cardiol. 2017;2017:7065759. doi:10.1155/2017/7065759
55. Sudasena D, Balanescu DV, Donisan T, et al. Fulminant Vascular and Cardiac Toxicity Associated with Tyrosine Kinase Inhibitor Sorafenib. Cardiovasc Toxicol. 2019;19(4):382–387. doi:10.1007/s12012-018-9499-2
56. Destere A, Merino D, Lavrut T, et al. Drug-induced cardiac toxicity and adverse drug reactions, a narrative review. Therapie. 2023. doi:10.1016/j.therap.2023.10.008
57. Roden DM. A current understanding of drug-induced QT prolongation and its implications for anticancer therapy. Cardiovasc Res. 2019;115(5):895–903. doi:10.1093/cvr/cvz013
58. Pabinger I, van Es N, Heinze G, et al. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol. 2018;5(7):e289–e298. doi:10.1016/S2352-3026(18)30063-2
59. Scheiner B, Northup PG, Gruber AB, et al. The impact of ABO blood type on the prevalence of portal vein thrombosis in patients with advanced chronic liver disease. Liver Int. 2020;40(6):1415–1426. doi:10.1111/liv.14404
60. Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol (Oxf). 2017;219(1):22–96. doi:10.1111/apha.12646
61. Moslehi JJ. Cardiovascular Toxic Effects of Targeted Cancer Therapies. N Engl J Med. 2016;375(15):1457–1467. doi:10.1056/NEJMra1100265
62. Quintanilha JCF, Racioppi A, Wang J, et al. PIK3R5 genetic predictors of hypertension induced by VEGF-pathway inhibitors. Pharmacogenomics J. 2022;22(1):82–88. doi:10.1038/s41397-021-00261-5
63. Miller TW, Cherney MM, Lee AJ, et al. The effects of nitroxyl (HNO) on soluble guanylate cyclase activity: interactions at ferrous heme and cysteine thiols. J Biol Chem. 2009;284(33):21788–21796. doi:10.1074/jbc.M109.014282
64. Yu Q, Li K, Zhao A, et al. Sorafenib not only impairs endothelium-dependent relaxation but also promotes vasoconstriction through the upregulation of vasoconstrictive endothelin type B receptors. Toxicol Appl Pharmacol. 2021;414:115420. doi:10.1016/j.taap.2021.115420
65. Abdel-Samad D, Bkaily G, Magder S, Jacques D. ETA and ETB receptors contribute to neuropeptide Y-induced secretion of endothelin-1 in right but not left human ventricular endocardial endothelial cells. Neuropeptides. 2016;55:145–153. doi:10.1016/j.npep.2016.01.001
66. Clozel M, Gray GA, Breu V, Loffler BM, Osterwalder R. The endothelin ETB receptor mediates both vasodilation and vasoconstriction in vivo. Biochem Biophys Res Commun. 1992;186(2):867–873. doi:10.1016/0006-291x(92)90826-7
67. Houde M, Desbiens L, D’Orleans-Juste P. Endothelin-1: biosynthesis, Signaling and Vasoreactivity. Adv Pharmacol. 2016;77:143–175. doi:10.1016/bs.apha.2016.05.002
68. Van Leeuwen MT, Luu S, Gurney H, et al. Cardiovascular Toxicity of Targeted Therapies for Cancer: an Overview of Systematic Reviews. JNCI Cancer Spectr. 2020;4(6):pkaa076. doi:10.1093/jncics/pkaa076
69. Imran TF, Shah R, Ha AS, Thomas R, Joseph J. Heart failure associated with small molecule tyrosine kinase inhibitors. Int J Cardiol. 2016;206:110–111. doi:10.1016/j.ijcard.2016.01.059
70. Li Y, Xia J, Shao F, et al. Sorafenib induces mitochondrial dysfunction and exhibits synergistic effect with cysteine depletion by promoting HCC cells ferroptosis. Biochem Biophys Res Commun. 2021;534:877–884. doi:10.1016/j.bbrc.2020.10.083
71. Kawabata M, Umemoto N, Shimada Y, et al. Downregulation of stanniocalcin 1 is responsible for sorafenib-induced cardiotoxicity. Toxicol Sci. 2015;143(2):374–384. doi:10.1093/toxsci/kfu235
72. French KJ, Coatney RW, Renninger JP, et al. Differences in effects on myocardium and mitochondria by angiogenic inhibitors suggest separate mechanisms of cardiotoxicity. Toxicol Pathol. 2010;38(5):691–702. doi:10.1177/0192623310373775
73. Ma W, Liu M, Liang F, et al. Cardiotoxicity of sorafenib is mediated through elevation of ROS level and CaMKII activity and dysregulation of calcium homoeostasis. Basic Clin Pharmacol Toxicol. 2020;126(2):166–180. doi:10.1111/bcpt.13318
74. Bouitbir J, Panajatovic MV, Krahenbuhl S. Mitochondrial Toxicity Associated with Imatinib and Sorafenib in Isolated Rat Heart Fibers and the Cardiomyoblast H9c2 Cell Line. Int J Mol Sci. 2022;23(4). doi:10.3390/ijms23042282
75. Dai N, Ye R, He Q, Guo P, Chen H, Zhang Q. Capsaicin and sorafenib combination treatment exerts synergistic anti‑hepatocellular carcinoma activity by suppressing EGFR and PI3K/Akt/mTOR signaling. Oncol Rep. 2018;40(6):3235–3248. doi:10.3892/or.2018.6754
76. Yang Y, Zhou Q, Gao A, Chen L, Li L. Endoplasmic reticulum stress and focused drug discovery in cardiovascular disease. Clin Chim Acta. 2020;504:125–137. doi:10.1016/j.cca.2020.01.031
77. Ren J, Bi Y, Sowers JR, Hetz C, Zhang Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat Rev Cardiol. 2021;18(7):499–521. doi:10.1038/s41569-021-00511-w
78. Wang H, Wang Y, Li J, et al. Three tyrosine kinase inhibitors cause cardiotoxicity by inducing endoplasmic reticulum stress and inflammation in cardiomyocytes. BMC Med. 2023;21(1):147. doi:10.1186/s12916-023-02838-2
79. Gatica D, Chiong M, Lavandero S, Klionsky DJ. The role of autophagy in cardiovascular pathology. Cardiovasc Res. 2022;118(4):934–950. doi:10.1093/cvr/cvab158
80. Zhang K, Zhang Q, Jia R, Xiang S, Xu L. A comprehensive review of the relationship between autophagy and sorafenib-resistance in hepatocellular carcinoma: ferroptosis is noteworthy. Front Cell Dev Biol. 2023;11:1156383. doi:10.3389/fcell.2023.1156383
81. Fornari F, Giovannini C, Piscaglia F, Gramantieri L. Elucidating the Molecular Basis of Sorafenib Resistance in HCC: current Findings and Future Directions. J Hepatocell Carcinoma. 2021;8:741–757. doi:10.2147/JHC.S285726
82. Booth LA, Roberts JL, Dent P. The role of cell signaling in the crosstalk between autophagy and apoptosis in the regulation of tumor cell survival in response to sorafenib and neratinib. Semin Cancer Biol. 2020;66:129–139. doi:10.1016/j.semcancer.2019.10.013
83. Liang F, Zhang K, Ma W, et al. Impaired autophagy and mitochondrial dynamics are involved in Sorafenib-induced cardiomyocyte apoptosis. Toxicology. 2022;481:153348. doi:10.1016/j.tox.2022.153348
84. Li DL, Hill JA. Cardiomyocyte autophagy and cancer chemotherapy. J Mol Cell Cardiol. 2014;71:54–61. doi:10.1016/j.yjmcc.2013.11.007
85. Li J, Cao F, Yin HL, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. doi:10.1038/s41419-020-2298-2
86. Xia Y, Wang G, Jiang M, et al. A Novel Biological Activity of the STAT3 Inhibitor Stattic in Inhibiting Glutathione Reductase and Suppressing the Tumorigenicity of Human Cervical Cancer Cells via a ROS-Dependent Pathway. Onco Targets Ther. 2021;14:4047–4060. doi:10.2147/OTT.S313507
87. Wu X, Li Y, Zhang S, Zhou X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021;11(7):3052–3059. doi:10.7150/thno.54113
88. Li Y, Yan J, Zhao Q, Zhang Y, Zhang Y. ATF3 promotes ferroptosis in sorafenib-induced cardiotoxicity by suppressing Slc7a11 expression. Front Pharmacol. 2022;13:904314. doi:10.3389/fphar.2022.904314
89. Frontiers Editorial O. Retraction: overexpression of SERCA2a alleviates cardiac microvascular ischemic injury by suppressing Mfn2-mediated ER/mitochondrial calcium tethering. Front Cell Dev Biol. 2022;10:1006540. doi:10.3389/fcell.2022.1006540
90. Zaafar D, Khalil HMA, Rasheed RA, Eltelbany RFA, Zaitone SA. Hesperetin mitigates sorafenib-induced cardiotoxicity in mice through inhibition of the TLR4/NLRP3 signaling pathway. PLoS One. 2022;17(8):e0271631. doi:10.1371/journal.pone.0271631
91. Grabowska ME, Chun B, Moya R, Saucerman JJ. Computational model of cardiomyocyte apoptosis identifies mechanisms of tyrosine kinase inhibitor-induced cardiotoxicity. J Mol Cell Cardiol. 2021;155:66–77. doi:10.1016/j.yjmcc.2021.02.014
92. Abdelgalil AA, Mohamed OY, Ahamad SR, Al-Jenoobi FI. The protective effect of losartan against sorafenib induced cardiotoxicity: ex-vivo isolated heart and metabolites profiling studies in rat. Eur J Pharmacol. 2020;882:173229. doi:10.1016/j.ejphar.2020.173229
93. Lewinter C, Nielsen TH, Edfors LR, et al. A systematic review and meta-analysis of beta-blockers and renin-angiotensin system inhibitors for preventing left ventricular dysfunction due to anthracyclines or trastuzumab in patients with breast cancer. Eur Heart J. 2022;43(27):2562–2569. doi:10.1093/eurheartj/ehab843
94. Nagasawa T, Hye Khan MA, Imig JD. Captopril attenuates hypertension and renal injury induced by the vascular endothelial growth factor inhibitor sorafenib. Clin Exp Pharmacol Physiol. 2012;39(5):454–461. doi:10.1111/j.1440-1681.2012.05699.x
95. Szmit S, Zaborowska M, Wasko-Grabowska A, et al. Cardiovascular comorbidities for prediction of progression-free survival in patients with metastatic renal cell carcinoma treated with sorafenib. Kidney Blood Press Res. 2012;35(6):468–476. doi:10.1159/000338175
96. Chen Z, Yuan T, Yan F, et al. CT-707 overcomes hypoxia-mediated sorafenib resistance in Hepatocellular carcinoma by inhibiting YAP signaling. BMC Cancer. 2022;22(1):425. doi:10.1186/s12885-022-09520-5
97. Mendez-Blanco C, Fondevila F, Garcia-Palomo A, Gonzalez-Gallego J, Mauriz JL. Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med. 2018;50(10):1–9. doi:10.1038/s12276-018-0159-1
98. Zhang Z, Tan X, Luo J, Yao H, Si Z, Tong JS. The miR-30a-5p/CLCF1 axis regulates sorafenib resistance and aerobic glycolysis in hepatocellular carcinoma. Cell Death Dis. 2020;11(10):902. doi:10.1038/s41419-020-03123-3
99. Xu R, Yuan W, Wang Z. Advances in Glycolysis Metabolism of Atherosclerosis. J Cardiovasc Transl Res. 2023;16(2):476–490. doi:10.1007/s12265-022-10311-3
100. Zhang X, Zheng B, Zhao L, et al. KLF4-PFKFB3-driven glycolysis is essential for phenotypic switching of vascular smooth muscle cells. Commun Biol. 2022;5(1):1332. doi:10.1038/s42003-022-04302-y
101. Schnitzler JG, Hoogeveen RM, Ali L, et al. Atherogenic Lipoprotein(a) Increases Vascular Glycolysis, Thereby Facilitating Inflammation and Leukocyte Extravasation. Circ Res. 2020;126(10):1346–1359. doi:10.1161/CIRCRESAHA.119.316206
102. Yu Q. Sorafenib Aggravates Atherosclerotic Progression Involving With Vascular Smooth Muscle Cell (VSMCs) Phenotypic Switching and Proliferation by PKM2-Mediated Glycolysis. Circulation. 2023;148(Suppl_1):10009–17322.
103. He Y, Wang X, Lu W, et al. PGK1 contributes to tumorigenesis and sorafenib resistance of renal clear cell carcinoma via activating CXCR4/ERK signaling pathway and accelerating glycolysis. Cell Death Dis. 2022;13(2):118. doi:10.1038/s41419-022-04576-4
104. Tan XP, Xiong BH, Zhang YX, Wang SL, Zuo Q, Li J. FXYD5 promotes sorafenib resistance through the Akt/mTOR signaling pathway in hepatocellular carcinoma. Eur J Pharmacol. 2022;931:175186. doi:10.1016/j.ejphar.2022.175186
105. Zhu Y, Xu J, Hu W, et al. TFAM depletion overcomes hepatocellular carcinoma resistance to doxorubicin and sorafenib through AMPK activation and mitochondrial dysfunction. Gene. 2020;753:144807. doi:10.1016/j.gene.2020.144807
106. Xu J, Ji L, Ruan Y, et al. UBQLN1 mediates sorafenib resistance through regulating mitochondrial biogenesis and ROS homeostasis by targeting PGC1beta in hepatocellular carcinoma. Signal Transduct Target Ther. 2021;6(1):190. doi:10.1038/s41392-021-00594-4
107. Tan W, Luo X, Li W, et al. TNF-alpha is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma. EBioMedicine. 2019;40:446–456. doi:10.1016/j.ebiom.2018.12.047
108. Jiang Y, Chen P, Hu K, et al. Inflammatory microenvironment of fibrotic liver promotes hepatocellular carcinoma growth, metastasis and sorafenib resistance through STAT3 activation. J Cell Mol Med. 2021;25(3):1568–1582. doi:10.1111/jcmm.16256
109. Lo J, Lau EY, Ching RH, et al. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology. 2015;62(2):534–545. doi:10.1002/hep.27859
110. Neves KB, Montezano AC, Lang NN, Touyz RM. Vascular toxicity associated with anti-angiogenic drugs. Clin Sci (Lond). 2020;134(18):2503–2520. doi:10.1042/CS20200308
111. Abdel-Qadir H, Ethier JL, Lee DS, Thavendiranathan P, Amir E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta-analysis. Cancer Treat Rev. 2017;53:120–127. doi:10.1016/j.ctrv.2016.12.002
112. Jiang L, Ping L, Yan H, et al. Cardiovascular toxicity induced by anti-VEGF/VEGFR agents: a special focus on definitions, diagnoses, mechanisms and management. Expert Opin Drug Metab Toxicol. 2020;16(9):823–835. doi:10.1080/17425255.2020.1787986
113. Zhong L, Li Y, Xiong L, et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021;6(1):201. doi:10.1038/s41392-021-00572-w
114. Brinda BJ, Viganego F, Vo T, Dolan D, Fradley MG. Anti-VEGF-Induced Hypertension: a Review of Pathophysiology and Treatment Options. Curr Treat Options Cardiovasc Med. 2016;18(5):33. doi:10.1007/s11936-016-0452-z
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.