Back to Journals » International Journal of Women's Health » Volume 15
Umbilical Artery Thrombosis After Selective Termination in Dichorionic Diamniotic Twin Pregnancy: A Case Report
Authors Liu H, Zeng Z, Liao H, Hu Q, Yu H
Received 28 May 2023
Accepted for publication 4 August 2023
Published 10 August 2023 Volume 2023:15 Pages 1327—1332
DOI https://doi.org/10.2147/IJWH.S423242
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Everett Magann
Hongyan Liu,1,2 Zhaomin Zeng,1,2 Hua Liao,1,2 Qing Hu,1,2 Haiyan Yu1,2
1Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, People’s Republic of China
Correspondence: Haiyan Yu, Department of Obstetrics and Gynecology, West China Second University Hospital, No. 20, 3rd Section, South Renmin Road, Chengdu, Sichuan, 610041, People’s Republic of China, Email [email protected]
Introduction: Umbilical artery thrombosis is a rare complication associated with poor perinatal outcomes. The incidence of umbilical artery thrombosis in pregnancy is estimated from 0.0025% to 0.045%. Prenatal screening and diagnosis of umbilical artery thrombosis is usually performed by ultrasonography. Up to now, no treatment consensus has been achieved.
Case Presentation: We present a case of dichorionic diamniotic twin pregnancy complicated with selective termination of one twin with lymphoid cystic tumor at 14 weeks and 2 days of gestation and the alive twin occurred single umbilical artery thrombosis at 35 weeks and 6 days of gestation. The emergency cesarean section was performed after emergency admission. A healthy male baby was delivered weighing 2690g with Apgar scores of 10 and 10 at 1 and 5 minutes, respectively, whereas the dead fetus weighed 10 g. Thrombosis was observed throughout one of the umbilical artery of the alive fetus. The woman and the infant are followed up closely and both in good condition.
Conclusion: The number and morphology of umbilical arteries should be carefully observed during pregnancy. Individualized management of umbilical artery thrombosis should be based on clinical conditions. Timely termination of pregnancy if necessary could be suitable to improve adverse pregnancy outcomes.
Keywords: umbilical artery thrombosis, adverse pregnancy outcome, twin pregnancy
Introduction
The umbilical cord connects the fetus to the placenta and plays the role of gas exchange, nutrition, excretion and metabolism.1 The incidence of umbilical artery thrombosis (UAT) is estimated from 0.0025% to 0.045% of gestations.2 Umbilical artery thrombosis is a rare complication associated with poor perinatal outcomes.3 Prenatal screening and diagnosis of UAT is usually performed by three dimensional ultrasonography with color Doppler and power Doppler. The difficulty in treatment lies in choosing the right time of delivery. Up to now, no consensus has been reached. Umbilical artery abnormalities should be detected in time to minimize the incidence of adverse outcomes. Timely termination of pregnancy can be selected to reduce the adverse pregnancy outcome.
Herein, we reported a rare case of dichorionic diamniotic twin pregnancy complicated with selective termination of one twin and the alive twin with single umbilical artery thrombosis in the third trimester. Additionally, we used a list of keywords including “umbilical artery thrombosis”, “adverse pregnancy outcome”, “pregnancy”, and “twin pregnancy” to an extensive Medline search and conducted a literature review in English and Chinese. The study was approved by the ethical committees of the West China Second University Hospital.
Case Presentation
A 29-year-old woman, gravida 1, para 0, conceived dichorionic diamniotic twin pregnancy by in vitro fertilization and embryo transfer. At 12 weeks of gestation, ultrasound examination showed one twin with cystic neck mass (twin A), which was considered as lymphocystic tumor (Figure 1). Given this condition, the patient was transferred to our department. Fetal ultrasonography confirmed that one twin with lymphocystic tumor and generalized edema (twin A) and indicated one umbilical vein and two umbilical arteries in another twins’ umbilical cord (twin B) (Figure 2A). After extensively counseled by the multidisciplinary team, the couple opted for selective termination of the lymphocystic tumor fetus (twin A). Selective fetal reduction by ultrasound-guided intracardiac injection of potassium chloride was performed on twin A at 14 weeks and 2 days of gestation. The couple refused chromosome examination in twin A, and amniocentesis was performed on twin B. The result of chromosome microarray analysis of twin B was normal. The patient had no history of thrombotic disease or related family history. The couple had no reported history of medication, hereditary disease, substance abuse or family history of congenital anomalies.
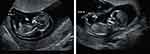 |
Figure 1 Images of twin A and twin B at 12 weeks. |
 |
Figure 2 (A) Images of umbilical arteries in twin B at 14+2 weeks. (B) Images of umbilical arteries in twin B at 35+6 weeks. |
After selective termination, the woman was followed up closely. Prenatal examination was performed regularly in our department. Ultrasound examination during following pregnancy remained one umbilical vein and two umbilical arteries in alive twin (twin B). The maternal prothrombin time, partially activated thrombin time and fibrinogen were all normal.
Ultrasound imaging of the umbilical cord in twin B revealed single umbilical artery at 35 weeks and 6 days of gestation (Figure 2B). After emergency admission to hospital, an emergent cesarean section was performed. A healthy male baby was delivered weighing 2690 g with Apgar scores of 10 and 10 at 1 and 5 minutes, respectively, whereas the dead fetus weighed 10 g. The umbilical cord was about 70cm long and was extremely torsional in 30 laps. Thrombosis was observed throughout one of the umbilical artery (Figure 3). Placental pathological examination revealed mild chorioamnionitis, placental villous parenchyma with punctate calcification and small infarcts, excessive helices in the umbilical cord and umbilical artery thrombosis.
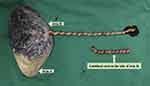 |
Figure 3 Images of the dichorionic diamniotic placenta and the umbilical cord of twin B. |
The patient was discharged three days later. The baby is now 2 years five months old and is in good health.
Discussion
The umbilical cord connects the fetus to the placenta and plays a role of gas exchange, nutrition, excretion and metabolism.1 The umbilical cord comprises one umbilical vein and two umbilical arteries. Umbilical cord thrombosis is a rare complication associated with poor perinatal outcomes. Umbilical cord thrombosis has been reported to occur in approximately 1 in 1300 births, 1 in 1000 postnatal autopsies, and 1 in 250 high-risk pregnancies.4 Venous thrombosis alone occurred in 70% of cases, venous and arterial thrombosis in about 20%, and arterial thrombosis in 10% of cases.5 The incidence of umbilical artery thrombosis (UAT) in pregnancy is estimated from 0.0025% to 0.045%.2
The etiology of UAT remains unclear. According to Virchow’s triad, conditions that predispose thrombosis include reduced blood flow (stasis), changes in the vascular endothelial, and hypercoagulability.6 Abnormal umbilical cord may cause umbilical artery blood flow stagnation and thrombosis,7,8 including long cords, short cords, twining, compression, hypercoiling, hypocoiling, strictures, cystics, vascular malformations and abnormal cord insertion. Hypercoagulability may be associated with maternal genetic or acquired factors.9 Inflammation can cause vascular endothelial damage, leading to the pathogenesis of umbilical artery thrombosis, such as materno-fetal infections and some obstetric complications.8 UAT may result from umbilical cord vessels puncture or intrauterine transfusion. Donepudi et al reported a case of UAT after repeated intrauterine transfusion due to severe fetal anemia secondary to Rh alloimmunization.10 Shubha P et al also reported a case of dichorionic and diamniotic pregnancy, in which one of the twins died of UAT caused by vascular endothelial damage.11 Alhousseini et al reported a case of UAT with severe fetal growth restriction, and the newborn was seriously deficient in protein S after birth. They speculated that protein S may play an important role in the formation of UAT.12 Although our case underwent selective fetal reduction in twin A and amniocentesis in twin B, umbilical cord vessel puncture was not performed. We think that the etiology of UAT in this case was that the umbilical cord was extremely torsional.
Prenatal screening and diagnosis of UAT is usually performed by ultrasonography. It can be confirmed by postpartum pathological examination of the umbilical cord. According to the number of umbilical artery at the level of the fetal bladder or in an axial view of the umbilical cord, it can be easy to identify.6 The transverse two-dimensional ultrasound scan of the normal umbilical cord showed three rings – one large ring, umbilical vein, and two small rings, umbilical artery.7 The two umbilical arteries should be described and recorded in detail during pregnancy so that a sudden absence of blood flow signals can be used to detect intrauterine thrombosis.6 Tanaka K et al13 reported the characteristic ultrasound finding of UAT, the occluded artery in parallel with the remaining artery and surrounded by the uterine vein, which resembled an orange grabbed by a hand. We did not find this characteristic ultrasound image in our case. We considered the diagnosis of UAT by the sudden disappearance of umbilical artery blood flow signal.
UAT is easily misdiagnosed as single umbilical artery (SUA). SUA is the most common true congenital anomaly present in humans.1,14 The incidence of SUA is between 0.63% and 1% of newborns,14 in which 15.4% presented other malformations and/or chromosomal abnormalities.1,14 The number of the umbilical arteries is widely used to detect SUA during the first-trimester.14 Although the number of umbilical arteries can be determined by evaluating fetal bladder level blood flow by color Doppler, it is difficult to distinguish primary hypoplasia from secondary obstruction in the case of single umbilical artery. The “orange grab sign” is completely different from the cross section of primary hypoplasia and can be appropriately distinguished.13 In addition, increasing the diameter of umbilical artery without changing the diameter of vein is considered as the prenatal ultrasound feature of single umbilical artery.1 Umbilical artery thrombosis should be considered when the number of umbilical vessels decreases or a sudden absence of blood flow signals in the third trimester.
Umbilical artery thrombosis is associated with adverse pregnancy outcomes.6 Shilling C reported 7 cases UAT in about 116,000 deliveries during 13 years. None of the live births had a normal neonatal course. Intrauterine death occurred in 2 of 7 cases and 3 cases had intrauterine growth restriction.3 UAT increases the risk of fetal death, intrauterine growth retardation, meconium in amniotic fluid, acute fetal distress during delivery and emergency caesarean section.2 Fetal thrombotic vasculopathy Newborns with fetal thrombotic vasculopathy are most likely to have thrombocytopenia in the first three days after birth, proving the severe systemic sequelae of diseases involving placental thrombosis.15 Sato et al we reported 11 cases UAT, in which 3 cases occurred intrauterine growth restriction and 2 cases occurred intrauterine fetal death.5 UAT may result in severe intrauterine growth restriction and chronic UAT should be considered when no cause is found.6
Regarding the treatment of UAT, the difficulty lies in choosing the right time of delivery. Up to now, no consensus has been reached. If ultrasonographic scans and fetal monitoring are stable, pregnancy can be prolonged under close supervision. Selective cesarean section can be selected following antenatal corticosteroid therapy to prompt fetal lung maturation.16 But Ying Zhu9 pointed out that because the adverse outcomes, emergency section is still recommended in the third trimester of pregnancy once UAT is diagnosed. Jing Wei et al8 reported two cases of UAT which were given low molecular weight heparin per day until the day of cesarean section. It is a trial treatment and the prophylactic use of low molecular weight heparin in these pregnancies needs further evaluation.
Conclusion
In conclusion, UAT is a rare complication that results in poor fetal prognosis. Sonographers play an important role in prenatal diagnosis. The number and morphology of umbilical arteries should be carefully observed during pregnancy. In our case, an emergency cesarean section was performed immediately after the diagnosis of UAT with no complication in the fetus and the neonate. Timely detection of umbilical artery abnormalities is important. Individualized management should be based on clinical conditions and timely termination of pregnancy if necessary could be suitable to improve adverse pregnancy outcomes.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical Approval and Informed Consent
This study was approved by the ethics committees at the West China Second University Hospital of Sichuan University (2022-194). Written informed consent was obtained from the parents of the patient for publication of this case report and any accompanying images.
Acknowledgments
We feel grateful for the doctors and staff who have been involved in this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Natural Science Foundation of Sichuan (2022NSFSC0659) and the Science Foundation of Chengdu (No. 2021-YF05-01532-SN).
Disclosure
The authors report no conflicts of interest associated with this work.
References
1. Sherer DM, Al-Haddad S, Cheng R, Dalloul M. Regina cheng, mudar dalloul. Current perspectives of prenatal sonography of umbilical cord morphology. Int J Womens Health. 2021;13:939–971. doi:10.2147/IJWH.S278747
2. Kitano T, Ohgitani A, Takagi K, et al. A case of severe neonatal asphyxia due to umbilical artery thrombosis. J Obstet Gynaecol. 2018;38(8):1164–1165. doi:10.1080/01443615.2017.1404012
3. Shilling C, Walsh C, Downey P, Mooney E. Umbilical artery thrombosis is a rare but clinically important finding: a series of 7 cases with clinical outcomes. Pediatr Dev Pathol. 2014;17(2):89–93. doi:10.2350/13-11-1407-OA.1
4. Stephen AH. Thrombosis of the umbilical cord: analysis of 52 cases and literature review. Pediatr Dev Pathol. 1988;8:37–54. doi:10.3109/15513818809022278
5. Sato Y, Benirschke K. Umbilical arterial thrombosis with vascular wall necrosis: clinicopathologic findings of 11 cases. Placenta. 2006;27(6–7):715–718. doi:10.1016/j.placenta.2005.05.008
6. Klaritsch P, Haeusler M, Karpf E, Schlembach D, Lang U. Spontaneous intrauterine umbilical artery thrombosis leading to severe fetal growth restriction. Placenta. 2008;29(4):374–377. doi:10.1016/j.placenta.2008.01.004
7. Li H, Qufeng W, Wei W, Lin X, Zhang X. Umbilical artery thrombosis: two case reports. Medicine. 2019;98(48):e18170. doi:10.1097/MD.0000000000018170
8. Wei J, Qiaoyun L, Zhai H. Umbilical artery thrombosis diagnosed at different gestational ages and fetal outcomes: a case series. BMC Pregnancy Childbirth. 2021;21(1):788. doi:10.1186/s12884-021-04264-9
9. Zhu Y, Beejadhursing R, Liu Y. 10 cases of umbilical cord thrombosis in the third trimester. Arch Gynecol Obstet. 2021;304(1):59–64. doi:10.1007/s00404-020-05910-x
10. Donepudi RV, Kj M. Intrauterine transfusion complicated by umbilical artery thrombosis. Case Rep Obstet Gynecol. 2019;2019:5952326. doi:10.1155/2019/5952326
11. Bhat SP, Paul R, Srinivas T, et al. Thrombosis of both umbilical arteries of a female fetus in a twin gestation causing fetal demise. J Health Allied Sci NU. 2021;(2). doi:10.1055/S-0040-1721527
12. Alhousseini A, Jaiman S, Hernandez-Andrade E, Zeineddine S, Qureshi F. Umbilical artery thrombosis with associated acute and severe fetal growth restriction and transient severe protein S deficiency: report of a case with prenatal ultrasound diagnosis allowing for timely intervention and good outcome. Case Rep Obstet Gynecol. 2018;2018:6324362. doi:10.1155/2018/6324362
13. Tanaka K, Tanigaki S, Matsushima M, et al. Prenatal diagnosis of umbilical artery thrombosis. Fetal Diagn Ther. 2014;35(2):148–150. doi:10.1159/000355601
14. Hasegawa J. Ultrasound screening of umbilical cord abnormalities and delivery management. Placenta. 2018;62:66–78. doi:10.1016/j.placenta.2017.12.003
15. Redline RW. Thrombophilia and placental pathology. Clin Obstet Gynecol. 2006;49(4):885–894. doi:10.1097/01.grf.0000211957.68745.6b
16. Lutfallah F, Oufkir N, Markou GA, Frimigacci D, Poncelet C. A case of umbilical artery thrombosis in the third trimester of pregnancy. Am J Case Rep. 2018;19:72–75. doi:10.12659/ajcr.906859
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
