Back to Journals » Journal of Pain Research » Volume 12
Ultrasound-guided subcostal transversus abdominis plane block with liposomal bupivacaine compared to bupivacaine infiltration for patients undergoing robotic-assisted and laparoscopic hysterectomy: a prospective randomized study
Authors Hutchins J, Argenta P, Berg A , Habeck J, Kaizer A, Geller MA
Received 8 November 2018
Accepted for publication 25 June 2019
Published 4 July 2019 Volume 2019:12 Pages 2087—2094
DOI https://doi.org/10.2147/JPR.S193872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Erica Wegrzyn
Jacob Hutchins,1 Peter Argenta,2 Aaron Berg,1 Jason Habeck,1 Alexander Kaizer,3 Melissa A Geller2
1Department of Anesthesiology, University of Minnesota, Minneapolis, MN, USA; 2Department of Obstetrics, Gynecology and Women’s Health, Minneapolis, MN, USA; 3Department of Biostatistics and Informatics, Colorado School of Public Health, University of Colorado, Aurora, CO, USA
Purpose: To determine if a transversus abdominis plane (TAP) block with liposomal bupivacaine reduces total postoperative opioid use in the first 72 hrs following laparoscopic or robotic hysterectomy compared to port-site infiltration with 0.25% bupivacaine.
Methods: Patients received either a true TAP block procedure with 266 mg liposomal bupivacaine and 50 mg of 0.25% bupivacaine and sham port infiltration or sham TAP block procedure with true port-site infiltration with 100–125 mg of 0.25% bupivacaine. All patients had a standardized, scheduled, non-opioid pain management plan. The primary outcome was total IV morphine equivalents used in the first 72 hrs following surgery. Secondary outcomes included assessment of postoperative pain over the study period and quality of recovery measures.
Results: Patients undergoing TAP blockade required fewer total opioid equivalents during the observation period than patients allocated to infiltration (median 21 versus 25 mg IV Morphine equivalents, P=0.03). Opioid use was highest in the first 24 hrs after surgery, with less difference between the groups during days 2 and 3 postoperatively. There were 5 in the TAP group and 0 in the infiltration group were opioid free at 72 hrs. Those in the TAP group had improved quality of recovery (QoR15) with no change in overall benefit of analgesia score.
Conclusion: TAP blockade reduced the requirement for opioid pain medication in the first 72 hrs after surgery, had more patients opioid free at 72 hrs, and improved patients’ quality of their recovery.
Keywords: acute pain, regional pain, TAP block, liposome bupivacaine
Introduction
The United States is in the midst of an opioid crisis which has placed even more importance on finding novel ways to minimize the amount of opioids patients need after surgery. Multimodal analgesia involves using two or more non-opioid medications to both minimize pain and minimize opioids after surgery. One component of that is the utilization of local anesthetics for postoperative pain control. It has been shown that using local anesthetics in transversus abdominis plane (TAP) blocks effectively provides analgesia for surgery-associated pain following abdominal surgery.1–4 Blocks can be performed before or after surgery and provide somatic analgesia for the anterior abdomen while decreasing the potential need for opioid pain medication.5–7 Our group recently reported both retrospective and prospective studies demonstrating that a group-wide transition from either bupivacaine local infiltration or bupivacaine TAP blocks to TAP blocks with liposomal bupivacaine was associated with lower postoperative pain scores, decreases in perioperative nausea, shorter length of hospitalization, and analgesia up through 72 hrs after surgery.8–10
The current study was undertaken to more rigorously test the hypothesis that regional TAP block, using liposomal bupivacaine and bupivacaine reduces perioperative opioid requirements compared to the most common technique, local port-site infiltration using 0.25% bupivacaine.
Methods
After obtaining approval from the University of Minnesota Institutional Review Board (IRB protocol 1508M77443) and registration with clinicaltrials.gov (NCT 02519023), we conducted a single institution, randomized, double-blinded, placebo-controlled study comparing TAP blocks with liposomal bupivacaine and 0.25% bupivacaine to port-site infiltration with 0.25% bupivacaine at a large, metropolitan, teaching hospital. We chose to compare TAP blocks with liposomal bupivacaine and 0.25% bupivacaine to port-site infiltration with 0.25% bupivacaine and not a higher concentration of bupivacaine because previous studies have demonstrated no difference in wound infiltration when using a higher concentration of local anesthetic.11,12 Patient consent was written informed consent and this trial was conducted in accordance with the Declaration of Helsinki. We do not intend to share deidentified participant data. Patients were randomly assigned after obtaining informed consent by a research assistant using a randomization sequence from www.random.org. Treatment allocation was 1:1 using block allocation. All women undergoing planned hysterectomy using a minimally invasive approach (laparoscopy or robotic-assisted) who were over the age of 18 and had an American Society of Anesthesiologists physical status of I–III were eligible for inclusion. Exclusion criteria included: contradiction to regional anesthesia, history of opioid usage for >3 weeks of continuous usage prior to surgery, patients with known chronic pain syndrome, and patients with an inability to understand the consent process. Because discharge rate was an endpoint, patients with a planned surgical start time after 5 PM were excluded from participation. All patients were operated on by one of six fellowship-trained gynecologic oncology surgeons. Patients who required conversion of a planned minimally invasive surgery to an open procedure were excluded from analyses. Demographic and medical histories were abstracted from the patients’ charts.
Each patient underwent both a TAP procedure prior to initiation of surgery and local infiltration of the port sites at the conclusion of surgery. Patients randomized to the experimental arm underwent bilateral infiltration in the TAP as previously described5 using 10 mL of 0.25% bupivacaine with 1:200,000 epinephrine followed by 10 mL of 1.3% liposomal bupivacaine and 10 mL of saline per side (with the liposomal bupivacaine and saline injected as a 50/50 mixture) on each side. Under ultrasound guidance, a 22-gauge 30° beveled echogenic needle was inserted until the tip pierced the transversus abdominis fascia. There the injectate was deposited between the fascia and the transversus abdominis muscle. The port sites were infiltrated with up to 10 mL of sham treatment (normal saline) at the conclusion of surgery. Patients randomized to the control arm had a sham TAP procedure performed using 30 mL of normal saline per side before surgery and underwent local injections at each port site using 10 mL of 0.25% bupivacaine with 1:200,000 epinephrine per port site prior to extubation.
Both the patient and the treating team (surgeon, anesthesiologist, and nurses) were blinded to the treatment assignment; and medications were repackaged by our investigational pharmacy to precluded incidental identification. There were no alterations in the protocol during the course of this study.
During the surgery and the postoperative care, all opioids were recorded. Pain was assessed using a 10-point numerical scale at 1 and 2 hrs postoperatively by the post-anesthesia unit nurses and at 6, 24, 48, and 72 hrs postoperatively by a research assistant; all assessors were also blinded to treatment allocation. Oxycodone (5 mg oral tablet) was given in the post-anesthesia care unit for a pain score of 5 or greater. Postoperatively, all subjects were instructed to take acetaminophen (1 g every 6 hrs), ibuprofen (800 mg every 8 hrs) scheduled for 72 hrs. They also were instructed to take oxycodone (5–10 mg every 4 hrs) only as needed for pain that rated above 5 on a 10-point numerical scale.
Phone interviews were conducted for patients that were discharged prior to planned assessment points. Medication usage and complications, including respiratory suppression and modality-related complications, were assessed at each time point. All patients were administered a Quality of Recovery-15 (QoR15)13 and the Overall Benefit of Analgesia Score (OBAS)14 instruments to assess their subjective sense of recovery at 72 hrs. Complications and/or readmissions occurring up to postoperative day 17 (2 weeks from the conclusion of planned data acquisition) were recorded.
The primary endpoint was total opioid usage by 72 hrs postoperatively. Secondary endpoints included: postoperative pain scores at 24, 48, and 72 hrs; daily opioid usage, QoR15 score at 72 hrs, OBAS score at 72 hrs, incidence of nausea and emesis within 72 hrs, length of time in recovery, postoperative admission rates, and complication rates.
Sample size was determined using median and range of opioid consumption data from our previous trial, in which patients undergoing TAP procedure with liposomal bupivacaine for hysterectomy used a median of 24.9 mg IV morphine equivalentwith ranges of 0–86.9 Using the methods of Hozo et al to estimate the mean and standard deviation of that group using the median and range, we had an estimate mean of 34 and standard deviation of 24.15 Using an α of 0.05, we determined that 62 patient endpoints (31 in each group) were required to provide a β of 0.8 to detect an effect size of at least 0.8 in opioid usage among patients receiving the experimental arm relative to the control arm. Assuming a dropout rate of 20% and conversion to open at 10%, the study was approved to enroll 80 patients. Due to an increased number of conversions to an open procedure, loss to follow-up and screen fails, approval to enroll to up to 95 was obtained.
Most continuous outcomes were not normally distributed (P<0.05 for Shapiro–Wilk test for normality), therefore comparisons used the Wilcoxon rank sum test. Chi-squared tests were used to compare dichotomous variables. P-values for secondary analyses were not adjusted for multiple comparisons. All analyses were completed with R-project Software version 3.3.1.
Results
A total of 87 patients were enrolled between July 2016 through April 2017, 49 in experimental and 38 in control arm. There were 201 patients evaluated with 62 reaching final analysis (Figure 1). Six patients did not receive the allocated intervention due to a change in surgical plan after allocation. One patient discontinued intervention and seven were lost to follow-up. Eleven patients required a conversion to laparotomy (nine in the experimental and two in the bupivacaine infiltration groups). Twenty-seven patients underwent laparoscopy and 35 patients underwent a robotic-assisted approach. Patient demographics are described in Table 1. The groups were well balanced for relevant risk factors.
 |
Table 1 Demographics |
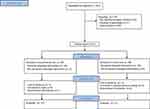 |
Figure 1 CONSORT flow diagram. |
Total opioid use over the 72 hrs following surgery was lower for the experimental group compared to the control group (median of 20.8 mg versus 25 mg IV morphine equivalents respectively, P=0.03) (Table 2). Opioid requirements were highest immediately following surgery and lower on postoperative days 2 and 3 for most patients. Notably, five patients (16.1%) assigned to the experimental group were opioid free at 72 hrs all while all patients in the control group required at least some opioid pain medication (P=0.062). Non-opioid pain management was similar for both groups, with no significant difference in acetaminophen or ibuprofen use. We examined both acetaminophen and ibuprofen use because while the patients were instructed to take these medications as scheduled and oxycodone as needed we discovered during our data analysis that not every patient took the non-opioid medications as scheduled for the full 72 hrs. Thus, we compared the amount used of each medication by each group to ensure the use of acetaminophen and ibuprofen did not influence the end results. There was a significant reduction in median maximal pain scores among patients allocated to the experimental group versus control on postoperative days 1 and 3 but were similar on postoperative day 2 (Table 3). The total maximal pain score which was the sum of maximal pain scores from day 1 through day 3 was significantly lower in the experimental group vs the control group (8.0 (0,29) vs 13.0 (3,30); P=0.022).
 |
Table 2 Opioid use and presence of nausea and vomiting |
 |
Table 3 Maximum pain scores |
Global measures of clinical effectiveness were mixed; there were significant differences in the QoR15 scores favoring the experimental group over the control group (median 126 versus 115, P=0.02), but no significant difference in the OBAS score (median 2 versus 2, P=0.11). Indirect measures of pain management including patient satisfaction and length of PACU or hospital stay were similar between the groups (Table 4).
 |
Table 4 Postoperative and satisfaction values |
Complications were rare and not significantly different between the groups. Four patients in the control group had urinary retention versus one in the experimental group. One patient in the control group had constipation and one patient in the experimental group had a postoperative urinary tract infection. The incidence of nausea and/or emesis were similar between the experimental and control groups during the study window (33% vs 53%, P=0.19).
The Cohen’s d effect size for the primary outcome of 0–72 hrs total opioid equivalents using the nonparametric Kruskal–Wallis test is 0.559 (ie, what would be considered a medium effect size).16
Discussion
This prospective randomized, double-blinded trial demonstrates that preoperatively applied TAP block with liposomal bupivacaine reduced opioid consumption in the first 72 hrs compared to port-site infiltration with bupivacaine in patients undergoing elective robotic or laparoscopic hysterectomy. Patients in the experimental group reported less pain, required fewer total opioids, had a higher quality of recovery score, and were more likely to be opioid free 72 hrs after surgery compared to the control group. Despite these improvements, patient-assessments of the overall benefit of analgesia score were not significantly different, possibly reflecting the general tolerability of minimally invasive approaches to hysterectomy or the multimodal approach to pain management applied to both study arms.
These data suggest that regional anesthesia may effectively improve postoperative recovery across a spectrum of surgeries, and are consistent with multiple reports suggesting benefit from including regional anesthesia (specifically TAP blocks) in enhanced recovery after surgery (ERAS) protocols in laparoscopic abdominal procedures.17–24 Most of these reports compare a pre- and post-intervention cohort, a strategy with multiple inherent biases, but multiple prospective head-to-head comparisons have been reported as well, making some comparisons possible.
Despite multiple studies suggesting the efficacy of ERAS principles, there remains no clear consensus regarding a single “optimal” strategy for perioperative pain management after minimally invasive hysterectomy or the ideal outcome measure. A recent study by Gasanova et al showed the analgesic superiority of liposomal bupivacaine infiltration when compared to bupivacaine TAP blocks for open hysterectomy, finding that patients treated with liposomal bupivacaine infiltration had improved pain scores through 48 hrs and less opioid use through 24 hrs when compared to bupivacaine TAP.25 Similarly, Barron et al compared infiltration with liposomal bupivacaine to bupivacaine in laparoscopic hysterectomy, and found the liposomal bupivacaine group to have superior analgesic results on both postoperative day 2 and day 3 but no difference in opioid use.26 These data combined with our current results and previous study results from our institution suggest that the use of liposomal bupivacaine irrespective of modality provides superior analgesia compared to bupivacaine when used in gynecologic procedures.8,9
Similar results have been recently reported in the urologic and general surgery literature; Hutchins et al reported a prospective randomized trial that TAP blockade with liposomal bupivacaine provided superior analgesic effect in patients undergoing donor nephrectomy compared to bupivacaine TAP blocks.10 Another study by Fayezizadeh et al compared the use of liposomal bupivacaine TAP blocks to no TAP blocks in abdominal wall reconstruction and again found superior analgesic effects in the liposomal bupivacaine TAP block group.6 Additionally, Stokes et al showed in a retrospective analysis that patients who received liposomal bupivacaine TAP blocks for colorectal surgery had improved pain control compared to those who received bupivacaine TAP blocks.27 All of these studies illustrate what we found in our study that the use of a TAP block with liposomal bupivacaine tends to provide superior analgesia than when either no local anesthetic or bupivacaine is used.
Our group previously reported a prospective trial demonstrating that liposomal bupivacaine provided superior pain control to bupivacaine hydrochloride when used in a TAP block for pain control after robotic hysterectomy.4 The TAP blockade technique was identical to that used in the present study and, reassuringly, the 72 hrs opioid usage was similar to what we observed in the TAP group of the current trial, demonstrating reproducibility of the results. Interestingly, analysis of secondary endpoints in both studies observed the highest pain scores and greatest discrepancy between the experimental and control arms in the first 24 hrs postoperatively. In both studies, we compared liposomal bupivacaine to 0.25% bupivacaine and not 0.5% or 0.75% bupivacaine. This is because previous studies have shown no difference in outcomes with the higher local anesthetic concentrations in either TAP blocks or wound infiltration.11,12,28,29 However, this has not been studied and compared to liposomal bupivacaine as such it is not known if there is a difference in comparing a higher concentration of bupivacaine infiltration to liposomal bupivacaine TAPs. The higher local anesthetic dose in our experimental group could explain the decreased opioid use and decreased pain scores. Thus, future studies should attempt to have a more similar total dose of local anesthetic in both groups to see if that has an impact on pain and opioid use. Gasanova et al did compare 0.5% bupivacaine TAP blocks to liposomal bupivacaine infiltration and still found the liposomal bupivacaine infiltration to be superior to the 0.5% TAP blocks.25
The strengths of this study include the prospective, randomized, sham-controlled, and blinded design. The balance of known risk factors suggests that the randomization was effective and the central blinding of the medications should preclude incidental observer or reporter bias. The effect size was defined a priori and at a level which was felt to be clinically relevant. The inclusion of multiple surgeons as well as both laparoscopic and robotic-assisted approaches suggests the data can be extrapolated to the most common used minimally invasive approaches. Finally, the study was completed over a relatively short period of time, precluding the potential impact of changes in surgical practice.
Despite these strengths, there are multiple weaknesses which should be considered in analyzing these data. All surgeries were performed by experienced surgeons, but specific strategies, including the number of ports used were not mandated. It is hoped that randomization and blinding would mitigate these differences, but standardization would potentially have assured this. Though the infiltration strategies were specified in the protocol, compliance was not measured; however as both the TAP infusate and local infusate were labeled to preclude identification, it is thought that non-compliance was unlikely to bias our results. Another weakness is that we used between 100 and 125 mg of bupivacaine in the control group site vs 50 mg of bupivacaine and 266 mg of liposomal bupivacaine in the experimental group and this disparity of dosing may have impacted outcomes. Also, the sham TAP procedure may have resulted in unintended pain just from the saline expansion of the TAP space. Finally, as most patients were treated in an outpatient setting, we relied on patient reporting of opioid use. While the accuracy of the reports cannot be confirmed, blinding of both the observer and reporter in this case is thought to decrease potential bias.
In conclusion, our study provides high-level evidence that TAP blockade with liposomal bupivacaine prior to a minimally invasive hysterectomy results in lower opioid usage in the first 72 hrs following surgery, without impairment of the quality of recovery. The procedure adds both time and cost to a patient’s perioperative experience relative to local infusion but did not impact their operative time or complication rate. These findings are critical in light of the urgent need to decrease prescription opioids with the ongoing opioid epidemic due to the high risk of addiction with such drugs. Further study will be necessary to optimize both the technique and patient selection to optimize the cost:benefit ratio of using perioperative TAP blocks.
Implication statement
This study illustrates the analgesic and patient satisfaction benefit of TAP blocks with liposome bupivacaine when compared to surgeon infiltration with bupivacaine in robotic and laparoscopic hysterectomy procedures.
Acknowledgments
The authors would like to acknowledge Melissa Cohen (data collection), Britt Erickson (contributing surgeon and study design), Sally Mullany (contributing surgeon and study design), Colleen Rivard (contributing surgeon and study design), and Boris Winterhoff (contributing surgeon and study design) for their contributions to this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Interim data from this work were presented at the 2017 European Society of Regional Anesthesia meeting on September 13-16, 2017 in Lugano, Switzerland.
Author contributions
J Hutchins and AB contributed to design, interpretation, writing, and editing. PA, MAG, J Habeck contributed to design, implementation of study, writing, and editing. AK contributed to data analysis and writing and editing. All authors contributed to drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
J Hutchins is a speaker and consultant for, and has received research funds from Pacira Pharmaceuticals, he is a consultant for and owns stock with Insitu Biologics, he is also a consultant and speaker for Acel RX, a consultant for Worrell and Johnson and Johnson, speaker for Sonosite, and a consultant and speaker for, and has received research funds from Avanos. AB is a consultant for Avnanos and Pacira Pharmaceuticals inc. and reports personal fees from Pacira Pharmaceuticals Inc., Halyard Health, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Rafi AN. Abdominal field block: a new approach via the lumbar triangle. Anaesthesia. 2001;56:1024–1026.
2. McDonnell JG, O’Donnell BD, Tuite D, Farrell T, Power C. The regional abdominal field infiltration (RAFI) technique computerized tomographic and anatomical identification of a novel approach to the transversus abdominis neuro-vascular fascial plain. Anesthesiology. 2004;101:A899.
3. McDonnell JG, O’Donnell B, Curley G, Heffernan A, Power C, Laffey JG. The analgesic efficacy of transversus abdominis plane block after abdominal surgery: a prospective randomized controlled trial. Anesth Analg. 2007;104:193–197. doi:10.1213/01.ane.0000250223.49963.0f
4. Hebbard P, Fujiwara Y, Shibata Y, Royse C. Ultrasound-guided transversus abdominis plane (TAP) block. Anaesth Intensive Care. 2007;35:616–617.
5. Hebbard P. Subcostal transversus abdominis plane block under ultra-sound guidance. Anesth Analg. 2008;106:674–675. doi:10.1213/ane.0b013e318161a88f
6. Fayezizadeh M, Majumder A, Neupane R, Elliott HL, Novitsky YW. Efficacy of transversus abdomninis plane block with liposomal bupivacaine during open abdominal wall reconstruction. A J Surg. 2016;212:399–405. doi:10.1016/j.amjsurg.2015.12.026
7. Abdallah FW, Laffey JG, Halpern SH, Brull R. Duration of analgesic effectiveness after the posterior and lateral transversus abdominis plane block techniques for transverse lower abdominal incisions: a meta-analysis. Br J Anaesth. 2013;111:721–735. doi:10.1093/bja/aet214
8. Hutchins J, Isaksson Vogel R, Ghebre R, et al. Ultrasound-guided subcostal transversus abdominis plane infiltration with liposomal bupivacaine for patients undergoing robotic-assisted hysterectomy. Int J Gynecol Cancer. 2015;25:937–941. doi:10.1097/IGC.0000000000000429
9. Hutchins J, Delaney D, Isaksson Vogel R, et al. Ultrasound guided subcostal transversus abdominis (TAP) infiltration with liposomal bupivacaine for patients undergoing robotic assisted hysterectomy: a prospective randomized controlled study. Gynecol Onc. 2015;138:609–613. doi:10.1016/j.ygyno.2015.06.008
10. Hutchins JL, Kesha R, Blanco F, Dunn T, Hochhalter R. Ultrasound-guided subcostal transversus abdominis plane blocks with liposomal bupivacaine vs. non-liposomal bupivacaine for postoperative pain control after laparoscopic hand-assisted donor nephrectomy: a prospective randomised observer-blinded study. Anaesthesia. 2016;71:930–937. doi:10.1111/anae.13502
11. Pettersson N, Berggren P, Larsson M, Westman B, Hahn RG. Pain relief by wound infiltration with bupivacaine or high-dose ropivacaine after inguinal hernia repair. Reg Anesth Pain Med. 1999;24:569–575.
12. Mulroy MF, Burgess FW, Emanuelsson BM. Ropivacaine 0.25% and 0.5% buty not 0.125% provide effective wound infiltration analgesia after outpatient hernia repair but with sustained plasma drug levels. Reg Anesth Pain Med. 1999;24:136–141.
13. Stark PA, Myles PS, Burke JA. Development and psychometric evaluation of a postoperative quality of recovery score: the QoR-15. Anesthesiology. 2013;118:1332–1340. doi:10.1097/ALN.0b013e318289b84b
14. Lehmann N, Joshi GP, Dirkmann D, et al. Development and longitudinal validation of the overall benefit of analgesia score: a simple multi-dimensional quality assessment instrument. Br J Anaesth. 2010;105:511–518. doi:10.1093/bja/aeq186
15. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi:10.1186/1471-2288-5-27
16. Ivarsson A, Andersen MB, Johnson U, Lindwall M. To adjust or not adjust: nonparametric effect sizes, confidence intervals, and real-world meaning. Pyschol Sport Exerc. 2013;14:97–102. doi:10.1016/j.psychsport.2012.07.007
17. Keller DS, Tahilramani RN, Flores-Gonzalez JR, Ibarra S, Haas EM. Pilot study of a novel pain management strategy: evaluating the impact on patient outcomes. Surg Endosc. 2016;30:2192–2198. doi:10.1007/s00464-015-4459-4
18. Alvarez MP, Foley KE, Zebley DM, Fassler SA. Comprehensive enhanced recovery pathway significantly reduces postoperative length of stay and opioid usage in elective laparoscopic colectomy. Surg Endosc. 2015;29:2506–2511. doi:10.1007/s00464-014-4006-8
19. Keller DS, Ermlich BO, Schiltz N, et al. The effect of transversus abdominis plane blocks on postoperative pain in laparoscopic colorectal surgery: a prospective, randomized, double-blind trial. Dis Colon Rectum. 2014;57:1290–1297. doi:10.1097/DCR.0000000000000211
20. Pisarka M, Pedziwiatr M, Major P, et al. Laparoscopic gastrectomy with enhanced recovery after surgery protocol: single-center experience. Med Sci Monit. 2017;23:1421–1427. doi:10.12659/msm.898848
21. Helander EM, Webb MP, Bias M, Whang EE, Kaye AD, Urman RD. Use of regional anesthesia techniques: analysis of institutional enhanced recovery after surgery protocols for colorectal surgery. J Laparoendosc Adv Surg Tech. 2017;27:898–902. doi:10.1089/lap.2017.0339
22. Pirrera B, Alagna V, Lucchi A, et al. Transversus abdominis plane (TAP) block versus epidural analgesia (TEA) in laparoscopic colon surgery in the ERAS program. Surg Endosc. 2018;32:376–382. doi:10.1007/s00464-017-5686-7
23. Pedrazzani C, Menestrina N, Moro M, et al. Local wound infiltration plus transversus abdominis plane (TAP) block versus local infiltration in laparoscopic colorectal surgery and ERAS program. Surg Endosc. 2016;30:5117–5125. doi:10.1007/s00464-016-4862-5
24. Kim AJ, Young RJ, Urman RD. The role of transversus abdominis plane blocks in enhanced recovery after surgery pathways for open and laparoscopic colorectal surgery. J Laparoendosc Adv Surg Tech. 2017;27:909–914. doi:10.1089/lap.2017.0337
25. Gasanova I, Alexander J, Ogunnaike B, et al. Transversus abdominis plane block versus surgical site infiltration for pain management after open total abdominal hysterectomy. Anesth Analg. 2015;121:1383–1388. doi:10.1213/ANE.0000000000000909
26. Barron KI, Lamvu GM, Schmidt RC, Fisk M, Blanton E, Pantanwala I. Wound infiltration with extended-release versus short-acting bupivacaine before laparoscopic hysterectomy: a randomized controlled trial. JMIG. 2017;24:286–292.
27. Stokes AL, Adhikary SD, Quintili A, et al. Liposomal bupivacaine use in transversus abdominis plane blocks reduces pain and postoperative intravenous opioid requirement after colorectal surgery. Dis Colon Rectum. 2017;60:170–177. doi:10.1097/DCR.0000000000000747
28. Ng SC, Habib AS, Sodha S, Carvalho B, Sultan P. High-dose versus low-dose local anaesthetic for transversus abdominis plane block post-caesarean delivery analgesia: a meta-analysis. Br J Anaesth. 2018;120:252–263. doi:10.1016/j.bja.2017.11.084
29. Jalil RMA, Yahya N, Sulaiman O, et al. Comparing the effectiveness of ropivacaine 0.5% versus ropivacaine 0.2% for transabdominis plane block in providing postoperative analgesia after appendectomy. Acta Anaesthesiol Taiwan. 2014;42:49–53. doi:10.1016/j.aat.2014.05.007
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
