Back to Journals » Journal of Pain Research » Volume 13
Ultrasound-Guided Rectus Sheath Block Combined with Butorphanol for Single-Incision Laparoscopic Cholecystectomy: What is the Optimal Dose of Ropivacaine?
Authors Fu H, Fu Y , xu X, Gao Y
Received 29 June 2020
Accepted for publication 14 September 2020
Published 15 October 2020 Volume 2020:13 Pages 2609—2615
DOI https://doi.org/10.2147/JPR.S265418
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Huimin Fu, Yu Fu, Xingguo Xu, Yongtao Gao
Department of Anesthesiology, Affiliated Hospital of Nantong University, Nantong, Jiangsu, People’s Republic of China
Correspondence: Xingguo Xu; Yongtao Gao
Department of Anesthesiology, Affiliated Hospital of Nantong University, No. 20 Xisi Road, Nantong, Jiangsu 226001, People’s Republic of China
Tel +86 15162771638
; +86 13962988003
Fax +86 051381160318
Email [email protected]; [email protected]
Purpose: In recent years, ultrasound-guided rectus sheath block (RSB) has been widely used in postoperative analgesia of abdominal operation. However, there is no uniform standard for the optimal dose of local anesthetics (LA) under ultrasound-guided rectus sheath block. This study aimed to determine the dose of ropivacaine combined with butorphanol that is effective in 50% (ED50) and 95% (ED95) of subjects for successful pain-free ultrasound-guided RSB in single-incision laparoscopic cholecystectomy (SILC).
Patients and Methods: Twenty-four patients scheduled to undergo single-incision laparoscopic cholecystectomy received an ultrasound-guided RSB. The initial dose of ropivacaine injected was 1.7 mg/kg, which was subsequently increased or decreased by 0.2 mg/kg, depending on whether the previous patient was free from pain (numeric rating scale (NRS) score of incisional pain at rest within 12 h after operation ≤ 3). All patients were treated with butorphanol 0.02 mg/kg as preemptive analgesia. The ED50 and ED95 were calculated using a probit regression model.
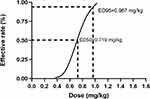
Results: The ED50 and ED95 of ropivacaine combined with butorphanol in ultrasound-guided rectus sheath block for analgesia in SILC, which were calculated by the probit regression model, were 0.719 mg/kg (95% confidence interval (CI), 0.553 mg/kg− 0.873 mg/kg) and 0.967 mg/kg (95% CI, 0.835 mg/kg− 1.91 mg/kg), respectively.
Conclusion: As part of a multimodal analgesia strategy, a dose of 0.719 mg/kg ropivacaine provided successful RSB under ultrasound guidance in 50% of the patients who underwent SILC. A dose of 0.967 mg/kg would be successful in 95% of patients.
Keywords: ED50, ED95, single-incision laparoscopic cholecystectomy, rectus sheath block
Introduction
As we all know, laparoscopic cholecystectomy is an effective method in the treatment of cholecystolithiasis.1–4 Compared with conventional laparoscopic cholecystectomy, single-incision laparoscopic cholecystectomy (SILC) involves only a 2-cm incision into the umbilicus between the T7 and T11 intercostal nerves, which is becoming increasingly popular because it has an outstanding cosmetic effect, but postoperative pain is still the main problem complained by patients.
Acute pain after SILC is mainly composed of incision pain and visceral pain, which has been proven to be an important risk factor for chronic pain.5,6 Due to the diversity of pain sources after SILC, multimodal analgesia is appropriate. In recent years, with the popularization and application of visualization technology in clinical anesthesia and postoperative analgesia, tissue planes, the bowel and the spread of local anesthetics can be seen, which can increase accuracy and safety, and ultrasound-guided rectus abdominis sheath block (RSB) has been popularized in multimodal analgesia after abdominal operation.7–11 To the best of our knowledge, no previous studies have determined the ideal dose of ropivacaine, reported concentration and volumes used vary widely, and anesthesiologists usually choose a higher dose to ensure the anesthetic effect.12 However, ultrasound can reduce the dose of local anesthetics (LA) because of its precise localization.13,14 Yasumura et al13 reported that maximum plasma concentration depended on the dose administered but not the procedure, and the toxic plasma concentration of ropivacaine has been reported to be 4 μg/mL after transversus abdominis plane (TAP) block.15 The effect of RSB is generally recognized, which performed before an operation can inhibit reflection of the skin incision, yet visceral pain cannot be effectively relieved.16 Visceral pain is a complex disorder that can be caused by mechanical traction, dilation, spasm, inflammation, ischemia and chemical stimulation.17,18 Several studies and our previous research have demonstrated that butorphanol, a κ-agonist, can effectively relieve visceral pain.
A high dose of LA may increase the incidence of serious adverse events. It is urgent to fix a minimum effective dose of ropivacaine. Therefore, we designed this prospective study using an up-and-down method to explore the optimal dose of ropivacaine combined with butorphanol required for successful analgesia using ultrasound-guided RSB for patients undergoing SILC.
Patients and Methods
Patients and Study Design
This prospective study was approved by the Ethics Committee of Affiliated Hospital of Nantong University (number: 2019-K075), registered with the Chinese Clinical Trial Registry (reg no. CHiCTR2000029008), and carried out in accordance with the Declaration of Helsinki, and written informed consent was obtained from all patients scheduled for elective SILC who were enrolled in this study from January 2020 to April 2020 at the Affiliated Hospital of Nantong University.
Male or female patients aged from 18 to 59 years with an American Society of Anesthesiology (ASA) score of I or II and a body mass index (BMI) of 18–30 kg/m2 were included in this study. The exclusion criteria included preexisting neuropathy, coagulopathy, local skin infection, hepatic, renal or cardiorespiratory failure, local anesthetic allergy, pregnancy, complications of gallstones with gallbladder perforation, diffuse peritonitis or acute pyogenic cholangitis.
The sensation of visceral pain and incisional pain is different; incisional pain was defined as superficial pain on the abdominal wall, and visceral pain was defined as pain inside the abdomen, which may be continuous, delayed and more difficult to localize. During a preoperative visit, patients had an adequate understanding of visceral pain, incisional pain and numeric rating scale (NRS) score (NRS; 0 =no pain; 10 =worst pain).
After reviewing different doses used in previous reports, the dose chosen to be administered to the first patient was 1.7 mg/kg ropivacaine, a concentration of 0.5% ropivacaine was achieved by adding 10 mL of 0.9% saline.11 The validity or invalidity of the study dose of ropivacaine was assessed using the NRS score of incisional pain at rest within 12 h after operation ≤ 3. Two results were considered: success (NRS score of incisional pain at rest within 12 h after operation ≤ 3), and failure (NRS score of incisional pain at rest within 12 h after operation > 3). Dose assignment was carried out using the up-and-down method, where the total dose of local anesthetic administered to each patient depended on the response of the previous one. If the previous patient’s block is successful, the next patient was decreased by 0.2 mg/kg. If it failed, the next patient was randomized to a 0.2 mg/kg incremental increase. According to our previous clinical experience, it is usually sufficient to use a total dose of 1.7 mg/kg ropivacaine for the first patient, but to prevent the occurrence of the risk of local anesthetic toxicity due to unexpected results, we decided a priori not to exceed 2.5 mg/kg injectate.
Anesthesia
In the anesthesia preparation room, all patients had intravenous access inserted in a peripheral arm vein, routine monitors including oxygen saturation (SpO2), heart rate (HR), noninvasive blood pressure (NIBP), and electrocardiogram (ECG) were used, and then all patients were performed with RSB 30 min before anesthesia induction, the transducer (HFL38x/13-6 MHz Transducer; SonoSite Inc., Bothell, WA, USA) applied in a sterile fashion at the lateral level of the umbilicus (Figure 1A). Using the in-plane technique, the needle was advanced until its tip was positioned in the location between the posterior rectus muscle and the posterior sheath. No blood and no gas were drawn back; furthermore, a small volume of saline (<2 mL) was injected in this potential space, and if the needle tip was correctly positioned, the ropivacaine was injected bilaterally (Figure 1B). The mixed solution for RSB was prepared by an investigator who was not involved in the study. All patients were treated by the same experienced anesthesiologist, who specialized in ultrasound-guided regional anesthesia and did not participate in the data collection. Neither the anesthesiologist performing the RSB and subsequent assessment and management nor the patients were aware of the ropivacaine dose administered and group allocation.
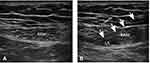 |
Figure 1 Ultrasound images (A) before and (B) after rectus sheath block. The arrow points to the path of the needle. |
After entering the operating room, all patients were administered butorphanol 0.02 mg/kg. Then, anesthesia was induced with midazolam (0.1 mg/kg), propofol (2 mg/kg) and sufentanil (0.2 µg/kg), followed by cisatracurium (0.15 mg/kg) to facilitate tracheal intubation. Anesthesia was maintained with propofol at 4–5 mg/kg/h, remifentanil at 0.2 µg/kg/min and 1–2% sevoflurane.
Patients defined as failure or NRS > 3 after surgery received butorphanol PCIA set at a background rate of 170 µg/h and a demand dose of 170 µg every 15 min as rescue analgesia for postoperative pain management.
Statistical Analysis
Due to the characteristics of the up-and-down sequential method, the data distribution was not independent and uncertain, which makes it difficult for us to estimate the sample size accurately.19,20 Previous studies have shown that reliable results can be obtained when the sample size reaches 20–40. In other research, at least >6 pairs of reversal of sequence were required at the end of the study. Finally, we chose 24 people to calculate the effective dose in 50% of subjects (ED50), effective dose in 95% of subjects (ED95) and 95% confidence interval (95% CI). Data were analyzed using the probit regression model to calculate the ED50, ED95 and 95% CI.
Statistical analysis was performed using SPSS 21.0 (IBM Corporation, Armonk, NY, USA). Data were presented as mean±SD, median (range) or frequency as appropriate. Categorical data were analyzed by the chi-squared (χ2) test or the Fisher exact test. Means were analyzed by Student’s t-test if they are normally distributed, and medians and means with non-normally distributed were analyzed by Mann–Whitney U-test. P<0.05 was considered statistically significant.
Results
The study flow diagram is presented in Figure 2. Three patients were excluded from the study, including two patients due to complications involving gallstones with gallbladder perforation during surgery and one patient due to the change of operation mode. The study was stopped after the enrolment of 24 consecutive patients. Patients' characteristics are presented in Table 1. There were no significant differences in patient characteristics, duration of surgery and total dose of propofol and remifentanil between the success and failure groups (P>0.05).
 |
Table 1 Patient Characteristics and Surgery Data |
 |
Figure 2 CONSORT flow diagram. |
The sequence of the dose of ropivacaine is illustrated in Figure 3. Our research had 8 pairs of reversal of sequence, which showed that our sample size was enough. As shown in Figure 3, the first four volumes were successful, and the maximum dose of 1.7 mg/kg was reached one time. The corresponding dose varied from 1.7 mg/kg to 0.5 mg/kg. The doses were calculated as 0.719 mg/kg (95% CI, 0.553 mg/kg–0.873 mg/kg) for ED50 and 0.967 mg/kg (95% CI, 0.835 mg/kg–1.91 mg/kg) for ED95. Dose–response curves for ropivacaine derived from probit regression model analysis are shown in Figure 4. The percentages of success and failure dosages of ropivacaine for postoperative pain are shown in Figure 5.
 |
Figure 5 The percentage of success and failure at different doses of ropivacaine. |
After surgery, none of the patients in the success group required rescue analgesia, while 90% of patients in the failure group received rescue analgesia. The NRS scores for incisional pain at rest were lower in the success group at 12 h after surgery than in the failure group (P<0.05).
Because the RSB procedure was carried out under visualization, there was not a case that had local anesthetic toxicity, and no adverse effects of ropivacaine (visual and hearing disturbances, dizziness, QRS modification) were recorded.
Discussion
Thus far, no previous research has recommendations regarding the minimal effective dose of LA in ultrasound-guided RSB, and this study is the first attempt to investigate the optimal dose for this technique. According to our study, it indicated that the ED50 and ED95 of ropivacaine in ultrasound-guided RSB for analgesia in SILC were 0.719 mg/kg (95% CI, 0.553 mg/kg–0.873 mg/kg) and 0.967 mg/kg (95% CI, 0.835 mg/kg–1.91 mg/kg), respectively. The above results are much less than the dose regular used in clinical treatment. To our minds, the dose of LA should not be too high for postoperative analgesia as long as it can block the sensation without muscle relaxation.
The up-and-down sequential method, a classical method to study the dose–response relationship, can make full use of the information provided by a lower number of patients and get the results quickly and accurately.19 Using the up-and-down method to determine ED50, once 6 pairs of reversal of sequence were achieved, it was possible to consider the sample size as adequate. Therefore, a sequential approach is widely used to study the minimum effective anesthetic dose. The ED50 is located in the most sensitive position of the dose–response curve, and is the most sensitive index reflecting the relationship between the dose and the efficiency, but the ED95 may be more useful in clinical practice than the ED50.
In theory, the success factors of the RSB procedure include three main aspects: correct anatomic location, appropriate concentration and volume.20 Schleich first used RSB in 1899 to provide muscle relaxation and analgesia; RSB mainly blocks the sheath nerve plexus between the rectus abdominis and posterior sheath of the rectus muscles, which is dominated by the ventral rami of the 6th to 11th intercostal nerves, providing analgesia for the peritoneum, muscle and skin involved in anterior abdominal wall incisions.21 An increased LA concentration can shorten the onset time due to the improved neural penetration by LA molecules, increasing the concentration gradient so as to promote the permeability of drug molecules to the nerve, and speed up the onset time. But we usually performed RSB for preemptive analgesia 30 min before the operation, and this is the same as the onset time of ropivacaine with different concentrations. So we do not think it is necessary to have a high concentration of LA; increasing the volume of LA can promote the spread of LA. Ropivacaine, a long-acting, pure S-enantiomer amide local anesthetic, is less toxic than bupivacaine. The maximal recommended dose of ropivacaine is 200–250 mg.22,23 Although the toxicity is lower than bupivacaine, the incidence of QRS prolongation, arrhythmia and LA toxicity increase with the dose of ropivacaine. Bupivacaine has a long-lasting action that leads to delayed absorption.24 The arteries, which carry blood to the anterior abdominal wall, are transferred to anastomose within the rectus sheath.17,25 We chose 12 h after surgery as the end point of observation because ropivacaine generally wears off after 12 h.
Butorphanol, a mixed agonist–antagonist opioid, induces analgesia by opioid pathways.17,26,27 Some studies have shown that butorphanol relieves visceral pain by indirectly suppressing cyclooxygenase activity and thus preventing prostaglandin formation in response to injury. In addition, the main metabolite of butorphanol activates κ-receptors and has dual effects of excitation and antagonism on μ-receptors. Therefore, butorphanol was injected 30 min before the end of the operation to relieve visceral pain.
We report that none of the patients in the success group required rescue analgesia, while 90% of patients in the failure group received rescue analgesia. The NRS scores for incisional pain at rest were significantly lower in the success group at 12 h after surgery than in the failure group. Our data suggest that successful pain management can significantly relieve pain during the perioperative period.
Limitations
There are several limitations in the current study. The anesthesiologist who performed the procedure was not blinded to volume, which increased the risk of bias. However, he was not in charge of the data collection of subsequent experiments. In our research the level of blood concentration of ropivacaine has not been checked, and the conclusion can be better confirmed by combining with laboratory examination in the future.
Conclusions
As an important part of multimodal analgesia, ultrasound-guided administration of 0.719 mg/kg (0.553 mg/kg–0.873 mg/kg) and 0.967 mg/kg (0.835 mg/kg–1.91 mg/kg) of ropivacaine in the RSB provided efficient preemptive analgesia in 50% and 95% of patients who underwent SILC. Although the ED50 and ED95 are not close to the toxicity threshold, we should still be careful to prevent potential systemic toxicity. Determining the optimal dose can improve safety, reduce the economic burden of patients and provide the possibility for implementation of accurate anesthesia.
Abbreviations
RSB, rectus sheath block; LA, local anesthetics; SILC, single-incision laparoscopic cholecystectomy; NRS, numeric rating scale; ED50, 50% effective dose; ED95, 95% effective dose; 95% CI, 95% confidence interval; HR, heart rate; BP, blood pressure; ECG, electrocardiogram; SpO2, blood oxygen saturation; NIBP, blood pressure; ASA, American Society of Anesthesiology; BMI, body mass index.
Data Sharing Statement
All necessary data supporting our findings have been presented within the manuscript. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Approval for this study was obtained from the Ethics Committee of the Affiliated Hospital of Nantong University (approval number: 2019-K075), and each patient provided a written informed consent.
Funding
This study was supported by grants from the Social Development Foundation of Nantong City (MS12019023).
Disclosure
The authors declare that we have no competing interests for this work.
References
1. Lirici MM, Tierno SM, Ponzano C. Single-incision laparoscopic cholecystectomy: does it work? A systematic review. Surg Endosc. 2016;30(10):4389–4399. doi:10.1007/s00464-016-4757-5
2. Omar MA, Redwan AA, Mahmoud AG. Single-incision versus 3-port laparoscopic cholecystectomy in symptomatic gallstones: a prospective randomized study. Surgery. 2017;162(1):96–103. doi:10.1016/j.surg.2017.01.006
3. Rizzuto A, Serra R, Mignogna C, et al. Single incision laparoscopic cholecystectomy in geriatric patients. Int J Surg. 2016;35:83–87. doi:10.1016/j.ijsu.2016.09.075
4. Kim JS, Choi JB, Lee SY, et al. Pain related to robotic cholecystectomy with lower abdominal ports: effect of the bilateral ultrasound-guided split injection technique of rectus sheath block in female patients: a prospective randomised trial. Medicine. 2016;95(31).
5. Mitra S, Khandelwal P, Roberts K, et al. Pain relief in laparoscopic cholecystectomy–a review of the current options. Pain Pract. 2012;12(6):485–496. doi:10.1111/j.1533-2500.2011.00513.x
6. Blichfeldt-Eckhardt MR, Ording H, Andersen C, et al. Early visceral pain predicts chronic pain after laparoscopic cholecystectomy. Pain. 2014;155(11):2400–2407. doi:10.1016/j.pain.2014.09.019
7. Hong S, Kim H, Park J. Analgesic effectiveness of rectus sheath block during open gastrectomy: a prospective double-blinded randomized controlled clinical trial. Medicine. 2019;98(15):e15159. doi:10.1097/MD.0000000000015159
8. Jin F, Li Z, Tan WF, et al. Preoperative versus postoperative ultrasound-guided rectus sheath block for improving pain, sleep quality and cytokine levels in patients with open midline incisions undergoing transabdominal gynecological surgery: a randomized-controlled trial. BMC Anesthesiol. 2018;18(1):19. doi:10.1186/s12871-018-0485-9
9. Cho S, Kim YJ, Jeong K, et al. Ultrasound-guided bilateral rectus sheath block reduces early postoperative pain after laparoscopic gynecologic surgery: a randomized study. J Anesth. 2018;32(2):189–197. doi:10.1007/s00540-018-2457-0
10. Kamei H, Ishibashi N, Nakayama G, et al. Ultrasound-guided rectus sheath block for single-incision laparoscopic cholecystectomy. Asian J Endosc Surg. 2015;8(2):148–152. doi:10.1111/ases.12178
11. Chung W, Yoon Y, Kim JW, et al. Comparing two different techniques of rectus sheath block after single port laparoscopic surgery in benign adnexal mass patients: surgical versus ultrasonography guidance-A randomized, single-blind, case-controlled study. Eur J Obstet Gynecol Reprod Biol. 2017;217:29–33. doi:10.1016/j.ejogrb.2017.08.020
12. Sola C, Menace C, Rochette A, et al. Ultrasound-guided tranversus abdominis plane block for herniorrhaphy in children: what is the optimal dose of levobupivacaine? Eur J Anaesthesiol. 2014;31(6):327–332. doi:10.1097/EJA.0000000000000040
13. Yasumura R, Kobayashi Y, Ochiai R. A comparison of plasma levobupivacaine concentrations following transversus abdominis plane block and rectus sheath block. Anaesthesia. 2016;71(5):544–549.
14. Griffiths JD, Barron FA, Grant S, et al. Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block. Br J Anaesth. 2010;105(6):853–856. doi:10.1093/bja/aeq255
15. Rajwani KM, Butler S, Mahomed A. In children undergoing umbilical hernia repair is rectus sheath block effective at reducing post-operative pain? Best evidence topic (bet). Int J Surg. 2014;12(12):1452–1455. doi:10.1016/j.ijsu.2014.11.007
16. Baranidharan G, Simpson KH, Dhandapani K. Spinal cord stimulation for visceral pain-a novel approach. Neuromodulation. 2014;17(8):753–758. doi:10.1111/ner.12166
17. Tsang BK, He Z, Wongchanapai W, et al. Visceral analgesic tolerance to intrathecal butorphanol in rats. Can J Anaesth. 1998;45(10):1019–1023. doi:10.1007/BF03012311
18. Houghton KJ, Rech RH, Sawyer DC, et al. Dose-response of intravenous butorphanol to increase visceral nociceptive threshold in dogs. Proc Soc Exp Biol Med. 1991;197(3):290–296.
19. Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007;107(1):144–152. doi:10.1097/01.anes.0000267514.42592.2a
20. Sviggum HP, Niesen AD, Sites BD, et al. Trunk blocks 101: transversus abdominis plane, ilioinguinal-iliohypogastric, and rectus sheath blocks. Int Anesthesiol Clin. 2012;50(1):74–92. doi:10.1097/AIA.0b013e31823bc2eb
21. Zhai W, Wang X, Rong Y, et al. Effects of a fixed low-dose ropivacaine with different volume and concentrations on interscalene brachial plexus block: a randomized controlled trial. BMC Anesthesiol. 2016;16(1):80. doi:10.1186/s12871-016-0248-4
22. Satsumae T, Tanaka M, Saito ST, et al. Convulsions after ropivacaine 300 mg for brachial plexus block. Br J Anaesth. 2008;101(6):860–862. doi:10.1093/bja/aen297
23. Lahlou-Casulli M, Chaize- Avril C, Pouliquen E, et al. The median effective analgesic dose (ED50) of ropivacaine in ultrasound-guided transversus abdominis plane block for analgesia in reversal of ileostomy: a double-blind up-down dose-finding study. Eur J Anaesthesiol. 2015;32(9):640–644. doi:10.1097/EJA.0000000000000198
24. Bowness J, Seeley J, Varsou O, et al. Arterial anatomy of the anterior abdominal wall: evidence-based safe sites for instrumentation based on radiological analysis of 100 patients. Clin Anat. 2020;33(3):350–354. doi:10.1002/ca.23463
25. Fu H, Zhong C, Fu Y, et al. Perioperative analgesic effects of preemptive ultrasound-guided rectus sheath block combined with butorphanol or sufentanil for single-incision laparoscopic cholecystectomy: a prospective, randomized, clinical trial. J Pain Res. 2020;13:1193–1200.
26. Johnson AC, Greenwood-van Meerveld B. The pharmacology of visceral pain. Adv Pharmacol. 2016;75:273–301.
27. Reddi D, Curran N. Chronic pain after surgery: pathophysiology, risk factors and prevention. Postgrad Med. 2014;90(1062):
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.