Back to Journals » Journal of Pain Research » Volume 15
Ultrasound-Guided Extraforaminal Thoracic Nerve Root Block Through the Midpoint of the Inferior Articular Process and the Parietal Pleura: A Clinical Application of Thoracic Paravertebral Nerve Block
Authors Pu S, Wu Y, Han Q, Chen J, Xu Y, Lv Y, Li C, Lu J, Wu J , Du D
Received 29 November 2021
Accepted for publication 12 February 2022
Published 19 February 2022 Volume 2022:15 Pages 533—544
DOI https://doi.org/10.2147/JPR.S351145
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jinlei Li
Shaofeng Pu,1 Yiyang Wu,1 Qingjian Han,2 Jie Chen,3 Yongming Xu,1 Yingying Lv,1 Chen Li,1 Jing Lu,4 Junzhen Wu,1,* Dongping Du1,*
1Department of Pain Management, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, 200233, People’s Republic of China; 2Institutes of Brain Science, Fudan University, Shanghai, 200032, People’s Republic of China; 3Shanghai Institute of Ultrasound in Medicine, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, 200233, People’s Republic of China; 4Radiology Department, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, 200233, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dongping Du, Department of Pain Management, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, 200233, People’s Republic of China, Tel +86 21 2405 8896, Fax +86 21 2405 8330, Email [email protected]
Purpose: Thoracic nerve root (TNR) block is performed primarily under computed tomography or X-ray fluoroscopy but is associated with radiation exposure. Ultrasound requires no radiation and distinguishes vessels, nerves, pleura, and other tissues. Few reports of ultrasound-guided TNR (US-TNR) block have been described, and the puncture end point has not been clearly defined. Herein, we evaluated the feasibility of US-TNR block using the midpoint of the inferior articular process (IAP) and parietal pleura (PP) as the puncture end point.
Patients and Methods: A prospective series of 10 patients with Herpes Zoster-associated pain underwent US-TNR-guided block performed using an in-plane technique with the midpoint of thoracic IAP and PP as the puncture end points of ultrasonography. The US-TNR block procedure was performed with ultrasound as the primary imaging tool followed by fluoroscopic confirmation.
Results: In all patients, the needle tips were visible at the lateral margin of the pedicle in the anteroposterior view and at the extraforaminal zone in the lateral view. The TNR and dorsal root ganglion (DRG) were delineated in all 10 patients. Furthermore, 2 mL of radiopaque agent could delineate the epidural space in 8 patients and the thoracic paravertebral (TPV) space in the other 2 patients. All patients developed numbness along the corresponding dermatome 30 min after injection of local anesthetics. The numeric rating scale (NRS) score at baseline, and at two- and four-week follow-ups were 6.50 ± 1.35, 3.50 ± 0.85 (vs NRS at baseline, P < 0.01), and 4.00 ± 0.82 (vs NRS at baseline, P < 0.01), respectively.
Conclusion: This study demonstrated the feasibility of US-TNR block using the in-plane technique with the midpoint of thoracic IAP and PP as the puncture end point to effectively block the TNR and DRG. This technique is an accurate clinical application of TPV nerve block and provides a potential therapeutic option for the treatment of neuropathic pain.
Keywords: ultrasound-guided, thoracic nerve root, thoracic paravertebral, midpoint, neuropathic pain
Introduction
Thoracic paravertebral (TPV) block was first described in 1905 and was introduced into clinical practice by Ethan and Wyatt in 1978.1 With the popularity of visualization technology, ultrasound-guided (US)-TPV block has been widely carried out in clinical practice,2,3 such as for anesthesia for thoracotomy,4 breast surgery,5 rib fracture surgery,6 cardiac surgery,7 and analgesia for herpes zoster neuralgia.8 Although US-TPV block is generally considered safe, complications such as pneumothorax, vasovagal episodes, symptomatic bradycardia, and hypotension have been reported.9 Many safe and feasible superficial needle insertion techniques, such as the erector spinae plane (ESP) block,10 the midpoint transverse process to pleura (MTP) block,11 and the subtransverse process interligamentary (STIL) plane block12 have been introduced into clinical practice. These techniques do not require piercing of the superior costotransverse ligament (SCTL), and the anatomical evidence supports paravertebral diffusion and clinical efficacy, suggesting their application as alternatives to traditional TPV block. The mechanism of paravertebral block involves the direct infiltration of medicine into the spinal nerve, including the dorsal branch, communicating branch, and sympathetic nerve chain.13 However, in the classic US-TPV block and superficial needle insertion techniques such as the ESP block, MTP block, and STIL plane block, the needle tip is located mainly near the transverse process but not near the thoracic nerve. All the block options described above may achieve effective anesthesia or analgesia, but these blocks required a large volume of local anesthetics (10–20 mL), which means that the block effect may partly depend on the diffusion of drugs. Treatment of neuropathic pain, such as herpes zoster neuralgia, often requires accurate interventional therapy. It has been reported that selective thoracic nerve root (TNR) stimulation could be performed under ultrasound, but its technical procedure has not been described in detail.14 Herein, we will introduce a novel TNR block under ultrasound guidance.
We used in-plane technology to elaborate on this puncture procedure. In view of the unclear puncture endpoint of US-TNR block in the past, we quantified the midpoint of the thoracic inferior articular process (IAP) and parietal pleura (PP) as the puncture end point, as this puncture end point would standardize and distinguish the puncture process. Second, we assumed that SCTL identification was not required in this novel US-TNR block, which simplified the procedure further. Third, we considered that our method could reduce the risk of the tip of the puncture needle entering the spinal canal and piercing the pleura, and thus, the approach could improve the safety of the puncture. Finally, we hypothesized that during this novel TNR block, the tip of the needle was not only approach the TNR, but also the dorsal root ganglion (DRG), so it could bring benefit to patients with neuropathic pain. The purpose of this study was to test these hypotheses and analyze the technical details.
Methods
Study Population
This trial was approved by the ethics committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, No. 2021-196-(1), and was registered in the Chinese Clinical Trial Registry with the identifier ChiCTR2100050408. All subjects were enrolled between September 20, 2021 and October 20, 2021 from Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. All procedures involving human studies were following the ethical standards of the national research committee. Written informed consent was obtained from all subjects according to the Helsinki declaration. All medical records were anonymous and no subject information was extracted except for the study purpose.
We recruited 10 consecutive patients with thoracic herpes zoster (HZ) within two weeks of rash. They all received at least one week of standard treatment (antiviral + analgesic), but their pain intensity was still >4 on a numerical rating scale (NRS). Patients with infection, scoliosis, coagulation disorders, and allergies to iodine or local anesthetics were excluded from the study.
Procedure of Ultrasound-Guided TNR Block
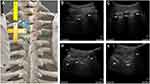
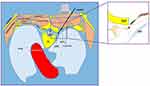
The US-TNR block procedure was performed with ultrasound as the primary imaging tool and with X-ray fluoroscopy confirmation. The patients were placed in the prone position when they received US-TNR block. A low frequency convex array ultrasound probe (2–5 MHz, EDGE-II; SonoSite, USA) was used to localize the thoracic segment with a four-step method (Figure 1A). First, the ultrasound probe was placed in the paraspinal sagittal position of the spine and then moved from the lower part of the cervical spine to the thoracic spine (from the cranial to the caudal end). The first hypoechoic shadow observed on ultrasound was the first rib (Figure 1B). Second, we continued to move the probe from the cranial to caudal end to define the segment of each rib. After determining the target rib (for example, if our target was the ventral branch of the 4th thoracic nerve, the 4th hypoechoic shadow was the target segment), we placed the target rib segment in the center of the ultrasound image (Figure 1C). We then performed the third step, which involved the rotation of the probe 90 degrees clockwise to form an axial position with the thoracic spine. Subsequently, the ultrasonic probe was slightly shifted to the thoracic vertebrae until we could simultaneously display the obvious transverse process, rib, and parietal pleura (PP) on the ultrasound image (Figure 1D). When the above typical images appeared, we moved the probe parallel from the cranial end to the caudal end, which allowed the transverse process to disappear. At this time, the image of the inferior articular process (IAP) appeared. The IAP cortex showed a linear hyperechoic image under ultrasound. On the lateral side of the IAP was the hyperechoic image of the parietal pleura (PP), and on the medial side was the hypoechoic image of the spinous process (Figure 1E).
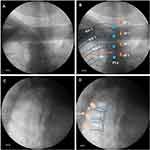
An in-plane puncture was performed from the lateral to medial end (Figure 2A). At this point, if there was a rib or scapula blocking the puncture outside the puncture route, the probe could rotate 5–15 degrees toward the caudal end to avoid the obstruction (Figure 2B). We drew an oblique line A along the PP and a vertical line B along the edge of the IAP on the ultrasound image. The starting point of line B was the edge of IAP, and the end point is the intersection with line A. The midpoint of line B was the puncture end point (Figure 2C). A 22-gauge Tuohy needle (TUORen, Co., Ltd. Henan, China) was then used to puncture from outside to inside using the in-plane technique (Figure 2D).
To analyze the technique, we performed anteroposterior (AP) and lateral X-ray fluoroscopy to observe the position of the needle tip when the ultrasonic procedure was completed. A 2-mL volume of radiopaque agent (Iohexol, 50 mL: 17.5g, Shanghai General Pharmaceutical Co., Ltd.) was then injected to observe its diffusion range by fluoroscopy. After injection of the radiopaque agent, patients received 2 mL of 1% lidocaine as the experimental dose for 5 minutes. If the patient did not experience any adverse reactions such as chest pain, local anesthesia poisoning or total spinal anesthesia, a single 5 mL mixture of 0.3% ropivacaine (AstraZeneca AB, Sweden) and 4 mg of dexamethasone palmitate (Mitsubishi Tanabe Pharma Corporation, Japan) was injected. All drugs were injected in real time under ultrasound.
All procedures were performed by two pain management physicians (Pu S and Wu J), who were trained and had more than 10 years of professional experience in pain management using interventional techniques.
Technical Assessment and Statistical Analysis
Fluoroscopic images were analyzed by an experienced radiologist (Lu J) specialized in radiological analysis for more than 10 years. The location of the needle tip in the AP view and the lateral view was assessed. To better understand the extend of US-TNR block, the diffusion of drugs under ultrasonography and radiopaque agents under X-ray fluoroscopy was also assessed. Sensation to pin-prick was tested after 30 minutes of block, with no pain sensation in the corresponding innervated dermatomes indicating numbness. The NRS scores of the patients were also recorded at the 2-week and 4-week follow-up.
All data were processed with SPSS software version 23.0 (IBM Corp., NY). Normally distributed continuous data relative to patient demographics and pain characteristics were reported as the mean ± standard deviation (SD), while non-normally distributed data were expressed as median (interquartile range). A generalized linear mixed model (GLMM) was performed to evaluate changes in pain NRS scores over repeated measurements. Statistical significance was defined with P-values <0.05.
Patients were asked to inform their doctors if they experienced any adverse symptoms at any time. Block-related complications, such as vascular puncture, Horner syndrome, and adverse reactions such as hematoma, infection, and back pain were recorded. Serious complications such as general spinal anesthesia, pneumothorax and motor deficits were reported to the ethics committee.
Results
Ten patients with HZ-associated pain who still presented severe pain after a week of standard treatment were enrolled in this study. The baseline characteristics of the included subjects are summarized in Table 1.
 |
Table 1 Demographic Characteristics |
When the radiopaque agent or drugs were injected at the endpoint, we were able to visualize the diffusion of the liquid below the IAP in all 10 patients under ultrasound. Pleural subsidence was observed in six patients, while for the remaining four patients only the diffusion of the liquid along the PP was visible, similar to a tide hitting the beach, but the phenomenon of pleural subsidence was not obvious.
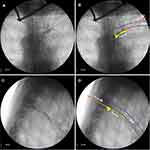
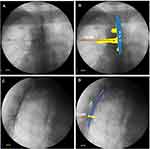
Under X-ray fluoroscopy, we determined that in all 10 patients, the needle tip was located at the lateral margin of the pedicle in the AP view of fluoroscopy and at the external space of the intervertebral foramen in the lateral view (Figure 3).
We found that a 2 mL volume of radiopaque agent could delineate the TNR in all patients. Typical neuroimaging showed that the thoracic nerve originated in the intraspinal region, extending outward from the inferior border of the pedicle and continuing along the inferior border of the rib on the AP fluoroscopy view. The nerve root appeared at the superior border of the foramen and ran along the inferior border of the rib on the lateral fluoroscopy view. The dorsal root ganglion (DRG) was visible as a radiopaque agent near the intervertebral foramen (Figure 4).
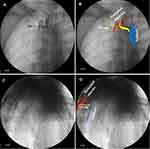
We also determined that the 2 mL radiopaque agent could delineate the transforaminal epidural space in 8 of 10 patients. When the agent delineated the epidural space, the agent was well distributed on the medial side of the pedicle on the AP view of fluoroscopy, and a smooth arcuate shadow appeared in the lateral view (Figure 5).
We found that the radiopaque agent delineated the TPV space but not the epidural space in the remaining 2 subjects. The dyed image in the TPV was very similar to the epidural image under fluoroscopy. However, there were some subtle differences between the two delineating images under fluoroscopy. When the agent delineated the TPV, the AP view under fluoroscopy showed that although the agent was well-distributed in the epidural space, and the lateral boundary of the diffusion was beyond the lateral edge of the pedicle. At this time, the arc shadow on the lateral view under fluoroscopy was relatively rough due to obstruction of the thoracic spine (Figure 6). These findings indicated that the agent did not spread in towards the spinal canal, but diffused outside the spinal canal.
All patients developed numbness along the corresponding dermatome 30 minutes after injection of the mixture of glucocorticoids and ropivacaine. The NRS score at baseline, at the two-week and four-week follow-up were 6.50±1.35, 3.50±0.85 (vs NRS at baseline, P<0.01) and 4.00±0.82 (vs NRS at baseline, P<0.01), respectively. There were no adverse symptoms such as hematoma, infection, and back pain at the 2-week and 4-week follow-up.
No other adverse symptoms were reported except that two patients experienced hypotension 30 min after injecting the mixture of glucocorticoid and ropivacaine.
Discussion
Ultrasound revealed not only the spinous process, transverse process, IAP, and ribs, but also the PP and blood vessels, which was superior to X-ray fluoroscopy. However, the TNR and intervertebral foramen were not visible under ultrasound, and there was no unified standard for the puncture end point during US-TNR. Therefore, we considered the midpoint of the IAP and the PP as our puncture end point. The objective of the procedure is to avoid penetrating the PP or into the subarachnoid space on the premise of effectively blocking the nerve root.
We found that all needle tips were located at the lateral margin of the pedicle in the AP view of fluoroscopy and the external space of the intervertebral foramen in the lateral view in 10 patients. It was confirmed that the needle tips were all located in the extraforaminal region; therefore, we named this puncture method the extraforaminal TNR block. This procedure may prevent the needle tip from entering the spinal canal, leading to spinal cord injury or total spinal anesthesia.
In this study, we found that a 2 mL volume of radiopaque agent was sufficient to delineate TNR in the 10 patients, which meant that a precise TNR block could be performed using our method. We also found that the radiopaque agent could delineate the DRG in the intervertebral foramen independently of the 2 mL radiopaque agent used to delineate the epidural area or the TPV area. At the thoracic level, the DRG is consistently placed under the vertebral pedicle.15 DRG is the key structure of sensory conduction and pain transmission, which is a therapeutic target for neuropathic pain, including herpes zoster neuralgia.16 Patients with herpes zoster mainly exhibit injury to the neurons in DRG. This study showed that the pain of 10 patients with herpes zoster was significantly relieved two and four weeks after a single low-dose mixture of glucocorticoids and local anesthetic injection, which may be evidence of the effect of the mixture on DRG. In the future, it will be meaningful to perform sensory and motor tests with a radiofrequency generator to confirm whether the puncture needle tip reaches or approaches the DRG.
In this study, the 2 mL-volume of radiopaque agent delineated the epidural space in 8 patients. What are the clinical implications? Failed epidural anesthesia or analgesia is more common than is generally recognized.17 In the case of epidural block failure, it is of great significance to find another local block technique as a substitute or remedial measure. Obviously, the transforaminal epidural block is a successful attempt to traditional trans ligamentum flavum epidural block. US-TNR block with the IAP-PP midpoint as the puncture may be used an alternative end point when epidural block cannot be implemented or when epidural block has failed in clinical anesthesia, although this should be confirmed in future studies.
In previous studies, the subsidence of the pleural cavity by drug injection observed under real-time ultrasound was often considered the standard of successful PTV block.18 However, pleural subsidence was not obvious in 4/10 patients in this study. There may be two reasons for this phenomenon. The first possibility is that the injection volume in our study is smaller than that in previous studies. Therefore, even if there was a slight pleural indentation, we could not observe it under real-time ultrasound. Another possible reason may be that the vertical distance between the IAP and the parietal pleura was much farther than that between the transverse process and the parietal pleura,2,19 so the subsidence of the pleural is not obvious when the agent was injected. Anyway, we believe that when using our method for TNR block, it is not necessary to take pleural subsidence as a sign of successful puncture, but only need to puncture the needle tip to the midpoint of IAP and PP.
Is it possible for drugs to penetrate the parietal pleura and infiltrate the chest? Obviously, this is an interesting question. In an observation study of US-TPV nerve block using thoracoscopy, Fujii et al found that the local anesthetics could not only diffuse along the vertebral axis and intercostal space, but also penetrated the parietal pleura and diffused into the thoracic cavity.20 This was an amazing discovery, which suggested that TPV block could be used to treat pleural diseases such as pleurisy in the future. Unfortunately, in our study, we did not find any sign of diffusion of the radiopaque agent into the chest under fluoroscopy. This difference from previous studies may be due to the difference in volumes used and in the dispersion of the radiopaque agent from that of local anesthetics.
When we perform an US-TNR block, we do not deliberately search for SCTL under ultrasonography, let alone experience the “sense of breakthrough”, which was often described as the standard for successful puncture in the classic TPV block. Anatomically, the TPV space is limited by a transverse process and the SCTL on the posterior side, which is the medial continuation of the thickened medial intercostal muscle aponeurosis from the lower side of transverse process to the upper side of inferior costal tubercle. However, a recent anatomical study revealed that the SCTL may not be a tight junction near the spine, and there is loose disunion of connective tissue which separates them,21 which may explain why our puncture process does not require a sense of breakthrough.
We also determined that during the TNR block, the radiopaque agent of 2 cases also infiltrated the paraspinal muscles (Figure 5). As mentioned above, there may be a separation between the SCTL and the intervertebral foramen, so the radiopaque agent could diffuse from the TPV to the superficial paravertebral muscles. Anatomically, the medial boundary of the TPV consisted of the vertebral body, the intervertebral disc, the zygapophyseal joint, and the foramen. The TPV was connected to adjacent contralateral paravertebral spaces and was also connected to the epidural space through the intervertebral foramen. Therefore, we believe that the US-guided IAP-PP midpoint TNR block belongs to the category of TPV block, but this puncture brings the tip of the needle closer to TNR and even the DRG, which may benefit patients with neuropathic pain.
In this study, two patients experienced hypotension 30 minutes after drug injection. In previous reports, hypotension often occurs during TPV block and epidural anesthesia. Part of the reason is that TPV block or epidural anesthesia can block the sympathetic nerve, causing the expansion of peripheral blood vessels, resulting in hypotension. Thus, we questioned whether there was evidence of a sympathetic block in our samples? An experienced radiologist carefully analyzed the extent of radiopaque drug staining and found no evidence of anterior staining of the thoracic vertebra, which indicated that the 2 mL drug volume could not reach the sympathetic ganglion of the thoracic vertebra. We did not perform infrared thermographic imaging to detect the presence of sympathetic block as previous studies have done.22 We look forward to further research in this field in the future.
TPV block has been considered as an alternative to epidural block with comparable analgesic effect and a better side effect profile. Richardson et al reported the use of bilateral TPV block in 541 patients with good results and a low rate of side effects and no serious complications.23 But because the TPV space is highly vascular, there is a potential for vascular injury and consequent bleeding risk. Vascular puncture has been reported to occur in 3.8% to 6.8% of patients, whereas the reported incidence of hematoma is around 2.4%.24,25 The resurgence of thoracic PVB is a part of the search to identify alternative regional anesthesia techniques and therapies to reduce this risk. No intravascular injection occurred in this study. However, whether this technique has fewer complications than traditional TPV block needs further study.
Above all, although current ultrasound technology could not directly reveal the TNR, we confirmed that it was feasible to use the midpoint of IAP-PP as the puncture target for TNR block under ultrasound-guidance. There was no need to identify SCTL or observe pleura subsidence during this procedure. The tip of the puncture needle was located outside the intervertebral foramen, which allow avoiding spinal cord injury as much as possible. Moreover, this study showed that US-TNR block with the midpoint of IAP-PP as the puncture end point could effectively block the TNR and DRG, bringing potential benefits to patients with neuropathic pain (Figure 7).
Conclusions
This study demonstrated the feasibility of US-TNR block using the in-plane technique with the midpoint of thoracic IAP and PP as the puncture end point to effectively block the TNR and DRG. This technique is an accurate clinical application of TPV nerve block and provides a potential therapeutic option for the treatment of neuropathic pain.
Data Sharing Statement
We all agree to share all the data about this article. The data will be available on: http://www.weibo.com/u/1259495587.
Funding
This work was supported through a grant from Shanghai Municipal Health Commission, No. 201840248 (Shaofeng Pu), the General Program of National Natural Science Foundation of China, No. 81672237 (Dongping Du) and 81871360 (Jie Chen).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Eason MJ, Wyatt R. Paravertebral thoracic block-a reappraisal. Anaesthesia. 1979;34(7):638–642. doi:10.1111/j.1365-2044.1979.tb06363.x
2. Krediet AC, Moayeri N, van Geffen GJ, et al. Different approaches to ultrasound-guided thoracic paravertebral block: an illustrated review. Anesthesiology. 2015;123(2):459–474. doi:10.1097/ALN.0000000000000747
3. Termpornlert S, Sakura S, Aoyama Y, et al. Distribution of injectate administered through a catheter inserted by three different approaches to ultrasound-guided thoracic paravertebral block: a prospective observational study. Reg Anesth Pain Med. 2020;45:866–871. doi:10.1136/rapm-2020-101545
4. Hegazy MA, Awad G, Abdellatif A, et al. Ultrasound versus thoracoscopic-guided paravertebral block during thoracotomy. Asian Cardiovasc Thorac Ann. 2021;29:98–104. doi:10.1177/0218492320965015
5. Santonastaso DP, de Chiara A, Russo E, et al. A possible future for anaesthesia in breast surgery: thoracic paravertebral block and awake surgery. A prospective observational study. Tumori J. 2021;107(2):125–131. doi:10.1177/0300891620951626
6. Wardhan R, Kantamneni S. The challenges of ultrasound-guided thoracic paravertebral blocks in rib fracture patients. Cureus. 2020;12:e7626. doi:10.7759/cureus.7626
7. Naganuma M, Tokita T, Sato Y, et al. Efficacy of preoperative bilateral thoracic paravertebral block in cardiac surgery requiring full heparinization: a propensity-matched study. J Cardiothorac Vasc Anesth. 2022;36(2):477–482. doi:10.1053/j.jvca.2021.05.001
8. Makharita MY, Amr YM. Effect of repeated paravertebral injections with local anesthetics and steroids on prevention of post-herpetic neuralgia. Pain Physician. 2020;23(6):565–572.
9. Pace MM, Sharma B, Anderson-Dam J, et al. Ultrasound-guided thoracic paravertebral blockade: a retrospective study of the incidence of complications. Anesth Analg. 2016;122:1186–1191. doi:10.1213/ANE.0000000000001117
10. Saadawi M, Layera S, Aliste J, et al. Erector spinae plane block: a narrative review with systematic analysis of the evidence pertaining to clinical indications and alternative truncal blocks. J Clin Anesth. 2021;68:110063. doi:10.1016/j.jclinane.2020.110063
11. Costache I, de Neumann L, Ramnanan CJ, et al. The mid-point transverse process to pleura (MTP) block: a new end-point for thoracic paravertebral block. Anaesthesia. 2017;72(10):1230–1236. doi:10.1111/anae.14004
12. Ince I, Dostbil A, Ozmen O, et al. Subtransverse process interligamentary (STIL) plane block for postoperative pain management after breast surgery. J Clin Anesth. 2020;61:109649. doi:10.1016/j.jclinane.2019.109649
13. Cowie B, McGlade D, Ivanusic J, et al. Ultrasound-guided thoracic paravertebral blockade: a cadaveric study. Anesth Analg. 2010;110(6):1735–1739. doi:10.1213/ANE.0b013e3181dd58b0
14. Gargya A, Singh H, Lin T, et al. Extraforaminal thoracic and lumbar spinal nerve ultrasound-guided percutaneous peripheral nerve stimulation. Pain Med. 2020;21(Supplement_1):S38–S40. doi:10.1093/pm/pnaa166
15. Leng L, Liu L, Si D. Morphological anatomy of thoracolumbar nerve roots and dorsal root ganglia. Eur J Orthop Surg Traumatol. 2018;28(2):171–176. doi:10.1007/s00590-017-2026-5
16. Kim JH, Apigo A, Fontaine C. Dorsal root ganglion stimulation for refractory post-herpetic neuralgia. Pain Pract. 2021;21(7):794–798. doi:10.1111/papr.13017
17. Hermanides J, Hollmann MW, Stevens MF, et al. Failed epidural: causes and management. Br J Anaesth. 2012;109(2):144–154. doi:10.1093/bja/aes214
18. Taketa Y, Fujitani T, Irisawa Y, et al. Ultrasound-guided thoracic paravertebral block by the paralaminar in-plane approach using a microconvex array transducer: methodological utility based on anatomical structures. J Anesth. 2017;31(2):271–277. doi:10.1007/s00540-016-2289-8
19. Taketa Y, Fujitani T. A novel paralaminar in-plane approach for ultrasound-guided continuous thoracic paravertebral block using microconvex array transducer. Reg Anesth Pain Med. 2015;40(4):390. doi:10.1097/AAP.0000000000000259
20. Fujii T, Shibata Y, Nishiwaki K. Observation of ultrasound-guided thoracic paravertebral block using thoracoscopy. Acta Anaesthesiol Taiwan. 2016;54(3):101–102. doi:10.1016/j.aat.2016.05.004
21. Cho TH, Kim SH, Jehoon O, et al. Anatomy of the thoracic paravertebral space: 3D micro-CT findings and their clinical implications for nerve blockade. Reg Anesth Pain Med. 2021;46(8):699–703. doi:10.1136/rapm-2021-102588
22. Kim YC, Bahk JH, Lee SC, et al. Infrared thermographic imaging in the assessment of successful block on lumbar sympathetic ganglion. Yonsei Med J. 2003;44:119–124. doi:10.3349/ymj.2003.44.1.119
23. Richardson J, Lonnqvist PA, Naja Z. Bilateral thoracic paravertebral block: potential and practice. Br J Anaesth. 2011;106:164–171. doi:10.1093/bja/aeq378
24. Lonnqvist PA, MacKenzie J, Soni AK, et al. Paravertebral blockade. Failure rate and complications. Anaesthesia. 1995;50:813–815. doi:10.1111/j.1365-2044.1995.tb06148.x
25. Naja Z, Lonnqvist PA. Somatic paravertebral nerve blockade. Incidence of failed block and complications. Anaesthesia. 2001;56:1184–1188. doi:10.1046/j.1365-2044.2001.02084-2.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.