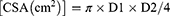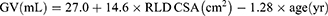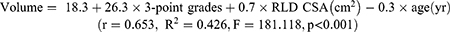Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Ultrasonographic Assessment of Gastric Volume in Fasted Patients Undergoing Gastrointestinal Endoscopy Under Sedation
Authors Rong H, Dai W , Qin Y, Meng Z, Zou X, Wang B, Wei Q, Xie Y
Received 7 June 2023
Accepted for publication 13 August 2023
Published 23 August 2023 Volume 2023:19 Pages 685—698
DOI https://doi.org/10.2147/TCRM.S424890
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Heng Rong,1,* Weixin Dai,1,* Yinying Qin,1,* Zhikeng Meng,2 Xia Zou,3 Binbin Wang,4 Qiufeng Wei,1 Yubo Xie1,5
1Department of Anesthesiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China; 2Department of Anesthesiology, The First People’s Hospital of Yulin, Yulin, People’s Republic of China; 3Department of Anesthesiology, The People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, People’s Republic of China; 4Department of Anesthesiology, The Second Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China; 5Guangxi Key Laboratory of Enhanced Recovery After Surgery for Gastrointestinal Cancer, The First Affiliated Hospital of Guangxi Medical University, Nanning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yubo Xie, Department of Anesthesiology, The First Affiliated Hospital of Guangxi Medical University, No. 6 Shuang-Yong Road, Nanning, Guangxi, 530021, People’s Republic of China, Email [email protected]
Purpose: In this prospective observational study, an ultrasonographic measurement of antral cross-sectional area (ACSA) was conducted to evaluate the gastric content and volume as well as to identify high-risk stomach in non-pregnant adult surgical patients adhering to preanesthetic fasting guidelines.
Patients and Methods: Fasted patients undergoing gastrointestinal endoscopy under sedation were included. Ultrasonographic measurements of ACSA were conducted in both semi-recumbent and right lateral decubitus positions before endoscopic procedures. Gastroscopy was employed to guide the measurement of suctioned gastric volume (GV). Ultrasonography was performed to assess gastric contents and identify patients with high-risk stomach. The relationship between ACSA and suctioned GV was also evaluated.
Results: ACSA was evaluated in 736 out of 782 patients. A significant positive correlation was discovered between ACSA in the right lateral decubitus position and suctioned GV, which was more reliable than in the semi-recumbent position. To analyze high-risk stomach with a GV > 100 mL, the cutoff value of ACSA in the right lateral decubitus was found to be 7.5 cm2, with the AUC, sensitivity and specificity of 0.80 (95% CI, 0.76– 0.82; P< 0.001), 82.4% and 67.3%, respectively. A novel mathematical model based on ACSA to estimate GV in non-pregnant fasted adults was presented.
Conclusion: Ultrasonographic measurement of ACSA can assist anesthesiologists in estimating the risk of pulmonary aspiration of gastric contents during general anesthesia and sedation.
Keywords: gastroscopy, antrum, gastric content, pulmonary aspiration, ultrasonography
Introduction
Pulmonary aspiration of gastric contents is a severe perioperative complication of anesthesia. It involves the inhalation of stomach contents into the lungs and can result in significant morbidity and mortality. In fact, mortality rates associated with this complication can be as high as 5%, and it is estimated to contribute to up to 9% of anesthesia-related fatalities.1–3 Both general anesthesia and sedation can have a depressant effect on the tone of the lower esophageal sphincter and the protective reflexes of the upper airway. When its tone is decreased, there is an increased risk of gastric contents refluxing into the esophagus. While adhering to strict fasting guidelines recommended by the American Society of Anesthesiologists (ASA) can significantly attenuate the risk of pulmonary aspiration, it does not provide absolute immunity.1,2,4 Previous studies found that 0.1% of patients who received Monitored Anesthesia Care (MAC) during endoscopy necessitated antibiotic treatment for aspiration.5,6 Gastroscopy stimulation, inappropriate anesthesia management during gastroscopy, and anatomical anomalies in the digestive tract can increase the risk of pulmonary aspiration. In certain cases where the risk of pulmonary aspiration is deemed high or uncertain, doctors and patients may opt for gastrointestinal endoscopy without sedation. This decision may be made to minimize the risk of sedation-related complications and reduce the chances of aspiration. However, undergoing endoscopy without sedation can be an uncomfortable experience for patients, and numerous interventions and examinations rely on sedation.
The antrum is the most appropriate site for gastric ultrasonography, and different features of the antrum can be used to judge the nature of gastric contents.7–9 Ultrasonography can provide qualitative assessments of solid gastric contents. It is a non-invasive imaging technique that can help determine the presence or absence of solid material in the stomach. However, for liquid gastroesophageal contents, assessing gastric volume (GV) becomes critical in preventing perioperative aspiration. The volume of gastric contents is an important factor in determining the risk of aspiration during anesthesia and surgical procedure. A GV > 1.5 mL/kg (equivalent to 100 mL for the average adult) is considered to be associated with an increased risk of aspiration.4,10–13 When the GV exceeds this threshold, the risk of regurgitation and subsequent aspiration of gastric contents during anesthesia becomes higher. The use of a composite ultrasound scale for the qualitative and quantitative assessment of GV has been proposed as a method to evaluate gastric contents.14–16 Additionally, a mathematical model-driven clinical algorithm has been suggested to aid in the assessment of GV.17,18 However, for quantitative assessment, the relationship between fasted patients’ gastric contents, the ultrasonographic assessment of antral cross-sectional area (ACSA), and the self-related factors warrants further investigation.
This study focuses on utilizing gastric antrum ultrasonography to evaluate gastric contents in patients who have followed fasting guidelines and are scheduled for gastrointestinal endoscopy with sedation. The ACSA of the gastric system and the volume of gastric contents were recorded and analyzed. In addition, we aimed to examine the clinical application of gastric antrum ultrasonography in evaluating gastric contents, while determining quantitative patterns for ultrasound analysis of the antrum in fasted patients. The objectives of our prospective observational study were as follows: (1) to evaluate the correlation between the ultrasonographic assessment of ACSA and actual measurements from gastroscopic suctioned volume of gastric contents in fasted patients; and (2) to identify gastric high-risk stomach during the preoperative period, as defined by the volume of gastric contents at risk of clinical consequences for pulmonary aspiration. The primary outcome of our study was to identify a cutoff value of ACSA that could be used to diagnose high-risk stomach. Finally, a mathematical model was developed to estimate GV.
Materials and Methods
Ethics and Consent of Participants
This observational study was performed in accordance with the Declaration of Helsinki, and was approved by the Research Ethics Committee of the First Affiliated Hospital of Guangxi Medical University in February 2020 (No. 2020KY-E-007). It was also registered on the International Clinical Trial Registry Platform (No. ChiCTR2000033984) in June 2020, and was conducted between June 2020 and December 2021 at the Department of Gastroenterology Outpatient Endoscopy Center of the same hospital. Written informed consent was obtained from all the participants. Before accessing the data, a statistical analysis plan was documented, timestamped with permanent electronic signatures, and filed for investigators’ records.
Participants
Fasted patients undergoing gastrointestinal endoscopy under sedation were enrolled into this study for ultrasonographic assessment of gastric contents. The inclusion criteria were as follows: patients aged 16–85 years old, with ASA physical status I–III, undergoing gastrointestinal endoscopy, and able to comprehend the rationale of the study. The exclusion criteria were as follows: recent upper gastrointestinal bleeding (within the preceding 1 month), gastrointestinal dysfunction (including prior gastric or lower esophageal surgeries, diabetes, severe liver and kidney dysfunction), documented upper gastrointestinal tract abnormalities, gastric tumors, hiatal hernias, pregnancy, poor-quality gastric antrum ultrasound images and the presence of solid food in the gastric system. All subjects provided written informed consent. Patients were prepared according to standard institutional guidelines.
Protocol
In line with standard institutional guidelines, all patients fasted for more than 8 h without solid food and 4 h without fluids before undergoing gastroscopy. The patient’s medical history, characteristics and general conditions were documented. The ultrasound examinations were carried out by an anesthetist with a minimum of 100 prior gastric ultrasound exams performed. The anesthetist performed the ultrasound using a FUJIFILM SonoSite M-Turbo™ machine (Bothell, USA) equipped with a low-frequency convex probe (C60 x/2-5 MHz).
Ultrasound Examinations of the Gastric Antrum
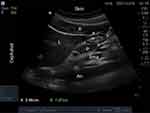
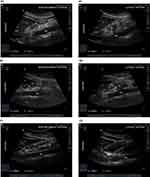
All examinations were conducted with the patient lying down in semi-recumbent positions (in a semi-reclined posture with the head of the bed elevated to an angle of 45 degrees) and right lateral decubitus (RLD) positions (with no elevation of the head-of-bed). Ultrasound scanning was conducted in accordance with a standardized procedure in the two positions.10,19 A standardized scanning level was achieved by identifying a sagittal cross-section of the antrum using the abdominal aorta and the left lobe of the liver as internal landmarks.20 The antrum was identified with a sagittal plane between the liver cephalad and anteriorly, the pancreas and aorta posteriorly (Figure 1). This ensured consistency and accuracy in locating the sagittal plane for antral assessment. All images were acquired during the intervals between peristaltic contractions when the antrum was in a state of rest, ensuring accurate assessment of the antral area and preventing underestimation. Care was taken to avoid excessive probe rotation, which could lead to oblique views and potentially result in an overestimation of the antrum size. During each position, the gastric contents were qualitatively analyzed and categorized as solid/thick liquid, clear liquid, or empty. The gastric antrum that could not be measured or located was then excluded. Ultrasound images were saved for study review and verification.
Interpretation of Gastric Ultrasound
A semi-quantitative assessment was performed based on the qualitative 3-point grade assessments as follows: Grade 0: The antrum appears devoid of any content in both RLD and semi-recumbent positions, indicating an empty stomach. Grade 1: Gastric fluid is visible only in the RLD position, indicating a small volume of fluid. Grade 2: Gastric fluid is detected in the antrum in both RLD and semi-recumbent positions, indicating a large volume of fluid. This grade is associated with patients who experienced regurgitation upon emergence from anesthesia.14 The ACSA was quantitatively analyzed by measuring the anteroposterior (D1) and longitudinal (D2) diameters of a single cross-section of the gastric antrum at the standardized scanning level. The measurement of gastric wall thickness encompassed the full thickness of the gastric wall, and the diameters were determined from the outer serosa layer to the inner serosa layer (Figure 2).
The diagnosis of high-risk stomach was defined by the presence of gastric contents with a volume that posed clinical consequences for pulmonary aspiration, gastric fluid volume exceeding (GV/body weight GV/W) 1.5 mL/kg or exceeding 100 mL per person.21 The anteroposterior (D1) and longitudinal (D2) diameters of the antral section were determined, and the cross-sectional area (CSA) was calculated as follows:
In cases where clear liquid was present in the RLD position, GV was subsequently determined using Perlas’ mathematical model.17
All data were collected in a prospective manner and summarized using percentages and ratios for discrete variables, while continuous variables were reported as mean (standard deviation, SD).
Anesthetic and Gastroscopic Management
Anesthetic and gastroscopy management followed pre-existing plans based on established clinical practices. In cases where the antrum contained solid material that was visible by ultrasound, it was considered as a full stomach and appropriately excluded and managed. Once the ultrasound scan was completed, patients with an estimated gastric fluid volume of <100 mL underwent gastrointestinal endoscopy under sedation according to a standard institutional practice. The anesthetic management involved the administration of IV propofol in increments of 2 mg/kg (maximum = 3 mg/kg) and IV fentanyl in increments of 50 µg (maximum = 100 µg) to achieve deep sedation. This approach, known as monitored anesthesia care (MAC), is commonly employed in endoscopic procedures. The administration of MAC was performed by a certified registered nurse anesthetist or an anesthesiologist.22 The patients maintained their own airway and cardiovascular status.23 For patients with a predicted gastric fluid volume exceeding 100 mL, complete suctioning of the esophagus and stomach through gastroscopy was performed before examining the gastrointestinal tract with MAC.
Measurement of GVs by Endoscopy
Gastroscopy was conducted by a gastroenterologist utilizing an Olympus gastroscope. The actual GV was measured under a gastroscopic guidance, with suctioning done through a side port. All gastric fluids were thoroughly suctioned, and their volume was measured and recorded to the nearest milliliter, which was referred to as the “suctioned volume”. The liquid volume and nature of gastric contents and the adverse reactions to anesthesia, including regurgitation, bucking and pulmonary aspiration of gastric contents during the endoscopic procedures, were documented.
Statistical Analysis
Statistical tests were conducted with SPSS 25.0 and MedCalc version 20.1 for Windows. The normality of the data distribution was evaluated using the Shapiro–Wilk’s W-test. Normally distributed measurement data were expressed as means (SD) and analyzed using one-way ANOVA, while non-normally distributed data were presented as median (interquartile range, IQR), and analyzed using the nonparametric rank sum test (Kruskal–Wallis rank sum test). The incidence data were compared with Fisher’s exact test or Pearson’s chi-squared test, depending on the specific circumstances and requirements of the analysis.
Receiver operating characteristic (ROC) curves were plotted, and areas under the curves (AUCs) were calculated to estimate the discriminating power of ultrasonographic measurement of ACSA for diagnosing the volume of aspirated gastric contents > 1.5 mL/kg and 100 mL. Sensitivity and specificity were determined according to the cutoff value of ACSA to identify a high-risk stomach. Correlation analysis of variables affecting gastric fluid volume was performed, and a linear regression model was established. Bland–Altman analysis was utilized to evaluate the agreement between the calculated GV by ultrasound assessment and the suctioned GV, and to assess the performance of our model in estimating GV.24 For each test, p<0.05 was deemed significant.
Results
Patient Characteristics
A cohort of 782 patients was included in this study. Eight patients were withdrawn due to the inability to obtain standardized gastric images. Twenty patients were withdrawn due to failure to record the nature or liquid volume of gastric contents (five patients had solid contents in the stomach). Eighteen patients with comorbid gastrointestinal dysfunction (2.0% diabetes and 0.3% renal impairment) were excluded (Figure 3). Out of the initial cohort, 736 patients successfully completed all study assessments in accordance with the protocol and were subsequently included in the final analysis. The patient’s demographic data, gastric ultrasound examination, and GV data are listed in Table 1.
 |
Table 1 Patient Demographics, Gastric Ultrasound Examination, and Gastric Fluid Volume Data |
 |
Figure 3 Flow chart of the study. RLD represents right lateral decubitus. |
Comparison of the ACSA, Predicted GV, and Suctioned GV in Different Decubitus Positions
The ACSA, calculated GV, suctioned GV, and suctioned GV per unit body weight of patients in different qualitative 3-point grade assessments were remarkably different (p<0.05). In addition, a positive correlation was observed among them. The patients in Grade 2 group had a significantly larger calculated and suctioned GV compared to those in other two groups (p<0.001; Table 1).
ROC Curve Analysis
The ROC curves obtained for ultrasonographic diagnosis of a high-risk stomach and the optimal cutoff value of ACSA for GV > 1.5 mL/kg and 100 mL at both RLD and semi-recumbent positions are presented in Figure 4. For patients with the RLD position, a cutoff value of 6.4 cm2 predicted a GV of > 1.5 mL/kg, while a value of 7.5 cm2 predicted a GV of > 100 mL.
Reconstructed Model of the Predicted GV After Correction
Based on the Perlas’ mathematical model, the current calculated gastric fluid volume provides an accurate estimate compared with the suctioned GV under a gastroscopic guidance. However, for small calculated volumes (Grade 0), the calculated volume is four times higher than the observed value; for medium volumes (Grade 1), it is calculated twice as much, while the highest agreement is found for large volumes (Grade 2), with a difference of approximately 30% between the suctioned and calculated volume. The overall calculated volume is twice that of the suctioned volume in the entire population (Table 1). A scatter plot was constructed, where the difference between the two measurements for each patient was plotted against their corresponding mean values. The Bland–Altman analysis was then conducted (Figure 5). In this analysis, the differences between the calculated GV based on the current model and the observed suctioned GV during gastroscopy were plotted. The mean difference between these values for each subject was calculated to provide a clinical context for evaluating the differences. The 95% limits of agreement, determined through this method, define the range within which the differences between the two measurements are expected to lie with a 95% probability. The results of this analysis demonstrated that the current mathematical model exhibited a mean “bias” or systematic error of 33.2 mL (95% CI, 29.7–36.7 mL), indicating a tendency to overestimate GV. Additionally, there was a strong positive correlation between the calculated and observed volumes, with a correlation coefficient of r = 0.83 (95% CI, 0.74–0.92), and the upper limit of 95% agreement band was 127.9 mL. The observed difference, particularly in low volume states, has clinical significance and indicates that the current model may lack the necessary accuracy for practical clinical use. Consequently, a novel model was developed based on the updated dataset to improve accuracy and reliability.
Bivariate correlation analysis was conducted to measure each group’s linear relationship between the independent and dependent variables. Nine independent variables, such as sex, age, height, weight, BMI, ASA, the ACSA in both RLD and semi-recumbent positions, and 3-point grades were re-screened using forward regression to obtain a corrected mathematical model (Table 2). The correlation between the ACSA and GV was significantly higher in the RLD position than in the semi-recumbent position. We selected the best-fit model with ACSA in the RLD position, 3-point grades, and age as predictors, which was shown as follows:
 |
Table 2 Correlation Analysis Between Gastric Volume and Independent Variables |
Subsequently, we analyzed the consistency between the new calculated and suctioned GV via Bland-Altman analysis (Figure 6), which indicated a high level of agreement between the newly calculated and suctioned volumes, with a mean bias of 1.3 mL (95% CI, −0.2 to 2.8 mL). The correlation coefficient r = −0.52 (95% CI, −0.58 to −0.45) and a 95% agreement band ranging from −39.8 mL to 42.5 mL were also observed. The findings demonstrated that the model’s consistency was improved significantly after correction.
Discussion
Ultrasound plays vital roles in assessing the nature and volume of gastric contents, predicting the risk of perioperative aspiration, and guiding anesthesia management. The qualitative examination of the gastric antrum in the RLD and semi-recumbent positions can assess GV with high sensitivity.14,19 The suctioned GV exhibited a better correlation with ACSA in the RLD than in semi-recumbent positions in our work. The reason for this improved correlation between ACSA measurement and fluid volume is likely due to the movement of fluids toward the antrum when the subject assumes the RLD position. This positioning facilitates the pooling of fluids in the lower part of the stomach, resulting in a more accurate assessment of ACSA and its relationship to the actual volume of fluid present.25 The cutoff value of ACSA in the RLD position holds clinical significance in diagnosing a stomach at risk. Our study further emphasized that a GV threshold of up to 100 mL is more sensitive in identifying the risk compared to a GV threshold of up to 1.5 mL/kg.
Our mathematical model, derived from an observational study of 782 fasted non-pregnant outpatients undergoing gastrointestinal endoscopy with MAC, utilizes ACSA in the semi-recumbent and RLD position, as well as age, to predict gastric fluid volume. This model can be of valuable assistance to anesthesiologists in evaluating the risk of pulmonary aspiration and making well-informed decisions regarding the anesthetic strategy. Several researchers have reported the development of prediction models for ultrasound-based assessment of gastric fluid volume, primarily focusing on elective or emergency surgery within operating rooms. However, there have been limited reports in the outpatient setting, and these models have predominantly been developed using data from Western countries.16,17,19 Hence, it remains crucial to investigate and ascertain the suitability and relevance of these existing models to Eastern populations. The current models involve various techniques such as using predetermined known volumes of fluid after fasting, performing suctioning under gastroscopic vision, or inserting an 18-French multi-orifice Salem tube to directly assess gastric contents and volume.17,26 The previous studies that relied on data from fasted healthy individuals who ingested known volumes of fluid may not fully reflect the clinical reality of patients with low gastric contents. These studies do not consider the pre-existing gastric secretions prior to ingestion, which can lead to inaccurate interpretations of gastric contents and potentially compromise the reliability of aspirated gastric contents through a gastric tube. To minimize experimental error, we assessed fasted patients undergoing gastrointestinal endoscopy with sedation, after which gastric fluid was suctioned under gastroscopic vision and measured.
Nevertheless, this observational study has some limitations worth considering. The lack of inclusion of aspiration subjects hinders the ability to discuss the reflux aspiration threshold, which could have provided further insights into the relationship between gastric fluid volume and aspiration risk. Moreover, there may be unmeasured factors and confounders that could have influenced the results of our study. These factors could include underlying undiagnosed diseases or anxiety related to preoperative preparation, which could potentially impact gastric emptying, gastric juice secretion, and the ACSA. In a study of gastritis, gastric ulcer, and Helicobacter pylori infection, the ACSA was larger in these patients than in healthy individuals, indicating the possibility of overestimating GV using the aforementioned GV prediction model in such populations.27,28 Current studies have demonstrated that conditions such as pregnancy, diabetes, and severe liver and kidney dysfunction can impact gastric emptying, but the relationship between the gastric ACSA and GV remains unclear.29–34 To minimize bias, we excluded patients with diseases associated with gastric emptying and dysfunction.
Furthermore, a negative correlation was observed between age and GV, which may be attributed to the reduction in gastric juice secretion in elderly patients. However, elderly patients frequently suffer from chronic diseases, gastrointestinal dysmotility, deglutition difficulties, weak cough reflex, and other weakened protective functions. Therefore, old age should not be considered as a protective factor against pulmonary aspiration.35,36
With the assistance of gastric ultrasound-based risk assessment, anesthetic management can be tailored appropriately for each individual patient. Patients whose ultrasound reveals solid or thick material in the antrum, regardless of the quantity, are likely at a high risk of pulmonary aspiration. In such cases, no further assessment is needed, and anesthetists may choose to postpone or cancel the gastrointestinal endoscopy under sedation.
Quantification of gastric content has become essential when clear fluid is present, as ultrasound can detect a significant amount of clear fluid in about half of all fasting patients.14 The widely accepted upper limit of normal for gastric clear fluid content is 1.5 mL/kg or approximately 100 mL in the average adult.7,14,17,18 For patients with an ACSA >7.5 cm2 in the RLD position or a predicted gastric fluid volume exceeding 100 mL, it is advisable to perform complete suctioning of the esophagus and stomach via gastroscopy before undergoing gastrointestinal endoscopy examination with sedation or anesthesia.
When antral fluid is present, a volume <1.5 mL/kg may indicate baseline gastric secretions and is likely associated with a low risk of pulmonary aspiration. Safety measures can be enhanced by elevating the head of the bed by 45 degrees, maintaining the appropriate depth of anesthesia or sedation, using topical anesthesia in the throat to reduce the cough reaction, and performing complete suctioning of gastric juice before proceeding with further gastrointestinal endoscopy examination.
Considering that reflux aspiration is a complex process influenced by various physiological and pathological factors, such as the secretory function of the stomach and oropharynx, aspiration cannot be entirely avoided, even if the stomach is completely empty. The aspiration of gastric contents during general anesthesia encompasses various pathophysiological features, which involve a range of risk factors. These factors can include challenges in airway management or the use of inadequate anesthetic techniques. During general anesthesia, challenges in airway management or inadequate anesthetic techniques can result in unintended consequences. For example, coughing, bucking, and blowing air into the stomach can occur, leading to increased intra-abdominal pressure. This elevated pressure can trigger episodes of gastroesophageal reflux, where gastric contents flow back up into the esophagus and potentially reach the upper airway.37–39 Therefore, a comprehensive assessment of the patient’s physiological and pathological status is crucial in determining the appropriate anesthesia management and surgical approach based on the predicted GV, which can further enhance the clinical decision-making process.
Conclusion
In conclusion, our study establishes that an ACSA cutoff value of 7.5 cm2 predicts a gastric volume exceeding 100 mL in the RLD position. We introduce a novel prediction model: Volume = 18.3 + 26.3 × 3-point grades + 0.7 × RLD CSA (cm2) - 0.3 × age (yr) to estimate gastric volume based on sonographic measurements of RLD position ACSA. This model effectively quantifies the presence of clear fluid in the antral gastric region and can be applied to aid in evaluating the risk of pulmonary aspiration for non-pregnant adult patients.
Data Sharing Statement
The data that support the findings of this study will be available from the corresponding author, Yubo Xie, upon reasonable request. All of the individual participant data collected during the trial will be available after deidentification. The data will be made available immediately following article publication, with no end date.
Acknowledgments
We would like to thank all the patients and their families who participated in this study.
Funding
This research was funded by a grant from the Health Commission Self-Funded Scientific Research Projects of Guangxi (No. Z20200145); Key Research and Development Program of Guangxi (No. AB20159019); Guangxi Clinical Research Center for Anesthesiology (No. GK AD22035214), and the Key Project of Natural Science Foundation of Guangxi (No. 2020GXNSFDA238025).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during the perioperative period. Anesthesiology. 1993;78:56–62. doi:10.1097/00000542-199301000-00010
2. Lienhart A, Auroy Y, Péquignot F, et al. Survey of anesthesia-related mortality in France. Anesthesiology. 2006;105:1087–1097. doi:10.1097/00000542-200612000-00008
3. Landreau B, Odin I, Nathan N. Inhalation gastrique : e´pide´miologie et facteurs de risque [Pulmonary aspiration: epidemiology and risk factors]. Ann Fr Anesth Reanim. 2009;28:206–210. French. doi:10.1016/j.annfar.2009.01.020
4. Van de Putte P, Vernieuwe L, Jerjir A, Verschueren L, Tacken M, Perlas A. When fasted is not empty: a retrospective cohort study of gastric content in fasted surgical patients†. Br J Anaesth. 2017;118:363–371. doi:10.1093/bja/aew435
5. El Chafic AH, Eckert G, Rex DK. Prospective description of coughing, hemodynamic changes, and oxygen desaturation during endoscopic sedation. Dig Dis Sci. 2012;57:1899–1907. doi:10.1007/s10620-012-2057-z
6. Agostoni M, Fanti L, Gemma M, Pasculli N, Beretta L, Testoni PA. Adverse events during monitored anesthesia care for GI endoscopy: an 8-year experience. Gastrointest Endosc. 2011;74:266–275. doi:10.1016/j.gie.2011.04.028
7. Van de Putte P, Perlas A. Ultrasound assessment of gastric content and volume. Br J Anaesth. 2014;113:12–22. doi:10.1093/bja/aeu151
8. Koenig SJ, Lakticova V, Mayo PH. Utility of ultrasonography for detection of gastric fluid during urgent endotracheal intubation. Intensive Care Med. 2011;37:627–631. doi:10.1007/s00134-010-2125-9
9. Jacoby J, Smith G, Eberhardt M, Heller M. Bedside ultrasound to determine prandial status. Am J Emerg Med. 2003;21:216–219. doi:10.1016/S0735-6757(02)42243-7
10. Perlas A, Arzola C, Van de Putte P. Point-of-care gastric ultrasound and aspiration risk assessment: a narrative review. Can J Anaesth. 2018;65:437–448. doi:10.1007/s12630-017-1031-9
11. Agarwal A, Chari P, Singh H. Fluid deprivation before operation. The effect of a small drink. Anaesthesia. 1989;44:632–634. doi:10.1111/j.1365-2044.1989.tb13581.x
12. Read MS, Vaughan RS. Allowing pre-operative patients to drink: effects on patients’ safety and comfort of unlimited oral water until 2 hours before anaesthesia. Acta Anaesthesiol Scand. 1991;35:591–595. doi:10.1111/j.1399-6576.1991.tb03354.x
13. Phillips S, Hutchinson S, Davidson T. Preoperative drinking does not affect gastric contents. Br J Anaesth. 1993;70:6–9. doi:10.1093/bja/70.1.6
14. Perlas A, Davis L, Khan M, Mitsakakis N, Chan VW. Gastric sonography in the fasted surgical patient: a prospective descriptive study. Anesth Analg. 2011;113:93–97. doi:10.1213/ANE.0b013e31821b98c0
15. Bouvet L, Desgranges FP, Aubergy C, et al. Prevalence and factors predictive of full stomach in elective and emergency surgical patients: a prospective cohort study. Br J Anaesth. 2017;118:372–379. doi:10.1093/bja/aew462
16. Bouvet L, Chassard D. Ultrasound assessment of gastric contents in emergency patients examined in the full supine position: an appropriate composite ultrasound grading scale can finally be proposed. J Clin Monit Comput. 2020;34:865–868. doi:10.1007/s10877-019-00452-3
17. Perlas A, Mitsakakis N, Liu L, et al. Validation of a mathematical model for ultrasound assessment of gastric volume by gastroscopic examination. Anesth Analg. 2013;116:357–363. doi:10.1213/ANE.0b013e318274fc19
18. Perlas A, Van de Putte P, Van Houwe P, Chan VW. I-AIM framework for point-of-care gastric ultrasound. Br J Anaesth. 2016;116:7–11. doi:10.1093/bja/aev113
19. Bouvet L, Cordoval J, Barnoud S, Berlier J, Desgranges FP, Chassard D. Diagnostic performance of qualitative ultrasound assessment for the interpretation of point-of-care gastric ultrasound to detect high gastric fluid volume: a prospective randomized crossover study. J Clin Anesth. 2022;81:110919. doi:10.1016/j.jclinane.2022.110919
20. Bouvet L, Barnoud S, Desgranges FP, Chassard D. Effect of body position on qualitative and quantitative ultrasound assessment of gastric fluid contents. Anaesthesia. 2019;74:862–867. doi:10.1111/anae.14664
21. Van de Putte P, Perlas A. The link between gastric volume and aspiration risk. In search of the Holy Grail? Anaesthesia. 2018;73:274–279. doi:10.1111/anae.14164
22. Ng M, Dhanani R, Galadima H, Burgess J. Moderate sedation or monitored anesthesia care for colonoscopies: is there a difference? Am Surg. 2018;84:1284–1287. doi:10.1177/000313481808400837
23. Faigel DO, Baron TH, Goldstein JL, et al. Guidelines for the use of deep sedation and anesthesia for GI endoscopy. Gastrointest Endosc. 2002;56:613–617. doi:10.1016/S0016-5107(02)70104-1
24. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. doi:10.1016/S0140-6736(86)90837-8
25. Perlas A, Chan VW, Lupu CM, Mitsakakis N, Hanbidge A. Ultrasound assessment of gastric content and volume. Anesthesiology. 2009;111:82–89. doi:10.1097/ALN.0b013e3181a97250
26. Bouvet L, Mazoit JX, Chassard D, Allaouchiche B, Boselli E, Benhamou D. Clinical assessment of the ultrasonographic measurement of antral area for estimating preoperative gastric content and volume. Anesthesiology. 2011;114:1086–1092. doi:10.1097/ALN.0b013e31820dee48
27. Tan Y, Wang X, Yang H, et al. Ultrasonographic assessment of preoperative gastric volume in patients with dyspepsia: a prospective observational study. BMC Anesthesiol. 2022;22:21. doi:10.1186/s12871-021-01559-4
28. Steinsvik EK, Valeur J, Hausken T, Gilja OH. Postprandial symptoms in patients with functional dyspepsia and irritable bowel syndrome: relations to ultrasound measurements and psychological factors. J Neurogastroenterol Motil. 2020;26:96–105. doi:10.5056/jnm19072
29. Arzola C, Perlas A, Siddiqui NT, Downey K, Ye XY, Carvalho JCA. Gastric ultrasound in the third trimester of pregnancy: a randomised controlled trial to develop a predictive model of volume assessment. Anaesthesia. 2018;73:295–303. doi:10.1111/anae.14131
30. Xiao MZX, Englesakis M, Perlas A. Gastric content and perioperative pulmonary aspiration in patients with diabetes mellitus: a scoping review. Br J Anaesth. 2021;127:224–235. doi:10.1016/j.bja.2021.04.008
31. Chen C, Liu L, Wang CY, Choi SW, Yuen VM. A pilot study of ultrasound evaluation of gastric emptying in patients with end-stage renal failure: a comparison with healthy controls. Anaesthesia. 2017;72:714–718. doi:10.1111/anae.13869
32. Steinsvik EK, Sangnes DA, Søfteland E, et al. Gastric function in diabetic gastroparesis assessed by ultrasound and scintigraphy. Neurogastroenterol Motil. 2022;34:e14235. doi:10.1111/nmo.14235
33. Ishizu H, Shiomi S, Kawamura E, et al. Gastric emptying in patients with chronic liver diseases. Ann Nucl Med. 2002;16:177–182. doi:10.1007/BF02996298
34. Wang Y, Tu Y, Liu Z, et al. Effects of preoperative oral carbohydrate on cirrhotic patients under endoscopic therapy with anesthesia: a randomized controlled trial. Biomed Res Int. 2021;2021:1405271. doi:10.1155/2021/1405271
35. Orr WC, Chen CL. Aging and neural control of the GI tract: IV. Clinical and physiological aspects of gastrointestinal motility and aging. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1226–31. doi:10.1152/ajpgi.00276.2002
36. Gidwaney NG, Bajpai M, Chokhavatia SS. Gastrointestinal dysmotility in the elderly. J Clin Gastroenterol. 2016;50:819–827. doi:10.1097/MCG.0000000000000650
37. Engelhardt T, Webster NR. Pulmonary aspiration of gastric contents in anaesthesia. Br J Anaesth. 1999;83:453–460. doi:10.1093/bja/83.3.453
38. Kluger MT, Short TG. Aspiration during anaesthesia: a review of 133 cases from the Australian Anaesthetic Incident Monitoring Study (AIMS). Anaesthesia. 1999;54:19–26. doi:10.1046/j.1365-2044.1999.00642.x
39. Sakai T, Planinsic RM, Quinlan JJ, Handley LJ, Kim TY, Hilmi IA. The incidence and outcome of perioperative pulmonary aspiration in a university hospital: a 4-year retrospective analysis. Anesth Analg. 2006;103:941–947. doi:10.1213/01.ane.0000237296.57941.e7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.