Back to Journals » International Journal of Women's Health » Volume 12
Ultraconservative, Fertility Sparing Treatment of Bilateral Borderline Ovarian Tumors: A Case Report of a 26-Year-Old, 0-Gravida with an Endometrioid Borderline Ovarian Tumor of the Right Ovary and a Sero-Mucinous Borderline Ovarian Tumor of the Left Ovary and a Review of the Literature
Received 16 April 2020
Accepted for publication 21 July 2020
Published 6 August 2020 Volume 2020:12 Pages 601—611
DOI https://doi.org/10.2147/IJWH.S258478
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Everett Magann
Stephanie Verta, Barbara Kipp
Department of Obstetrics and Gynecology, Lucerne Cantonal Hospital, Lucerne, Switzerland
Correspondence: Stephanie Verta Email [email protected]
Abstract: Endometrioid borderline ovarian tumors are rare, entailing a lack of data on their natural history as well as the safety of conservative and ultraconservative surgical management, especially in cases with bilateral borderline ovarian tumors including one of endometrioid differentiation. Therefore, we present such a case and provide a review of the literature on endometrioid borderline ovarian tumors. We report the case of a 26-year-old, 0-gravida with an endometrioid borderline ovarian tumor of the right and a sero-mucinous borderline ovarian tumor of the left ovary treated by fertility sparing, ultraconservative surgery with bilateral cystectomy, completed by staging procedures including omentectomy and peritoneal sampling, as well as endometrial sampling by means of curettage. Reviewing the literature and taking into account the course of our case, we propose the feasibility of an ultraconservative management, including endometrial sampling, in young patients with bilateral borderline ovarian tumors including one of endometrioid differentiation who desire to preserve fertility, followed by a closely monitored follow-up.
Keywords: endometrioid borderline ovarian tumor, bilateral borderline ovarian tumor, ultraconservative treatment, fertility sparing surgery, bilateral cystectomy
Introduction
Borderline ovarian tumors (BOTs) make up approximately 10–20% of all epithelial ovarian malignancies.1–5 In 15–40% they occur bilaterally.5–7 Because over one-third of the patients diagnosed with BOTs are under 40 years of age, fertility and its preservation are a very important issue to consider in the treatment planning and counseling of these patients.1,5,7,8
Radical surgery consisting of hysterectomy and bilateral salpingo-oophorectomy (BSOP), including staging procedures such as omentectomy and peritoneal sampling, has been the standard treatment of BOTs for years.5,9 However, regarding the excellent prognosis of BOTs in general, the often young age at the time of diagnosis and the high rate of bilateral occurrence, conservative treatment must be considered and offered, especially to young patients wanting to preserve their fertility.10–13 Therefore, it has become the gold standard for the management of unilateral and bilateral BOTs.2,9,14-16
The recurrence rate for unilateral disease lies between 0% and 5% following radical surgery including BSOP, and up to 25% following fertility sparing surgery with unilateral salpingo-oophorectomy (USOP) or unilateral cystectomy (UCE).5,7,9,13,16-18 A very recent study by Chevrot et al reported a slightly higher recurrence rate of 38% among conservatively or ultraconservatively treated BOTs, their study population however being more complex and at higher risk for recurrence.12 Even in the case of bilateral disease conservative or ultraconservative surgery comprising USOP plus UCE or bilateral cystectomy (BCE), respectively, shows similar recurrence rates of approximately 22–28%.17,18 Furthermore, it has been shown that recurrence itself has no impact on survival,5,6,12,18 amongst other things, because relapsing histology almost always represents borderline malignancy, making it possible to be managed by further surgical intervention.5,12,19
With regards to the occurrence of malignant transformation in BOTs, a large multicenter study on BOTs calculated an incidence rate of 2.3% for invasive relapses in a study population that already presented an overall low recurrence rate of 7%, thereby showing that only one-third of the occurring relapses were invasive.20
Among BOTs, endometrioid borderline ovarian tumors (eBOTs) constitute a rare group, comprising up to only 0.2% of all epithelial ovarian tumors21,22 and 2–10% of BOTs.1,23,24 Very little data is available on the prognosis and therefore recommendations concerning the surgical management with respect to fertility in specific.1,25-27 Even less information exists on bilateral eBOTs. In the literature, there have only been reports on 11 cases so far.1,23
Thus, due to the rare occurrence of eBOTs and bilateral BOTs including an eBOT, and the lack of data on the natural history as well as on the safety of conservative surgical management, especially in young patients wishing to preserve their fertility, we present in the following the case of a 26-year-old Asian woman with bilateral BOTs, an eBOT on the right ovary and a sero-mucinous BOT on the left ovary, and provide a review of the literature on eBOTs.
Case Report
Towards the end of the year 2017 a then 25-year-old, 0-gravida of Asian origin first came to our outpatient ward. She had an MRI scan in her home country, in which a complex cyst in the left ovary had been detected, suggesting an endometriotic origin. She wished to have a follow-up on the cyst. But the findings in the MRI could not be reproduced by ultrasound. Four months later, however, in the subsequent follow-up ultrasound scan, both of the ovaries as well as the rectovaginal septum showed newly developed, possible endometriotic lesions (Figure 1).
 |
Figure 1 Sonographic findings of 03/2018: possible, small endometriotic lesions in the (A) right (yellow arrow) and (B) left (green arrows) ovaries. |
In consequence of these findings, a hormonal treatment with norethisterone acetate was initiated. In the following months, she submitted herself twice to the emergency ward of a different clinic because of severe pain in her lower abdomen. Both times no correlating pathology, other than the already suspected endometriosis could be found which could have explained the severe symptoms.
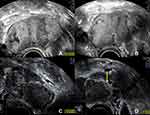
Between two subsequent scans in July and November 2018, made in the process of regular follow-up at our clinic, and under continuation of the prescribed hormonal treatment, a large, partially septated, endometriotic cyst had developed in the right ovary measuring a maximum diameter of 8 cm, showing a content of ground glass echogenicity as well as signs of fresh hemorrhages (Figure 2A and B). There had also been an increase in the size of the left ovary, with multiple cystic findings with ground glass echogenic content (Figure 2C), one of which had a possible papillary structure lining the cyst wall (Figure 2D).
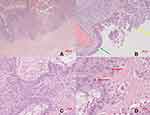
Only two days after her last regular check-up in November 2018, the patient presented herself in our clinic with acute abdominal pain. The ultrasound scan suggested a ruptured endometrioma of the right ovary, which was confirmed during emergency laparoscopic surgery. Intraoperative findings additionally revealed concomitant endometriosis on the cardinal ligaments as well as on the uterosacral ligaments and adhesions of both ovaries to the pelvic wall (rASRM IV°, ENZIAN B3). Besides being adherent to the left pelvic wall, the left ovary appeared to be enlarged and hard upon palpation. During enucleation of the endometriotic cyst on the right side (Figure 3A), unexpected papillary structures were found lining the bottom of the cyst (Figure 3B). Definitive histological examination revealed an endometrioid borderline ovarian tumor, pT1c2 (rupture of the cyst preoperatively), without stromal invasion (Figure 4).
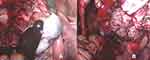 |
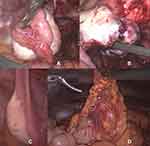
Figure 3 Intraoperative findings 11/2018: (A) chocolate like fluid spilling from the endometriotic cyst of the right ovary; (B) papillary structures found lining the bottom of the endometriotic cyst. |
Because of the intraoperative appearance of the left ovary, an ultrasound follow-up after 4 weeks was concluded, prior to planning the staging operation, in case the left ovary needed surgical intervention as well. A fact that would have to be considered in the treatment planning. Unfortunately, after this period of 4 weeks, sonographic imaging showed further enlargement of the left ovary itself as well as the cystic lesions, along with a more prominently appearing central solid part, all in all suspicious of contralateral borderline malignancy entailing further surgical investigation (Figure 5).
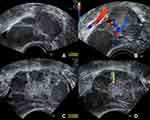 |
Figure 5 Ultrasound study of the left ovary 4 weeks postoperatively showing progressive enlargement of the ovary itself as well as the cystic structures. (A) the left ovary now measures 64 mm in the maximum diameter (in the previous exam it measured 51 mm, see Figure 2) with cystic lesions with ground glass echogenic content, suggestive of endometriomas; (B) no blood flow is detected within the cystic lesions; (C) in the center of the left ovary a solid part can be distinguished; (D) the solid center shows no increased vascularization (yellow arrow). |
Thus, suspecting bilateral involvement of the ovaries in this now 26-year-old patient, the decision was made to complete the staging operation by laparoscopic reevaluation of the right ovary (Figure 6C), cystectomy of the left ovary for histological diagnosis (Figure 6A and B) as well as peritoneal washing cytology, omentectomy (Figure 6D), peritoneal biopsies and endometrial sampling. Postoperative pathological examination revealed a borderline ovarian tumor of the left ovary as well, however of sero-mucinous differentiation, pT1c1. Omentum and peritoneal samplings showed no implants or invasive implants and endometrial sampling was unsuspicious for endometrial pathology. Peritoneal cytology showed very few slightly atypical cell groups with no further classification possible and no high grade atypia.
In conclusion, considering the bilateral involvement of the ovaries and the young age of the patient, the interdisciplinary tumor board recommended an ultraconservative, fertility sparing management with preservation of both ovaries and an aftercare including regular sonographic assessments of the ovaries. This treatment plan was discussed with the patient and she was informed of the higher risk of recurrence as a consequence of this management.
After 18 months of follow-up, under continued hormonal treatment of the endometriosis with Dienogest, the patient remains recurrence-free and also asymptomatic regarding the endometriosis. Taking into account the higher risk of recurrence and the young age of the patient, quick pursuit of family planning or, alternatively, timely oocyte freezing was recommended to the patient.
Method of Literature Search
A literature search was performed to provide an overview of recurrence risk and management aspects of eBOTs, bilateral eBOTs or bilateral BOTs including an eBOT. We conducted the search in the PubMed® database from the year 2000 to 2020 for the terms “endometrioid AND borderline”. This yielded a total of 381 citations. By reading the titles, the abstracts or the full text articles we excluded papers with the focus on ovarian cancer, non-endometrioid subtypes, molecular biology, non-ovarian localization and description of pathological features or ultrasound findings. Abstracts without full text availability were also excluded. In the end, 7 papers on eBOTs were selected from this search focusing on prognosis, recurrence rates, surgical management and synchronously appearing disorders or pathologies (Ref. Nr. 1, 21, 23, 25, 27, 28, 29). Two additional articles on eBOTs were selected due to their frequent citation in several of the eBOT papers (Ref. Nr. 26, 34). To complete the discussion part on bilateral eBOTs 2 articles on bilateral BOTs in general were selected because of the lack of data on bilateral eBOTs. Also, 4 articles on BOTs in general were included to discuss the prognostic factors for recurrence. Thus, a total of 9 articles on eBOTs and 6 articles on BOTs were taken into consideration to make up the discussion and review of literature part of this paper (Figure 7).
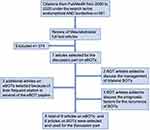 |
Figure 7 Selection process for the literature used for review and discussion. |
Discussion and Review of Literature
Currently, there is still limited data on the natural history and prognosis of eBOTs and especially bilateral eBOTs or bilateral BOTs including an eBOT. This case demonstrates an ultraconservative surgical approach (BCE) in a young patient with bilateral BOTs including an eBOT and a future childbearing desire who remains recurrence-free after a follow-up period of 18 months.
Due to the rare occurrence of this tumor entity, there is a paucity of data concerning the recommendations on surgical management, with respect to fertility in particular.1,25-27 Regarding the bilateral occurrence of eBOTs, literature has described all but 11 cases so far. Uzan et al mentioned 1 case,25 Snyder et al 1 case,26 Bell and Kurman et al 3 cases,28 Jia et al 5 cases1 and Yüksel et al 1 case.23 Thus, bilateral eBOTs comprise approximately 3–9% of all eBOT-cases.23
BOTs in general have an excellent prognosis, allowing for the consideration of conservative surgery. This has changed the gold standard from a radical procedure comprising BSOP to a fertility sparing management, especially in young patients.
For eBOTs there is still scarce data supporting such an approach. Regarding the prognosis of eBOTs, Jia et al were able to demonstrate a recurrence rate of approximately 17% for conservative, fertility sparing surgery in unilateral eBOTs (2x USOP and 3x UCE) and 13% for radical surgery including BSOP in their case series which included a total of 59 patients with eBOTs, 29 of them treated by conservative surgery. The difference in the recurrence rate did not reach statistical significance.1 Bell and Kurman et al reported a case series with 33 eBOTs in 31 patients, 13 of them undergoing conservative surgery (12 USOP and 1 CE), with no disease recurrence after 48 months of follow-up. However, only 11 of the 31 patients were even available for follow-up at all.28 Also, Snyder et al reported on 4 patients with conservatively treated eBOTs (USOP) who did not experience any recurrence. This series included a total of 29 patients with eBOTs followed up without evidence of disease.26 In the case series presented by Uzan et al 16 cases of eBOTs were reported, 7 of them undergoing conservative and 9 radical surgery. Of the 7 conservatively treated cases 5 were managed with USOP and 2 with UCE. 1 of the 7 (USOP) developed a recurrence on the side of initial USOP after 16 months.25 Zhang et al collected a total of 52 cases of eBOTs, 25 of them undergoing conservative surgery. Progression-free survival (PFS) in these cases showed no association with the type of surgery performed. Overall a disease progression or recurrence was observed in 17.3%.29 Another paper by Jia et al reported on 29 patients with eBOTs, half of them undergoing conservative surgery, followed up for a median of 54 months, experiencing a recurrence rate of 10.3% (3 patients).27 Of these 3 patients with recurrences, all 3 were initially treated conservatively.27 Finally, Yüksel et al described no recurrences in their study comprising 9 eBOTs in which 2 had undergone conservative surgery, over a follow-up period of 80–120 months.23
With regards to malignant transformation in the sense of invasive relapses in eBOTs, Jia et al suggested an incidence rate of approximately 5%,1 which is higher than the reported 2.3% in the large multicenter study on BOTs by Du Bois et al.20 A closer look at the characteristics of the groups the invasive relapses (6 in total) were encountered in reveals however that only one occurred in the conservatively managed group (USOP) comprising 29 patients and 5 in the radically managed group (BSOP) comprising 30 patients.1 In the paper by Uzan et al 1 case of an invasive relapse occurring 7 months after radical treatment of the first borderline recurrence was described, in a series of 7 initially conservatively managed eBOTs.25 Zhang et al reported on 2 invasive transformations into endometrioid ovarian cancer in a total of 52 patients with eBOTs occurring after 18 and 68 months, respectively.29 All in all these reports suggest that radical surgical management of eBOTs (including BSOP) does not provide a risk reduction towards invasive recurrence, compared to the fertility sparing procedures.1
Looking at the characteristics and the histology of the recurrences and invasive relapses, Uzan et al reported that in the one patient with recurring disease, the first recurrence was again of borderline histology, occurring on the same side. The second relapse was Grade 1 endometrioid ovarian carcinoma, also occurring on the same side as the initial eBOT.25 In the case series of Jia et al 9 patients with eBOTs experienced a total of 13 relapses, 4 in the form of an eBOT (one of them twice), 2 in the form of a BOT and 3 in the form of an endometrioid ovarian carcinoma (1 patient had 3 relapses, 1 patient 2). The eBOT recurrences relapsed mainly in the contralateral ovary (3/4) and only one in the ipsilateral ovary (1/4). It must be added that the 2 contralateral eBOT relapses following conservative surgery happened after USOP. The one ipsilateral eBOT relapse in the conservative group occurred after UCE.1 In the other paper by Jia et al the 3 reported recurrences after conservative surgery of an eBOT (1x UCE, 2x USOP) were all of eBOT- histology, 2 appearing in the contralateral ovary, after USOP, and one bilaterally, after initial UCE.27
Hence, in the case of a relapse after conservative surgery, the most common site for the recurring disease to appear is the spared ovary after USOP and the affected one or both after UCE. This in return makes repeat surgical management of the recurrence possible. However, more data is needed on the topic on how to treat recurrences.
As far as the recommendations for the surgical management of unilateral eBOTs go, the available data seems to support the feasibility of conservative surgery including USOP or even UCE with regards to recurrence risk and malignant transformation. This is why it should be recommended to young patients with a desire to preserve fertility.1,23,25-29
For bilateral eBOTs there is no available data to predict recurrence rates and prognosis following conservative or ultraconservative surgery.1 In terms of the surgical management of other bilaterally occurring histological subtypes, Palomba et al, conducting a prospective long-term extension study of a randomized controlled trial, assessed the risk–benefit ratio of an ultra-conservative fertility-sparing approach, in the sense of a bilateral cystectomy (BCE), in patients with bilateral BOTs compared to a conservative approach with USOP plus UCE.30 They were able to show that BCE enhances reproductive outcome significantly, without increasing the risk of recurrence. However, from the oncological point of view, the first recurrences occurred significantly faster in the ultra-conservative group, leading to a higher rate of radical surgery as a consequence.30 But as recurrence itself does not negatively impact survival in BOTs,5,6,9,12,18,19 ultraconservative surgical management of bilateral BOTs in young patients is feasible and provides reproductive advantages.30 A similar conclusion was reached by Delle Marchette et al. By analyzing the outcome of 535 patients, 271 undergoing USO and 264 undergoing CE, they concluded, that the type of surgical procedure, conservative or ultraconservative, did not influence the rate of recurrence in unilateral as well as bilateral BOTs.13
The only prognostic factor shown to be associated with the recurrence of eBOTs sofar is a young age at diagnosis.1 This coincides with the findings of Uzan et al who were able to demonstrate, for serous borderline ovarian tumors (SBOT), that a young age is the only independent prognostic factor for recurrence following conservative surgery.31 Also, large multicenter studies on BOTs have shown the association of recurrence and young age at diagnosis.32,33 A possible interfering or even causal factor could be the choice of surgical management depending on the age at diagnosis. Younger patients are more likely to undergo conservative or ultraconservative procedures.1,20
eBOTs have been shown to be frequently associated with endometriosis and endometrial lesions. In the literature, a synchronous occurrence of endometriosis or endometrial pathology has been commonly described in approximately one-third of the cases.27,29,34
In the study conducted by Jia et al including a total of 33 patients with eBOTs, 25 patients underwent an endometrial evaluation, revealing a total of 13 endometrial pathologies. This represented a prevalence of 52%. The 13 cases with endometrial pathologies comprised 6 endometrial carcinomas, 5 atypical hyperplasias and two endometrial hyperplasias without atypia.27 The authors also systematically reviewed the literature on eBOTs and synchronous endometrial disorders ultimately including 147 patients. Of these, 86 were evaluated for endometrial disorders, leading to the detection of 33 endometrial pathologies including 9 endometrial cancers. When joining the data with their own cases they were able to calculate a prevalence of 41.4% for endometrial pathologies found synchronously in eBOT patients.27 The risk factors identified for having a synchronous endometrial disorder in eBOTs were younger age, nulliparity and abnormal vaginal bleeding.27 Snyder et al26 reported a slightly higher occurrence rate of endometrial disorders of 68.4% in eBOT patients, while Bell and Kurman et al28 as well as Yüksel et al23 showed lower rates of 12.5% and 22%, respectively. Overall, endometrial lesions are found commonly in eBOT patients, entailing the recommendation for intraoperative endometrial sampling if fertility sparing surgery is planned. In the case of radical treatment, required by disease characteristics or by request of the patient, hysterectomy should be performed for the same logical argumentation.23,25,27
The possibility of a synchronous occurrence of endometriosis and eBOTs was already pointed out in 1988 by Snyder et al. Among their 31 cases of eBOTs, 16 presented with concomitant endometriosis (51.6%).26 Bell and Kurman et al reported on 12 patients with endometriosis in their series of 33 eBOTs (36.4%).28 Even a rate of 67% for the synchronous appearance of endometriosis in eBOTs was suggested by Roth et al.34 Uzan et al showed 3 cases of endometriosis in a total of 16 eBOTs (18.7%).25 In more recent studies Yüksel et al reported on synchronous endometriosis in 33% of their eBOTcases,23 and Zhang et al showed that 36.5%, that is 19 of a total of 52 patients with eBOTs, were affected by synchronous endometriosis.29 However, the goal of the study by Zhang et al was to compare the clinicopathological and outcome features of eBOTs associated with endometriosis (EAEBOT) and those without concomitant endometriosis (non-EAEBOT) in view of the impact on prognosis and thus recurrence rates.29 Summarizing, they found no significant differences, especially regarding PFS. In terms of differences in fertility outcome comparing EAEBOT and non-EAEBOT, no statement could be made because in their study population there was only one case of successful term birth.29 Thus they considered the two entities to be similar.29 Jia et al demonstrated a slightly higher recurrence risk for EAEBOTs, which, however, did not quite reach statistical significance.1 But, to further evaluate possible differences between EAEBOTs and non-EAEBOTs, studies with large sample sizes are needed.
Conclusions
EBOTs occur mainly unilaterally, are almost always diagnosed at an early stage and present a good overall prognosis.1,21,23,25,26,28,29 Conservative surgery with USOP or even UCE, including endometrial sampling, can thus be proposed to young eBOT patients with a future childbearing desire, with unilateral involvement of the ovaries, under careful follow-up. However, more data is needed on the natural history of bilateral eBOTs or bilateral BOTs including an eBOT, as well as on the prognosis and fertility outcomes following conservative and ultraconservative surgery in such cases.
Nevertheless, regarding the literature and the course of our case, we suggest an ultraconservative management by means of BCE and including endometrial sampling, for fertility preservation and optimization of the fertility outcome in young patients with bilateral BOTs including an eBOT and a future desire to conceive. A closely monitored follow-up should of course complement the surgical procedure.
Although follow-up in the present case has only been 18 months to date, this recurrence-free period has provided a significant amount of time for the patient to potentially fulfill her childbearing desire thanks to the ultraconservative (BCE), bilateral organ- and, thus, maximally fertility-sparing management of the bilateral BOTs, including an eBOT.
Patient counseling on surgical management, however, must include communicating the higher recurrence risk following conservative and ultraconservative surgery to reach an informed consent on how to manage the surgical therapy in the context of a potential future childbearing desire.
Author’s Information
Stephanie Verta: Senior physician at the Department of Obstetrics and Gynecology, Lucerne Cantonal Hospital, 6000 Lucerne.
Barbara Kipp: Chief physician at the Department of Obstetrics and Gynecology, Lucerne Cantonal Hospital, 6000 Lucerne.
Abbreviations
BCE, bilateral cystectomy; BOT, borderline ovarian tumor; BSOP, bilateral salpingo-oophorectomy; CE, cystectomy; EABOT, endometriosis associated endometrioid borderline ovarian tumor; eBOT, endometrioid borderline ovarian tumor; HE, hysterectomy; PFS, progression-free survival; SBOT, serous borderline ovarian tumor; UCE, unilateral cystectomy; USOP, unilateral salpingo-oophorectomy.
Data Sharing Statement
The data used for this article (complete patient history as well as the ultrasound and intraoperative images) are available from the corresponding author upon reasonable request.
Consent for Publication
Written informed consent was obtained from the patient for publication of her case as well as the accompanying images.
Acknowledgment
We would like to thank Walter Arnold, MD, for providing the microscopic images.
Author Contributions
Both authors made a significant contribution to the conception of the article, the acquisition of data, in drafting, revising and critically reviewing the manuscript, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
Both authors report no conflicts of interest in this work.
References
1. Jia S, Zhang J, Liang Z, et al. Safety and fertility outcomes after the conservative treatment of endometrioid borderline ovarian tumours. BMC Cancer. 2018;18(1):1160. doi:10.1186/s12885-018-5091-1
2. Cadron I, Leunen K, Van Gorp T, Amant F, Neven P, Vergote I. Management of borderline ovarian neoplasms. J Clin Oncol off J Am Soc Clin Oncol. 2007;25(20):2928–2937. doi:10.1200/JCO.2007.10.8076
3. Morris CR, Liu L, Rodriguez AO, Cress RD, Snipes K. Epidemiologic features of borderline ovarian tumors in California: a population-based study. Cancer Causes Control. 2013;24(4):665–674. doi:10.1007/s10552-013-0145-9
4. Skírnisdóttir I, Garmo H, Wilander E, Holmberg L. Borderline ovarian tumors in Sweden 1960–2005: trends in incidence and age at diagnosis compared to ovarian cancer. Int J Cancer. 2008;123(8):1897–1901. doi:10.1002/ijc.23724
5. Masciangelo R, Bosisio C, Donnez J, Amorim CA, Dolmans -M-M. Safety of ovarian tissue transplantation in patients with borderline ovarian tumors. Hum Reprod Oxf Engl. 2018;33(2):212–219. doi:10.1093/humrep/dex352
6. Zanetta G, Rota S, Chiari S, Bonazzi C, Bratina G, Mangioni C. Behavior of borderline tumors with particular interest to persistence, recurrence, and progression to invasive carcinoma: a prospective study. J Clin Oncol off J Am Soc Clin Oncol. 2001;19(10):2658–2664. doi:10.1200/JCO.2001.19.10.2658
7. Donnez J. Safety of conservative management and fertility outcome in women with borderline tumors of the ovary. Fertil Steril. 2003;79(5):1216–1221. doi:10.1016/S0015-0282(03)00160-2
8. Harter P, Gershenson D, Lhomme C, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian tumors of low malignant potential (borderline ovarian tumors). Int J Gynecol Cancer off J Int Gynecol Cancer Soc. 2014;24(9 Suppl 3):S5–8. doi:10.1097/IGC.0000000000000282
9. Morice P. Borderline tumours of the ovary and fertility. Eur J Cancer. 2006;42(2):149–158. doi:10.1016/j.ejca.2005.07.029
10. Kurman RJ, Trimble CL. The behavior of serous tumors of low malignant potential: are they ever malignant? Int J Gynecol Pathol off J Int Soc Gynecol Pathol. 1993;12(2):120–127. doi:10.1097/00004347-199304000-00006
11. Casey AC, Bell DA, Lage JM, Fuller AF, Nikrui N, Rice LW. Epithelial ovarian tumors of borderline malignancy: long-term follow-up. Gynecol Oncol. 1993;50(3):316–322. doi:10.1006/gyno.1993.1218
12. Chevrot A, Pouget N, Bats A-S, et al. Fertility and prognosis of borderline ovarian tumor after conservative management: results of the multicentric OPTIBOT study by the GINECO & TMRG group. Gynecol Oncol. 2020;157(1):29–35. doi:10.1016/j.ygyno.2019.12.046
13. Delle Marchette M, Ceppi L, Andreano A, et al. Oncologic and fertility impact of surgical approach for borderline ovarian tumours treated with fertility sparing surgery. Eur J Cancer. 2019;111:61–68. doi:10.1016/j.ejca.2019.01.021
14. Mangili G, Somigliana E, Giorgione V, et al. Fertility preservation in women with borderline ovarian tumours. Cancer Treat Rev. 2016;49:13–24. doi:10.1016/j.ctrv.2016.06.010
15. Morice P, Uzan C, Fauvet R, Gouy S, Duvillard P, Darai E. Borderline ovarian tumour: pathological diagnostic dilemma and risk factors for invasive or lethal recurrence. Lancet Oncol. 2012;13(3):e103–115. doi:10.1016/S1470-2045(11)70288-1
16. Daraï E, Fauvet R, Uzan C, Gouy S, Duvillard P, Morice P. Fertility and borderline ovarian tumor: a systematic review of conservative management, risk of recurrence and alternative options. Hum Reprod Update. 2013;19(2):151–166. doi:10.1093/humupd/dms047
17. Chen R-F, Li J, Zhu -T-T, Yu H-L LX. Fertility-sparing surgery for young patients with borderline ovarian tumors (BOTs): single institution experience. J Ovarian Res. 2016;9:16. doi:10.1186/s13048-016-0226-y
18. Vasconcelos I, de Sousa Mendes M. Conservative surgery in ovarian borderline tumours: a meta-analysis with emphasis on recurrence risk. Eur J Cancer. 2015;51(5):620–631. doi:10.1016/j.ejca.2015.01.004
19. Uzan C, Kane A, Rey A, Gouy S, Duvillard P, Morice P. Outcomes after conservative treatment of advanced-stage serous borderline tumors of the ovary. Ann Oncol off J Eur Soc Med Oncol. 2010;21(1):55–60. doi:10.1093/annonc/mdp267
20. Du Bois A, Ewald-Riegler N, de Gregorio N, et al. Borderline tumours of the ovary: a cohort study of the Arbeitsgmeinschaft Gynäkologische Onkologie (AGO) study group. Eur J Cancer. 2013;49(8):1905–1914. doi:10.1016/j.ejca.2013.01.035
21. Nakagawa E, Abiko K, Kido A, et al. Four cases of endometrioid borderline ovarian tumour: case reports and literature review. BJR Case Rep. 2018;4(1):20170062. doi:10.1259/bjrcr.20170062
22. Ellenson LH, Carinelli SG, Cho KR, et al. Endometrioid tumours. In:Kurman RJ, International Agency for Research on Cancer, World Health Organization, editor. WHO Classification of Tumours of Female Reproductive Organs.
23. Yüksel D, Çakır C, Kimyon Cömert G, et al. Uncommon borderline ovarian tumours: a clinicopathologic study of seventeen patients. J Turk Ger Gynecol Assoc. 2019;20(4):224–230. doi:10.4274/jtgga.galenos.2018.2018.0098
24. Hussin H, Ghani F. Ovarian endometrioid borderline tumor arising from an endometriotic cyst. J Interdiscip Histopathol. 2017;1. doi:10.5455/jihp.20161231122553.
25. Uzan C, Berretta R, Rolla M, et al. Management and prognosis of endometrioid borderline tumors of the ovary. Surg Oncol. 2012;21(3):178–184. doi:10.1016/j.suronc.2012.02.002
26. Snyder RR, Norris HJ, Tavassoli F. Endometrioid proliferative and low malignant potential tumors of the ovary. A clinicopathologic study of 46 cases. Am J Surg Pathol. 1988;12(9):661–671. doi:10.1097/00000478-198809000-00002
27. Jia S-Z, Zhang -J-J, Yang -J-J, Xiang Y, Liang Z, Leng J-H. Risk of synchronous endometrial disorders in women with endometrioid borderline tumors of the ovary. J Ovarian Res. 2018;11(1):30. doi:10.1186/s13048-018-0405-0
28. Bell KA, Kurman RJ. A clinicopathologic analysis of atypical proliferative (borderline) tumors and well-differentiated endometrioid adenocarcinomas of the ovary. Am J Surg Pathol. 2000;24(11):1465–1479. doi:10.1097/00000478-200011000-00002
29. Zhang W, Jia S, Xiang Y, Yang J, Jia C, Leng J. Comparative study of endometrioid borderline ovarian tumor with and without endometriosis. J Ovarian Res. 2018;11(1):67. doi:10.1186/s13048-018-0440-x
30. Palomba S, Falbo A, Del Negro S, et al. Ultra-conservative fertility-sparing strategy for bilateral borderline ovarian tumours: an 11-year follow-up. Hum Reprod Oxf Engl. 2010;25(8):1966–1972. doi:10.1093/humrep/deq159
31. Uzan C, Muller E, Kane A, et al. Prognostic factors for recurrence after conservative treatment in a series of 119 patients with stage I serous borderline tumors of the ovary. Ann Oncol off J Eur Soc Med Oncol. 2014;25(1):166–171. doi:10.1093/annonc/mdt430
32. Trillsch F, Mahner S, Woelber L, et al. Age-dependent differences in borderline ovarian tumours (BOT) regarding clinical characteristics and outcome: results from a sub-analysis of the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) ROBOT study. Ann Oncol off J Eur Soc Med Oncol. 2014;25(7):1320–1327. doi:10.1093/annonc/mdu119
33. Ouldamer L, Bendifallah S, Nikpayam M, et al. Improving the clinical management of women with borderline tumours: a recurrence risk scoring system from a French multicentre study. BJOG Int J Obstet Gynaecol. 2017;124(6):937–944. doi:10.1111/1471-0528.14577
34. Roth LM, Emerson RE, Ulbright TM. Ovarian endometrioid tumors of low malignant potential: a clinicopathologic study of 30 cases with comparison to well-differentiated endometrioid adenocarcinoma. Am J Surg Pathol. 2003;27(9):1253–1259. doi:10.1097/00000478-200309000-00009
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.