Back to Journals » Therapeutics and Clinical Risk Management » Volume 10
Udenafil for the treatment of erectile dysfunction
Received 6 September 2013
Accepted for publication 1 November 2013
Published 14 May 2014 Volume 2014:10 Pages 341—354
DOI https://doi.org/10.2147/TCRM.S39727
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Video abstract presented by Min Chul Cho.
Views: 5208
Min Chul Cho,1 Jae-Seung Paick2
1Department of Urology, Dongguk University College of Medicine, Goyang, Korea; 2Department of Urology, Seoul National University College of Medicine, Seoul, Korea
Abstract: Erectile dysfunction (ED) is often perceived by both patients and sexual partners as a serious problem that can jeopardize quality of life, psychosocial or emotional well-being, and the partnership in the long term. Since their introduction, oral phosphodiesterase type 5 inhibitors (PDE5Is) have been found to be highly effective and well tolerated, and are available as the first-line therapy for the treatment of ED. Udenafil is one of the selective PDE5Is made available in recent years for the treatment of ED. Udenafil has clinical properties of both relatively rapid onset and long duration of action due to its pharmacokinetic profile, thereby providing an additional treatment option for ED men to better suit individual needs. There is positive evidence that udenafil is effective and well tolerated in the treatment of ED of a broad spectrum of etiologies or severity. Udenafil is as effective in the treatment of diabetes mellitus-associated ED as other PDE5Is. Due to the clinical property of relatively long duration of action, udenafil may be another option in daily dosing treatment for ED, as suggested by its favorable efficacy and safety profile. Most adverse effects reported from clinical trials are mild or moderate in severity, without any serious adverse event, with headache and flushing being the most common. Also, the concomitant use of anti-hypertensive drugs or alpha-1-blockers does not significantly affect the efficacy and safety profile of udenafil. However, additional studies with larger cohorts including prospective, multicenter, comparative studies with patients of different ethnicities are needed to further validate the favorable findings of udenafil in the treatment of ED.
Keywords: udenafil, erectile dysfunction, therapy
Introduction
Erectile dysfunction (ED) is defined by the inability to achieve or maintain an erection sufficient for satisfactory sexual performance and is a common clinical entity that primarily affects men older than 40 years of age.1,2 ED is associated with various comorbidities or conditions, including advanced age, hypertension, hyperlipidemia, metabolic syndrome, lower urinary tract symptoms (LUTS) of benign prostatic hyperplasia (BPH), cardiovascular disease, central neuropathologic conditions, psychological factors, diabetes mellitus (DM), a result of radical prostatectomy, and the use of medications prescribed for the treatment of depression and hypertension.3–10 Furthermore, recent studies have suggested that ED can be a strong predictor of cardiovascular disease and that the development of symptomatic ED may precede the occurrence of a cardiovascular event by 2–3 years.11–15
Many treatment modalities have been introduced to address ED due to its considerable impact on overall quality of life and psychosocial aspects, such as emotional well-being or interpersonal relationships. Currently, treatment options available for ED include vacuum pump devices, intracavernosal injection of vasoactive agents, intraurethral suppositories, penile prosthesis, vascular surgery, hormones, and phosphodiesterase (PDE) type 5 inhibitors (PDE5Is).16,17 Among these, the advent of PDE5Is, which offer advantages over other treatment modalities in terms of ease of administration and costs, has revolutionized the medical treatment for ED.18,19 PDE5Is are considered to be the first-line therapy for most men with ED across a broad spectrum of underlying diseases and severity. To date, there are four PDE5Is (sildenafil, vardenafil, tadalafil, and avanafil) approved for the treatment of ED worldwide, and udenafil has been approved in 13 countries, including Korea.20,21 Mirodenafil has been approved only in Korea, and lodenafil and SLx-2101, new PDE5Is, are still under investigation.22–25
Udenafil (Zydena; Dong-A Pharmaceutical, Seoul, Korea), one of the selective PDE5Is, was developed in Korea and approved for the treatment of ED in 2005. Its pharmacokinetic profiles include a time to maximum drug plasma concentration (tmax) of 0.8–1.3 hours and a half-life (t1/2) of 9.9~12.1 hours, which would confer clinical properties of both relatively rapid onset and long duration of action.26 Its molecular structure is similar to that of sildenafil citrate (Viagra; Pfizer Inc, New York, NY, USA), and the isoenzyme selectivity profile of udenafil is comparable to that of sildenafil.26 Clinical efficacy and safety of udenafil have been evaluated in men with ED of a broad spectrum of etiologies or severity in several randomized, controlled trials;27–33 thus, the availability of a variety of PDE5Is, including udenafil, can assist clinicians in tailoring treatment regimens to the specific needs of each patient with ED and in prescribing the PDE5I that has the highest efficacy for erection or satisfaction and the least overall adverse effects in a given patient. In this review, we highlight the preclinical and clinical evidence for the efficacy and safety of udenafil in the treatment of ED.
Physiologic basis for effects and adverse effects
PDEs comprise a family of metallophosphohydrolases that cleave the 3′,5′-cyclic phosphate moiety of cyclic adenosine monophosphate (cAMP) and/or cyclic guanosine monophosphate (cGMP) to produce the corresponding 5′ nucleotide.34 PDE5 selectively cleaves cGMP, a key intracellular secondary messenger in penile erection, by which the effect of nitric oxide (NO) on relaxation of cavernous smooth muscles and dilation of helicine arterioles is mediated.34,35 PDE5I has a similar structure to cGMP and inhibits the breakdown of NO-derived cGMP by competitively binding the catalytic site of PDE5, thereby allowing the accumulation of cGMP in the cytoplasm of cavernosal and vascular smooth muscle cells for continuous activation of the NO/cGMP mechanism, which can lead to increased penile blood flow during sexual stimulation and, ultimately, enhanced penile erection.36
Given this physiologic background, therapeutic effects of PDE5Is such as udenafil depend on their specificity for PDE5 and their pharmacokinetics, as well as on the distribution of different PDE isoforms in the cavernous tissue. As different PDE isoforms are distributed throughout a variety of tissues and PDE5 is present in high concentration in the cavernous smooth muscle, the selectivity of PDE5Is for PDE5 over the other PDE isoforms is a prerequisite for an increased therapeutic window.34 Further, the selectivity for PDE5 over the other PDE isoforms and degree of inhibition of PDE5 in tissues other than corpora cavernosa are key factors determining the safety and tolerability of PDE5Is. If PDE5Is have a large enough difference in the affinity to PDE5 compared to other PDE isoforms, there is less likely to be a significant inhibition of the other PDE isoforms and, thereby, less likelihood of unwanted adverse effects at therapeutic plasma concentrations.
Table 1 summarizes the data comparing selectivity for various PDE isoenzymes between udenafil and the other three PDE5Is. A preclinical study showed that an inhibitory concentration for PDE5 of udenafil was approximately 150-, 17-, 9- and 10-fold lower than those for PDE1, PDE2, PDE3, and PDE6, respectively.26 Moreover, with regard to PDE1 associated with vasodilation and flushing, PDE1 selectivity of udenafil (selectivity ratio of 149) is similar to that of sildenafil (selectivity ratio of 111).26 Also, the selectivity data of udenafil for PDE5 over PDE2, PDE3, and PDE6 are comparable to those of sildenafil.26 As for PDE11, udenafil (selectivity ratio of 96) has higher selectivity than tadalafil (selectivity ratio of 7.1), but the clinical significance of PDE11 inhibition has not yet been established.31,37 Thus, udenafil is one of the selective PDE5Is, based on its low affinity for the other PDE isoforms.
Chemistry
Udenafil (5-[2-propyloxy-5-(1-methyl-2-pyrollidinylethylamidosulphonyl)phenhyl]-1-methyl-3-propyl-1,6-dihydro-7H-pyrazolo(4,3-d)-pyrimidin-7-one) is a pyrazolopyrimidine derivative and has a molecular structure similar to that of cGMP, like sildenafil (Figure 1).37,38 Udenafil has a molecular mass of 516.657 g/mol and its product is available in tablet formulation of 100 mg and 200 mg.
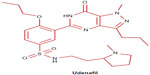  | Figure 1 Structural formula of udenafil. |
Pharmacological profile
Pharmacokinetics
A recent Phase I clinical trial has demonstrated that udenafil is rapidly absorbed, reaching peak plasma concentrations at 0.8~1.3 hours, then declining monoexponentially with a t1/2 from 9.9 to 12.1 hours, which can confer clinical properties of both relatively rapid onset and long duration of action.38 Also, the area under the time–concentration curve and the maximum concentrations in plasma (Cmax) of udenafil increased supraproportionally with increasing dose on single administration.38 Udenafil reached steady state by day 5 of regular repeated administrations, without significant drug accumulation.38 According to a previous preclinical study using rats, absolute oral bioavailability of udenafil was only 38% in rats, which was similar to that of sildenafil (23%~44%).39,40 This low oral bioavailability of udenafil appears to be mainly due to a considerable intestinal first-pass effect.39 On the other hand, according to a model of interspecies scale-up of pharmacokinetics of udenafil, it was predicted that its volume of distribution in humans was large, as extrapolated from data on other species.41 The time to Cmax of udenafil is delayed under a fat-fed condition;42 however, although the Cmax can be reduced by approximately 21% in a low-fat-fed state, overall bioavailability is not affected when taken with food.42 Table 2 summarizes the data comparing the pharmacokinetics between udenafil and the other three PDE5Is.
Pharmacodynamics
PDE5 is mainly found in the corpora cavernosa, the vascular and visceral smooth muscles, and platelets.43,44 Udenafil exhibits its inhibitory effect by binding competitively to the catalytic site of PDE5, thereby promoting the accumulation of cGMP in the smooth muscle cells of corpora cavernosa. The inhibition of various PDEs by udenafil was evaluated in comparison to that of sildenafil.26 The half-maximal inhibitory concentration (IC50) for PDE5 of udenafil is 8.25±2.90 nM, which is similar to that of sildenafil (8.50±2.05 nM).26,38 PDE1 inhibition is associated with vasodilation, flushing, and tachycardia. The inhibitory effect of udenafil on PDE5 is about 150-fold greater than that on PDE1 (selectivity ratio of 149), which is similar to the PDE1 selectivity of sildenafil (selectivity ratio of 111).26 Furthermore, PDE11 selectivity of udenafil (selectivity ratio of 96) is relatively high, indicating a very low probability of inhibition of PDE11 at therapeutic doses of udenafil.31,37 PDE11 is present in a variety of organs, including skeletal muscle, prostate, testis, corpora cavernosa, heart, and anterior pituitary, and its functions are not clearly known. On the other hand, the IC50 values for PDE2, PDE3, and PDE6 of udenafil were 101±15.1 nM, 52.0±3.53 nM, and 53.3±2.47 nM, which was similar to those of sildenafil (111±25.0 nM, 30.6±1.65 nM, and 72.4±2.94 nM), suggesting the selectivity ratios for PDE2, PDE3, and PDE6 were roughly 10 in both udenafil and sildenafil.26 PDE3 hydrolyzes cAMP and is mainly found in cardiomyocytes, as well as in the corpora cavernosa. Cross-reactions with PDE3 by udenafil might increase the level of cAMP in the heart, thereby inducing an increase in heart rate with a positive inotropic effect.32,45 Also, udenafil could cross-react with PDE6, as sildenafil does, and might partially inhibit it at therapeutic doses. As inhibition of PDE6, which controls levels of cGMP in the retina, may cause visual adverse effects, such as chromatopsia and blurred vision, reported in a minority of patients taking udenafil, attention should be paid to PDE6, which is predominantly expressed in the cones and rods of retina.31,32,38,45,46
Metabolism
Udenafil is mainly metabolized by cytochrome P450 3A4 (CYP3A4), and CYP3A5 partly contributes to its metabolism.37,47 There are three metabolites produced by the metabolism: DA-8164, M1 (hydroxyl DA-8159), and M2 (N-demethyl DA-819).39,48 Among these, DA8164 is known as the major active metabolite of udenafil.48,49 CYP3A4 is predominantly responsible for the formation of DA-8164 from udenafil.48 DA-8164 has a half in vitro potency for inhibition of PDE5 compared to that of udenafil, and t1/2 of DA-8164 is nearly similar to that of udenafil (12 hours).50 Udenafil appears to be mainly excreted into the feces, although this has not been conclusively proven, due to limited data on the pharmacological properties of udenafil.37,50 Urinary excretion of udenafil was low (<12%), and DA-8164 was extremely low (0.2%), suggesting both udenafil and DA-8164 are subject to nonrenal elimination.50 Table 2 summarizes the data comparing metabolism between udenafil and the other three PDE5Is.
Efficacy of udenafil in ED
The current American Urological Association and European Association of Urology guidelines recommend oral PDE5Is as a first-line treatment option for ED.17,51 Initially, the efficacy of udenafil was documented in a preclinical study using rat and dog models, which reported that oral or intravenous administration of udenafil increased the number of penile erections with increasing dosages, indicating significant therapeutic potential in the treatment of ED.52 In a Phase I trial, udenafil was found to be safe, well tolerated, and rapidly absorbed, and systemic exposure to udenafil increased in a dose-proportional manner with both single and multiple doses.50 A Phase II, double-blind, placebo-controlled, multicenter, parallel-group clinical trial showed that udenafil produced a highly significant improvement in erectile function, with an up to 91% vaginal penetration success rate.53 The clinical efficacy of udenafil in patients with ED of a variety of underlying etiologies, severities, and ages of the patients has been suggested in several randomized, placebo-controlled trials.27–29,31–33 Patient characteristics, outcome measures, and results of these trials are summarized in Tables 1 and 2.
A Phase III, 12-week, multicenter, double-blind, randomized, placebo-controlled, parallel-group study by Paick et al evaluated the efficacy and safety of udenafil at two doses (100 mg and 200 mg) in 167 Korean men aged 19 to 70 years with at least a 6-month history of ED from organic, psychogenic, or mixed etiology.27 The primary efficacy variable was the change from baseline in the erectile function domain (EF) scores of the International Index of Erectile Function (IIEF) questionnaire. Secondary efficacy variables included changes from baseline in scores on the IIEF for question 3 (penetration ability) and question 4 (maintenance frequency); changes from baseline in all domain scores of the IIEF; and patients’ responses to questions 2 and 3 of the Sexual Encounter Profile (SEP) (SEP2: successful vaginal penetration; SEP3: the ability to successfully complete intercourse). Patients’ responses to the Global Assessment Question (GAQ) were also assessed after 12 weeks of treatment. After 12 weeks of treatment, the patients treated with udenafil showed significantly greater change from baseline in the IIEF-EF scores compared with placebo (placebo: 0.20; 100 mg udenafil: 7.52; 200 mg udenafil: 9.93). Furthermore, the proportion of subjects exhibiting normal erection based on the IIEF-EF score after the 12-week period was 3.7%, 35%, and 48% in the placebo, 100 mg udenafil, and 200 mg udenafil treatment groups, respectively. Compared with placebo, udenafil significantly enhanced the rates of successful penetration (SEP2) (placebo: 53.38%; 100 mg udenafil: 88.83%; 200 mg udenafil: 92.40%) and maintenance of erection (SEP3) (placebo: 15.44%; 100 mg udenafil: 70.08%; 200 mg udenafil: 75.70%). Furthermore, significantly greater proportions of udenafil treatment groups responded positively to the GAQ compared with the placebo group (placebo: 25.9%; 100 mg udenafil: 81.5%; 200 mg udenafil: 88.5%). Thus, udenafil treatment resulted in a numerically dose-dependent increase in all efficacy variables, with mild-to-moderate treatment-related adverse events, indicating that udenafil may well be a reliable treatment option for ED of broad-spectrum etiology and severity.
A recent multicenter, randomized, double-blind, placebo-controlled, parallel-group study assessed the efficacy and safety of 8-week 100 mg udenafil treatment in 118 Turkish men aged 19 to 70 years with ED of organic or psychogenic etiology.33 The primary efficacy variable was change from baseline in IIEF-EF scores. The secondary efficacy variables included changes from baseline in the other domain scores of the IIEF, in SEP2 and SEP3, and in the patients’ responses to GAQ. The udenafil group showed significantly higher increase in the IIEF-EF score compared to the placebo group (placebo: 3.8; 100 mg udenafil: 7.7). Similarly, greater improvements were observed for SEP2, SEP3, and two other IIEF domains (sexual desire and overall sexual satisfaction). The proportion of positive responses to GAQ was significantly greater in the udenafil group than in the placebo group (72.2% versus 49.1%). All adverse events observed during the study period were of mild or moderate severity.
Recently, a randomized, double-blind, placebo-controlled, parallel-group, fixed-dose-design, multicenter study by Park et al showed that the effectiveness of udenafil to improve erectile function of the subjects could be sustained for up to 12 hours after a single dosage of 100 mg, indicating that flexibility and spontaneity in the sex-life could be achieved.28 In the study, participants were requested to attempt sexual intercourse at 12 hours after udenafil or placebo dosing, taken as needed, during the 4-week treatment period. The primary efficacy variable was response to SEP3. The secondary efficacy variables were response to SEP2 and change from baseline in IIEF-EF scores. Udenafil significantly enhanced the rate of maintenance of erection (placebo: 28.3% versus udenafil: 54.7% [SEP3]). Compared with the placebo, significant change in the IIEF-EF score was observed in the udenafil group (placebo: −0.58±0.67; 100 mg udenafil: 4.40±0.84). For SEP2, however, there was no difference from baseline, and no difference between the udenafil and placebo groups. Park et al concluded that udenafil could be a reliable treatment option in patients who are in need of a spontaneous recovery of erectile function and who desire sexual intercourse without concerns about the duration of efficacy.28
According to a recent meta-analysis of five randomized, placebo-controlled trials by Ding et al, the pooled analysis showed that udenafil treatment provided an average increase in change from baseline in the IIEF-EF score of 5.65 points compared with placebo, which was statistically significant.54 Similar results were observed in the comparison of SEP2 and SEP3, GAQ, and the proportion of shift to normal erection based on the IIEF-EF score (≥26). The pooled results showed average increases of 22.14% and 31.22% in positive responses to SEP2 and SEP3 respectively after the udenafil treatment compared with placebo. Also, the udenafil group showed greater increases in positive responses to GAQ and the proportion of shift to normal erection compared to placebo (risk ratios: 3.44 and 2.37, respectively).
Efficacy in ED patients with DM
The neurologic and vascular consequences of DM are considered to be a significant comorbidity for development of ED. DM contributes to ED through a complex interplay of elevated advanced glycation end-products; increased levels of oxygen free radicals; impaired NO synthesis; increased endothelin B receptor binding sites; upregulation of RhoA/Rho-kinase pathway; neuropathic damage; and impaired cGMP-dependent protein kinase-1, indicating that DM-associated ED is multifactorial.55 Despite considerable progress in the knowledge and understanding of pathophysiology of ED, the treatment of DM-associated ED is often difficult due to its multifactorial etiology.
Several preclinical studies using animal models of DM-associated ED have provided a rationale for the use of udenafil as treatment for diabetic ED.56–58 Also, in a rat model of DM-associated ED, chronic administration of udenafil was shown be a potential therapeutic strategy to prevent the progression of diabetic ED by enhancing gene and protein expression levels of neuronal NO synthase and endothelial NO synthase in diabetic corpora cavernosa.59 Interestingly, a recent study using a rat model of streptozotocin-induced diabetic ED has demonstrated that a time-dependent deterioration of erectile function during the course of diabetes was followed by decreased responsiveness to udenafil and severe ED refractory to udenafil, suggesting the necessity of early preventive treatment for DM-associated ED.60
A Phase III, 12-week, multicenter, placebo-controlled, randomized, double-blind, double-dummy, parallel-group-design, fixed-dose trial by Moon et al evaluated the efficacy and safety of udenafil at two doses (100 mg and 200 mg) in 174 Korean men with ED.29 The primary efficacy variable was change in the IIEF-EF score from baseline. The secondary parameters were IIEF questions 3 and 4, SEP2 and SEP3, rate of achieving normal erectile function, and patient response to GAQ. Both udenafil groups showed significantly greater change from baseline in the IIEF-EF score compared with placebo (placebo: 1.20; 100 mg udenafil: 7.00; 200 mg udenafil: 8.21). Similar results were observed in the comparison of IIEF questions 3 and 4, SEP2 and SEP3, and GAQ between the udenafil and placebo groups.
Efficacy in ED patients with hypertension
Hypertension is a prevalent condition in men with ED as well as a risk factor for the development of ED.2 ED can also occur as an adverse effect of several antihypertensive drugs such as beta-blockers and thiazide.2 Thus, given the close association between ED and hypertension, the issues of efficacy and safety of PDE5Is for treatment of ED in this patient population has assumed considerable importance.
A Phase III, 12-week, multicenter, double-blind, randomized, placebo-controlled, parallel-group study by Paick et al was performed to determine the efficacy and safety of udenafil of fixed doses (100 mg or 200 mg) in 165 Korean men with at least a 6-month history of ED who took one or more antihypertensive agents in a stable dose for arteriogenic hypertension.32 The primary efficacy variable was change from baseline in IIEF-EF scores. The secondary efficacy variables included change from baseline in scores on the IIEF question 3 and question 4, changes in SEP2 and SEP3, and patient responses to GAQ. The udenafil groups showed significantly greater increase in the IIEF-EF score (placebo: 1.98; 100 mg udenafil: 8.71; 200 mg udenafil: 10.04) and scores on the IIEF question 3 (placebo: 0.1; 100 mg udenafil: 1.3; 200 mg udenafil: 1.4) and question 4 (placebo: 0.7; 100 mg udenafil: 2.0; 200 mg udenafil: 2.5) compared with placebo group. Similarly, greater improvements were observed for changes from baseline in the SEP2 (placebo: 3.13%; 100 mg udenafil: 25.85%; 200 mg udenafil: 29.82%) and SEP3 (placebo: 12.55%; 100 mg udenafil: 57.86%; 200 mg udenafil: 71.82%). Compared to the placebo group, the proportion of positive responses to GAQ (placebo: 41.2%; 100 mg udenafil: 78.8%; 200 mg udenafil: 85.2%) and that of subjects who returned to normal erection (placebo: 15.7%; 100 mg udenafil: 44.2%; 200 mg udenafil: 54.5%) were significantly greater for the udenafil groups. The efficacy was maintained irrespective of baseline ED severity, the number of antihypertensive agents, and prior experience with PDE5Is. Udenafil was well tolerated with a low incidence of treatment-emergent adverse events.
Efficacy in ED patients with LUTS
A large body of epidemiologic evidence supports a causal relationship between ED and LUTS.61 Although the underlying mechanisms for the relationship between LUTS and ED in men with BPH are not fully understood, common links, such as the NO/cGMP pathway, RhoA/Rho-kinase signaling, pelvic atherosclerosis, and autonomic adrenergic hyperactivity, can be potential targets for PDE5Is.62 Thus, a recent meta-analysis of available randomized, placebo-controlled trials investigating the efficacy and safety of PDE5Is alone or in combination with alpha-blocker in men with LUTS/BPH showed favorable outcomes in terms of both erectile function and LUTS.62
An open, prospective, non-comparative study by Chung et al assessed the efficacy and safety of coadministered 100 mg udenafil and alpha-1-blockers in 120 patients with both LUTS/BPH and ED, who were already receiving stable alpha-1-blocker therapy.30 At the end of the treatment period (8 weeks), mean international prostatic symptom score (IPSS) significantly decreased from 14.3 at baseline to 11.5, and mean IIEF-5 score significantly increased from 11.9 at baseline to 19.3, without any additional adverse effects related to coadministration.
Chronic dosing of udenafil for ED
Several preclinical and clinical studies have demonstrated that chronic or daily use of PDE5Is in the treatment of ED can significantly improve endothelial dysfunction, suggesting a promising therapeutic potential.63–65 Potential benefits of chronic PDE5I dosing are as follows: 1) a natural and spontaneous sex-life as it used to be when erectile function was not impaired; 2) salvage treatment of nonresponder to on-demand use of PDE5Is; 3) endothelial and penile rehabilitation, especially in complicated cases; and 4) combined treatment of LUTS/BPH.2,19,66 Given the pharmacokinetic profiles of udenafil with tmax of 0.8~1.3 hours and t1/2 of 9.9~12.1 hours, udenafil may be a candidate for chronic dosing.
A Phase II, multicenter, randomized, double-blind, placebo-controlled, fix-dosed clinical trial evaluated the efficacy and safety of a once-daily low dose of udenafil (25 mg or 50 mg or 75 mg) in the treatment of ED and aimed to determine the optimal clinical dose and dosing schedule.31 The primary efficacy variable was change from baseline in IIEF-EF scores. The secondary efficacy variables included patient responses to SEP2, SEP3, and GAQ, and the percentage of patients exhibiting a “shift to normal.” Compared with placebo, patients who took 50 mg or 75 mg of udenafil had significantly greater increase in their IIEF-EF score, but those who took 25 mg of udenafil did not (placebo: 3.61; 25 mg udenafil: 5.75; 50 mg udenafil: 6.55; 75 mg udenafil: 8.71). A similar finding was observed when comparing change from baseline in patient response of SEP2 (placebo: 11.95%; 25 mg udenafil: 22.10%; 50 mg udenafil: 27.9%, 75 mg udenafil: 39.11%); however, compared to placebo, all udenafil groups showed significantly greater increases in patient response of SEP3 (placebo: 23.46%; 25 mg udenafil: 42.09%; 50 mg udenafil: 51.41%, 75 mg udenafil: 73.5%) and in the percentage of patients achieving normal IIEF-EF scores (placebo: 13.6%; 25 mg udenafil: 30.5%; 50 mg udenafil: 40.0%; 75 mg udenafil: 44.1%). Also, with respect to the proportion of positive responses to GAQ, all udenafil groups showed a significant difference compared with the placebo group (placebo: 35.6%; 25 mg udenafil: 69.5%; 50 mg udenafil: 75.0%, 75 mg udenafil: 88.1%). In general, udenafil was well tolerated, and all treatment-related adverse effects were mild or moderate in severity.
Efficacy in other populations with ED
Dyslipidemia, one component of metabolic syndrome, is an independent risk factor for endothelial dysfunction, which is believed to be a major contributor to the development of ED.67 Several clinical trials have demonstrated favorable outcomes of vardenafil and tadalafil in the treatment of ED patients with dyslipidemia.68,69 A preclinical study using a rat model of hypercholesterolemic ED showed that chronic udenafil treatment restored the erectile function, providing a rationale for the potential use of udenafil for treating ED secondary to hypercholesterolemia.70 To date, however, no clinical data on the efficacy of udenafil for treatment of ED patients with dyslipidemia have been published.
The role of PDE5Is in penile rehabilitation following radical prostatectomy has been an active area of debate ever since several preclinical studies suggested the protective role of PDE5Is in the prevention of endothelial damage due to vascular ischemic and cavernous nerve (CN) injuries.71 A few experimental studies using animal models of CN injury have shown that chronic administration of udenafil can preserve erectile function through amelioration of penile hypoxia and fibrosis induced by CN injury, indicating the beneficial role against the pathophysiological consequences of CN injury.72,73 There have, however, been no human data published on the protective role of udenafil in men with post-radical prostatectomy ED.
Safety and tolerability of udenafil in ED
Udenafil is well tolerated with few treatment-related adverse events. Most clinical investigations of udenafil for ED report that no adverse effects were shown in electrocardiogram or laboratory tests or on vital signs such as blood pressure (BP) and pulse rate in men with ED.27–29,31,32
Treatment-related adverse effects
All clinical trials evaluating the efficacy and safety of udenafil in men with ED reported that most adverse effects were mild-to-moderate in severity, without any serious adverse event during the study period.27–33 The most common udenafil-related adverse effects include flushing and headache.19 Common adverse effects are summarized in Table 3. The adverse effects are generally related to the vasodilatory effect, a known pharmacology of PDE5Is, and the safety profile of udenafil is largely a function of inhibition of PDE5 expressed in non-penile tissue or cross-reactivity with the other PDEs. Although there has been a report of a case of anterior ischemic optic neuropathy associated with the use of udenafil,74 the overall frequencies of visual adverse effects related to udenafil were very low (0.0%~0.6%).27–33 Udenafil appears not to be associated with adverse effects such as myalgia and back pain. Although a single case of nonaneurysmal subarachnoid hemorrhage has been reported in a patient after taking 50 mg udenafil,75 it is not clear that there was a causal relationship between intake of udenafil and the hemorrhage.
  | Table 3 Summary of treatment-related adverse effects caused by udenafil |
Safety and tolerability in ED patients with hypertension
Given the vasodilatory effect of PDE5Is, special attention has been paid to the safety and tolerability of udenafil in patients who took concomitant antihypertensive agents. In patients with ED who took concomitant antihypertensive drugs, significant decrease in diastolic BP was observed in both 100 mg (standing only: from 85.3 mmHg to 81.9 mmHg) and 200 mg (both standing and sitting: from 83.7 and 85.9 mm Hg to 81.1 and 83.0 mmHg, respectively) udenafil treatment groups, without a significant change in systolic BP.32 Interestingly, a significant reduction of diastolic BP was noted in both sitting and standing positions after the treatment with placebo; thus, there were no statistically significant differences in the sitting or standing BP profiles between the groups. Furthermore, concomitant use of udenafil with antihypertensive medication did not lead to increases in the frequency of udenafil-related adverse events, such as vasodilatory symptoms (headache, flushing) or significant hypotensive symptoms (dizziness, faintness, vertigo).32
Another class of drug requiring special caution, when taken in combination with udenafil, is alpha-blocker, which can be used for treatment of BPH or hypertension. It may induce significant hypotension when administered in combination with udenafil. A recent Phase I, randomized, double-blind, double-dummy, placebo-controlled trial showed that the coadministration of udenafil and tamsulosin was not associated with clinically significant hemodynamic changes such as systolic or diastolic BP in healthy volunteers.76 There have however, been no clinical data on safety and tolerability of udenafil when administered in combination with nonselective alpha-blockers, such as doxazosin and terazosin.
Discussion
Since their introduction in the treatment of ED, PDE5Is have rapidly gained wide acceptance among clinicians and patients due to several factors, such as reliable efficacy and tolerability, an apparently favorable safety profile, and ease of use. PDE5Is improve erectile function by increasing cGMP in corpora cavernosa, leading to relaxation of cavernosal smooth muscle cells. Clinical differences among these PDE5Is are mainly related to their different pharmacokinetics, particularly time to onset and duration of action. Thus, the advent of a variety of PDE5Is and other potential agents now under clinical development with varying pharmacokinetic and other properties can provide additional options for patients and thus better suit their individual needs. In this regard, because udenafil has clinical properties of both relatively rapid onset and long duration of action, it may be a better treatment option for ED according to patient-specific sex-life profiles.
There is positive evidence that udenafil is an effective, safe, and well-tolerated treatment option in the management of ED.27–29,31,32 The recent meta-analysis by Ding et al showed that 100 mg and 200 mg udenafil could increase IIEF-EF scores by 6.69 and 8.62 points, respectively.54 These are similar to the results of the pooled analyses of 100 mg sildenafil, 20~25 mg tadalafil, and 20 mg vardenafil (9.65, 8.52, and 7.50 points, respectively), although it may be difficult to directly compare the efficacy of different PDE5Is due to limited data on comparative analyses of their efficacy in the treatment of ED.77 Also, udenafil showed increases in SEP2 and SEP3 by 28.09% and 40.23%, respectively, which was similar to the results of the pooled analysis for sildenafil (10.48% and 29.10%, respectively), tadalafil (29.70% and 48.07%, respectively) and vardenafil (29.22% and 48.13%, respectively).21,54 In terms of GAQ, udenafil showed approximately twice the positive response to GAQ compared to placebo as the other PDE5Is have shown (placebo: 24%; udenafil: 69%; sildenafil: 73%; tadalafil: 75%; vardenafil: 73%).21 In addition, the effect of udenafil on improving erectile function was maintained for up to 12 hours after a single dosage of 100 mg udenafil, suggesting the potential of allowing spontaneity in the sex-lives of patients.28
Although the response to PDE5I in ED men is lower in those with DM than in those without DM, PDE5I is a helpful treatment option for the management of ED in diabetic men. According to a recent Cochrane Review, treatment with sildenafil resulted in a 7.19-point improvement in IIEF-EF score compared to placebo.78 A multicenter, randomized, double-blind, placebo-controlled, parallel-group trial by Sáenz de Tejada et al showed that 10 mg and 20 mg tadalafil improved the IIEF-EF score by 6.3 and 7.2 points compared with placebo, respectively.79 Also, treatment with 10 mg and 20 mg tadalafil significantly increased the positive response to SEP2 (10 mg and 20 mg tadalafil: 22.2% and 22.6%, respectively) and SEP3 (10 mg and 20 mg tadalafil: 28.4% and 29.1%, respectively) compared to baseline. A Phase III, multicenter, randomized, double-blind, fixed-dose, parallel-group trial by Goldstein et al demonstrated that 10 mg and 20 mg vardenafil improved the IIEF-EF score by 4.5 and 6.4 points compared with placebo, respectively.80 Furthermore, treatment with 10 mg and 20 mg vardenafil significantly increased the positive response to SEP2 (10 mg and 20 mg vardenafil: 30.0% and 23.0%, respectively) and SEP3 (10 mg and 20 mg vardenafil: 41.0% and 39.0%, respectively) compared to baseline. In accordance with these findings, 100 mg and 200 mg udenafil showed greater increase in the IIEF-EF score by 5.8 and 7.01 points compared with placebo, respectively.29 As for the positive response to SEP2, treatment with 100 mg and 200 mg udenafil demonstrated increases of 23.84% and 31.07% compared to baseline, respectively. As for the positive response to SEP3, treatment with 100 mg and 200 mg udenafil showed increases of 45.97% and 55.56% compared to baseline, respectively. Taken together, despite the differences in the pharmacokinetics and pharmacodynamics among different PDE5Is, the clinical efficacy of udenafil is comparable to that of other PDE5Is.
Udenafil can be an effective and well-tolerated treatment option for ED men with underlying HTN who take antihypertensive drugs. According to the recent data by Paick et al,32 100 mg and 200 mg udenafil showed a greater increase in the IIEF-EF score by 6.73 and 8.06 points compared with placebo, respectively, which was comparable to the results obtained with 25~100 mg sildenafil (6.2 points), 20 mg tadalafil (8.1 points), and 5~20 mg vardenafil (8.9 points).81–83 In terms of the positive response to SEP2 and SEP3, treatment with 100 mg and 200 mg udenafil showed increases of 25.85% and 57.88% and 29.82% and 71.11% compared to baseline, respectively, which were comparable to the results obtained with 20 mg tadalafil (34.3% and 45.1%) and 5~20 mg vardenafil (32.4% and 38.0%).32,81–83 Thus, although previous studies for other PDE5Is used different primary efficacy variables, thereby making direct comparison among different PDE5Is difficult, the body of evidence suggests that the clinical efficacy of udenafil is similar to that of other PDE5Is. Also, the results of udenafil for the treatment of ED in hypertensive men on antihypertensive drugs appear to be similar to those results found for the all-comer population with ED.27,32 In terms of safety and tolerability, the treatment with udenafil in ED men on concomitant antihypertensive medication did not result in any significant changes in the BP profiles or increases in the frequency of treatment-related adverse events, such as clinically significant vasodilatory symptoms or hypotensive symptoms, compared with placebo, which was similar to the results of other PDE5Is.32,84,85
Recent evidence indicates that ED men may prefer daily use of PDE5I to on-demand use, due to greater improvements in sexual self-confidence, time concerns, and spontaneity.86 The on-demand use of PDE5I requires scheduled sexual activities, which may not be favored either by ED men or their sexual partners. Several studies have shown that chronic administration of PDE5Is in the treatment of ED can significantly improve endothelial dysfunction and structural alteration in penis.63–65 Thus, daily use of PDEIs may have the advantage of potentially curing ED by interfering with pathophysiological factors of both psychogenic (anxiety due to scheduled sexual activity) and organic (endothelial dysfunction and penile structural alteration) origin, although further studies are needed. In this context, prolonged duration of action provides an ideal pharmacokinetic profile for daily dosing of PDE5I, thereby allowing constant steady-state concentrations of the drug. Tadalafil, the only drug currently approved for daily dosing in the treatment of ED, has a t1/2 of 17.5 hours, which is longer than that of sildenafil (3~4 hours) or vardenafil (4~5 hours).19 Although the t1/2 of udenafil (9.9~12.1 hours) is shorter than that of tadalafil, udenafil appears to be have a pharmacokinetic property of relatively longer t1/2 compared to sildenafil or vardenafil.19,25 Thus, udenafil may be considered another potential option for chronic dosing of PDE5I in the treatment of ED. According to the recent study by Zhao et al,31 daily dosing of 50 mg and 75 mg udenafil for 12 weeks showed significantly greater increase in the IIEF-EF score, by 3.45 and 5.20 points, compared with placebo, respectively, which was similar to the results of daily dosing of 2.5~10 mg tadalafil for 12~24 weeks (4.9~8.8 points).64,87 In terms of the positive response to SEP2 and SEP3, treatment with 50 mg and 75 mg udenafil showed increases of 27.9% and 51.41% and 39.11% and 73.5% compared to baseline, respectively, which was similar to the data obtained for 2.5~10 mg tadalafil (24.3%~39.4% for SEP2 and 31.2%~50.1% for SEP3).
Several randomized, placebo-controlled trials have investigated the efficacy and safety of PDE5I alone or in combination with alpha-blocker in the treatment of LUTS/BPH. According to a recent meta-analysis, the use of PDE5Is alone in patients with LUTS/BPH was associated with a significant improvement of the IIEF score (−5.487) and IPSS (−2.852), but not the maximum flow rate (Qmax) compared with placebo.62 The combined use of PDE5I and alpha-1-blocker significantly improved the IIEF score (−3.6), IPSS score (−1.8), and Qmax (−1.5) compared to alpha-1-blocker alone, without an increase in significant adverse effects. Consistent with these findings, Chung et al demonstrated that combination of 100 mg udenafil with alpha-1-blockers in patients with both LUTS/BPH and ED significantly improved the IPSS (−2.8) and IIEF-5 score (−7.4), without any additional adverse effects related to coadministration.30
There has been a scarcity of studies devoted specifically to the clinical properties (onset or duration of action) of udenafil. In the study by Park et al, however, participants were requested to attempt sexual intercourse at 12 hours after udenafil or placebo dosing to evaluate the duration of action of udenafil.28 The study showed that udenafil significantly enhanced the rate of maintenance of erection and improved the IIEF-EF score compared with the placebo, indicating that the effectiveness of udenafil could be sustained for up to 12 hours after a single dosage of 100 mg. For sildenafil, several studies have suggested its sustained efficacy of 4~12 hours after dosing.88–91 Also, a prospective randomized controlled trial showed the extended duration of action of flexibly dosed vardenafil in men with ED when taken 8 hours before intercourse.92 The t1/2 of udenafil (9.9~12.1 hours) is longer than that of sildenafil (3~4 hours) or vardenafil (4~5 hours), but shorter than that of tadalafil (17.5 hours). Thus, given the difference in t1/2 among the PDE5Is, it is possible that the effectiveness of udenafil to improve erectile function may persist even longer than 12 hours after dosing. Because there has been no study directly comparing the duration of action among the various PDE5Is, further studies are warranted.
A recent systemic review and meta-analysis for oral PDE5Is in the treatment of ED demonstrated that the adverse events caused by PDE5Is were generally mild and that there were no major differences in safety profiles among various PDE5Is.21 The adverse effects of udenafil are usually similar to those of sildenafil. The frequency of myalgia and back pain caused by udenafil is substantially low. Although some publications have suggested that PDE11 inhibition could account for the back pain and myalgia reported by some men taking tadalafil,46,93 the physiological significance of PDE11 and the possible consequences of its inhibition have not yet been established. Results from most studies on udenafil support the conclusion that, when used as recommended, udenafil is safe in a broad range of patient populations, including those with hypertension.27–33
Patient preference studies on various PDE5Is may be helpful in better suiting individual needs and increasing patient satisfaction. Although most of the currently available patient preference studies have reported preference rates favorable to tadalafil, mainly because of longer duration of action that increases the patient’s freedom in their sexual life, there are some possible sources of bias.94,95 To date, there have been no data on patient preference between udenafil and the other PDE5Is. Thus, although it is very difficult to evaluate an individual preference in an objective way in daily clinical practice, the results of preference studies on various PDE5Is, including udenafil, can be useful in determining the best tailored therapy.94
To date, there have been no data on long-term efficacy and tolerability of udenafil for the treatment of ED. Also, there have been sparse data on efficacy and safety of udenafil for the treatment of ED in other ethnic populations, since all studies, with the exception of a study by Ortaç et al,33 have been performed in East Asian countries. Additionally, further research is needed to evaluate the efficacy of udenafil for ED management in difficult-to-treat populations, such as nonresponders to other PDE5Is or post-radical prostatectomy ED men. Further studies devoted specifically to clinical properties such as onset of action are needed. Although not published yet, a recent Phase III, multicenter, randomized, placebo-controlled study reported the favorable efficacy and safety of udenafil at three doses (50 mg, 100 mg, and 150 mg) in the United States.96 Thus, research on ED treatment using udenafil is still ongoing.97 Therefore, further clinical trials are needed to confirm long-term efficacy and safety of udenafil for the treatment of ED in various ethnic populations other than East Asian.
Conclusion
Udenafil has proven to have high efficacy and a favorable safety profile for a broad spectrum of ED patients, which are comparable to those of other PDE5Is. Due to the clinical properties of relatively rapid onset and long duration of action, udenafil may be a better option for ED treatment according to patient-specific sex-life patterns. Udenafil is as effective in the treatment of DM-associated ED as other PDE5Is. Recent data suggest that the concomitant use of antihypertensive drugs does not significantly affect the efficacy and safety profile. Also, due to its clinical properties, udenafil can be a daily-dosing option for the treatment of ED, as suggested by its favorable efficacy and safety profile. Further studies are still required to validate the efficacy and safety of udenafil and to complement the scientific basis for rationale, evidence-based prescription, and dosing decisions.
Disclosure
Jae-Seung Paick has served as a consultant of Mezzion Pharma Co, Ltd, since 2012. The authors report no other conflicts of interest in this work.
References
[No authors listed]. Consensus development conference statement. Impotence. December 7–9, 1992. Int J Impot Res. 1993;5(4):181–284. | |
Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381(9861):153–165. | |
Kubin M, Wagner G, Fugl-Meyer AR. Epidemiology of erectile dysfunction. Int J Impot Res. 2003;15(1):63–71. | |
Kupelian V, Shabsigh R, Araujo AB, O’Donnell AB, McKinlay JB. Erectile dysfunction as a predictor of the metabolic syndrome in aging men: results from the Massachusetts Male Aging Study. J Urol. 2006;176(1):222–226. | |
McVary KT. Erectile dysfunction and lower urinary tract symptoms secondary to BPH. Eur Urol. 2005;47(6):838–845. | |
Rosen R, Altwein J, Boyle P, et al. Lower urinary tract symptoms and male sexual dysfunction: the multinational survey of the aging male (MSAM-7). Eur Urol. 2003;44(6):637–649. | |
Billups KL. Sexual dysfunction and cardiovascular disease: integrative concepts and strategies. Am J Cardiol. 2005;96(12B):57M–61M. | |
McVary KT, Carrier S, Wessells H; Subcommittee on Smoking and Erectile Dysfunction Socioeconomic Committee, Sexual Medicine Society of North America. Smoking and erectile dysfunction: evidence based analysis. J Urol. 2001;166(5):1624–1632. | |
Brown JS, Wessells H, Chancellor MB, et al. Urologic complications of diabetes. Diabetes Care. 2005;28(1):177–185. | |
McVary KT. Clinical practice. Erectile dysfunction. N Engl J Med. 2007;357(24):2472–2481. | |
Kapur V, Chien CV, Fuess JE, Schwarz ER. The relationship between erectile dysfunction and cardiovascular disease. Part II: The role of PDE-5 inhibition in sexual dysfunction and cardiovascular disease. Rev Cardiovasc Med. 2008;9(3):187–195. | |
Miner MM. Erectile dysfunction and the “window of curability”: a harbinger of cardiovascular events. Mayo Clin Proc. 2009;84(2):102–104. | |
Shabsigh R, Shah M, Sand M. Erectile dysfunction and men’s health: developing a comorbidity risk calculator. J Sex Med. 2008;5(5):1237–1243. | |
Montorsi P, Ravagnani PM, Galli S, et al. Association between erectile dysfunction and coronary artery disease. Role of coronary clinical presentation and extent of coronary vessels involvement: the COBRA trial. Eur Heart J. 2006;27(22):2632–2639. | |
Hodges LD, Kirby M, Solanki J, O’Donnell J, Brodie DA. The temporal relationship between erectile dysfunction and cardiovascular disease. Int J Clin Pract. 2007;61(12):2019–2025. | |
Wessells H, Joyce GF, Wise M, Wilt TJ. Erectile dysfunction. J Urol. 2007;177(5):1675–1681. | |
Montague D, Jarow J, Broderick G, et al; Erectile Dysfunction Guideline Update Panel. Chapter 1: The management of erectile dysfunction: an AUA update. J Urol. 2005;174(1):230–239. | |
Montorsi F, Adaikan G, Becher E, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med. 2010;7(11):3572–3588. | |
Bruzziches R, Francomano D, Gareri P, Lenzi A, Aversa A. An update on pharmacological treatment of erectile dysfunction with phosphodiesterase type 5 inhibitors. Expert Opin Pharmacother. 2013;14(10):1333–1344. | |
Bell AS, Palmer MJ. Novel phosphodiesterase type 5 modulators: a patent survey (2008–2010). Expert Opin Ther Pat. 2011;21(10):1631–1641. | |
Yuan J, Zhang R, Yang Z, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol. 2013;63(5):902–912. | |
Glina S, Toscano I, Gomatzky C, et al. Efficacy and tolerability of lodenafil carbonate for oral therapy in erectile dysfunction: a phase II clinical trial. J Sex Med. 2009;6(2):553–557. | |
Glina S, Fonseca GN, Bertero EB, et al. Efficacy and tolerability of lodenafil carbonate for oral therapy of erectile dysfunction: a phase III clinical trial. J Sex Med. 2010;7(5):1928–1936. | |
Hatzimouratidis K, Hatzichristou DG. Looking to the future for erectile dysfunction therapies. Drugs. 2008;68(2):231–250. | |
Palit V, Eardley I. An update on new oral PDE5 inhibitors for the treatment of erectile dysfunction. Nat Rev Urol. 2010;7(11):603–609. | |
Doh H, Shin CY, Son M, et al. Mechanism of erectogenic effect of the selective phosphodiesterase type 5 inhibitor, DA-8159. Arch Pharm Res. 2002;25(6):873–878. | |
Paick JS, Kim SW, Yang DY, et al. The efficacy and safety of udenafil, a new selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction. J Sex Med. 2008;5(4):946–953. | |
Park HJ, Park JK, Park K, Min K, Park NC. Efficacy of udenafil for the treatment of erectile dysfunction up to 12 hours after dosing: a randomized placebo-controlled trial. J Sex Med. 2010;7(6):2209–2216. | |
Moon DG, Yang DY, Lee CH, et al. A therapeutic confirmatory study to assess the safety and efficacy of Zydena (udenafil) for the treatment of erectile dysfunction in male patients with diabetes mellitus. J Sex Med. 2011;8(7):2048–2061. | |
Chung BH, Lee JY, Lee SH, Yoo SJ, Lee SW, Oh CY. Safety and efficacy of the simultaneous administration of udenafil and an alpha-blocker in men with erectile dysfunction concomitant with BPH/LUTS. Int J Impot Res. 2009;21(2):122–128. | |
Zhao C, Kim SW, Yang DY, et al. Efficacy and safety of once-daily dosing of udenafil in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2011;60(2):380–387. | |
Paick JS, Kim SW, Park YK, et al. The efficacy and safety of udenafil [Zydena] for the treatment of erectile dysfunction in hypertensive men taking concomitant antihypertensive agents. J Sex Med. 2009;6(11):3166–3176. | |
Ortaç M, Çayan S, Çalişkan MK, et al. Efficacy and tolerability of udenafil in Turkish men with erectile dysfunction of psychogenic and organic aetiology: a randomized, double-blind, placebo-controlled study. Andrology. 2013;1(4):549–555. | |
Corbin JD, Francis SH. Pharmacology of phosphodiesterase-5 inhibitors. Int J Clin Pract. 2002;56(6):453–459. | |
Lue TF. Erectile dysfunction. N Engl J Med. 2000;342(24):1802–1813. | |
Kim NN. Phosphodiesterase type 5 inhibitors: a biochemical and clinical correlation survey. Int J Impot Res. 2003;15 Suppl 5:S13–S19. | |
Kouvelas D, Goulas A, Papazisis G, Sardeli C, Pourzitaki C. PDE5 inhibitors: in vitro and in vivo pharmacological profile. Curr Pharm Des. 2009;15(30):3464–3475. | |
Kim BH, Lim HS, Chung JY, et al. Safety, tolerability and pharmacokinetics of udenafil, a novel PDE-5 inhibitor, in healthy young Korean subjects. Br J Clin Pharmacol. 2008;65(6):848–854. | |
Shim HJ, Kim YC, Park KJ, et al. Pharmacokinetics of DA-8159, a new erectogenic, after intravenous and oral administration to rats: hepatic and intestinal first-pass effects. J Pharm Sci. 2003;92(11):2185–2195. | |
Walker DK, Ackland MJ, James GC, et al. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29(3):297–310. | |
Shim HJ, Kim YC, Lee JH, et al. Interspecies pharmacokinetic scaling of DA-8159, a new erectogenic, in mice, rats, rabbits and dogs, and prediction of human pharmacokinetics. Biopharm Drug Dispos. 2005;26(7):269–277. | |
Kim TE, Kim BH, Kim JR, et al. Effect of food on the pharmacokinetics of the oral phosphodiesterase 5 inhibitor udenafil for the treatment of erectile dysfunction. Br J Clin Pharmacol. 2009;68(1):43–46. | |
Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phosphodiesterase families and the effect of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol. 1999;83(5A):3C–12C. | |
Francis SH, Turko IV, Corbin JD. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog Nucleic Acid Res Mol Biol. 2001;65:1–52. | |
Bischoff E. Potency, selectivity, and consequences of nonselectivity of PDE inhibition. Int J Impot Res. 2004;16(Suppl 1):S11–S14. | |
Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil – review of the literature. Eur J Med Res. 2002;7(10):435–446. | |
Ku HY, Ahn HJ, Seo KA, et al. The contributions of cytochromes P450 3A4 and 3A5 to the metabolism of the phosphodiesterase type 5 inhibitors sildenafil, udenafil, and vardenafil. Drug Metab Dispos. 2008;36(6):986–990. | |
Ji HY, Lee HW, Kim HH, et al. Role of human cytochrome P450 3A4 in the metabolism of DA-8159, a new erectogenic. Xenobiotica. 2004;34(11–12):973–982. | |
Kim J, Kim SJ, Ji HY, et al. Simultaneous determination of a new phosphodiesterase-5 inhibitor DA-8159 and its active metabolite in human plasma by high performance liquid chromatography with tandem mass spectrometry. Chromatographia. 2003;57(7–8):447–450. | |
Amakye D, Ward J, Bryson S, Han K. DA-8159-phase I studies to investigate the safety and pharmacokinetics in healthy male Caucasian subjects. Clin Pharmacol Ther. 2004;75:86. | |
Hatzichristou D, Amar E, Eardley I, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010;57(5):804–814. | |
Oh TY, Kang KK, Ahn BO, Yoo M, Kim WB. Erectogenic effect of the selective phosphodiesterase type 5 inhibitor, DA-8159. Arch Pharm Res. 2000;23(5):471–476. | |
Salem EA, Kendirci M, Hellstrom WJ. Udenafil, a long-acting PDE5 inhibitor for erectile dysfunction. Curr Opin Investig Drugs. 2006;7(7):661–669. | |
Ding H, Du W, Wang H, et al. Efficacy and safety of udenafil for erectile dysfunction: a meta-analysis of randomized controlled trials. Urology. 2012;80(1):134–139. | |
Thorve VS, Kshirsagar AD, Vyawahare NS, Joshi VS, Ingale KG, Mohite RJ. Diabetes-induced erectile dysfunction: epidemiology, pathophysiology and management. J Diabetes Complications. 2011;25(2):129–136. | |
Kang KK, Choi SM, Ahn GJ, Kwon JW, Kim WB. The effect of DA-8159 on corpus cavernosal smooth muscle relaxation and penile erection in diabetic rabbits. Urol Res. 2004;32(2):107–111. | |
Ahn GJ, Sohn YS, Kang KK, et al. The effect of PDE5 inhibition on the erectile function in streptozotocin-induced diabetic rats. Int J Impot Res. 2005;17(2):134–141. | |
Ahn GJ, Yu JY, Choi SM, et al. Chronic administration of phosphodiesterase 5 inhibitor improves erectile and endothelial function in a rat model of diabetes. Int J Androl. 2005;28(5):260–266. | |
Ahn GJ, Chung HK, Lee CH, Kang KK, Ahn BO. Increased expression of the nitric oxide synthase gene and protein in corpus cavernosum by repeated dosing of udenafil in a rat model of chemical diabetogenesis. Asian J Androl. 2009;11(4):435–442. | |
Cho SY, Park K, Paick JS, Kim SW. Change of erectile function and responsiveness to phosphodiesterase type 5 inhibitors at different stages of streptozotocin-induced diabetes in rats. J Sex Med. 2011;8(5):1352–1361. | |
Martínez-Salamanca JI, Carballido J, Eardley I, et al. Phosphodiesterase type 5 inhibitors in the management of non-neurogenic male lower urinary tract symptoms: critical analysis of current evidence. Eur Urol. 2011;60(3):527–535. | |
Gacci M, Corona G, Salvi M, et al. A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with α-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur Urol. 2012;61(5):994–1003. | |
Behr-Roussel D, Gorny D, Mevel K, et al. Chronic sildenafil improves erectile function and endothelium-dependent cavernosal relaxations in rats: lack of tachyphylaxis. Eur Urol. 2005;47(1):87–91. | |
Porst H, Giuliano F, Glina S, et al. Evaluation of the efficacy and safety of once-a-day dosing of tadalafil 5 mg and 10 mg in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2006;50(2):351–359. | |
Porst H, Rajfer J, Casabé A, et al. Long-term safety and efficacy of tadalafil 5 mg dosed once daily in men with erectile dysfunction. J Sex Med. 2008;5(9):2160–2169. | |
Bella AJ, Deyoung LX, Al-Numi M, Brock GB. Daily administration of phosphodiesterase type 5 inhibitors for urological and nonurological indications. Eur Urol. 2007;52(4):990–1005. | |
Miner M, Billups KL. Erectile dysfunction and dyslipidemia: relevance and role of phosphodiesterase type-5 inhibitors and statins. J Sex Med. 2008;5(5):1066–1078. | |
Lewis RW, Sadovsky R, Eardley I, et al. The efficacy of tadalafil in clinical populations. J Sex Med. 2005;2(4):517–531. | |
Miner M, Gilderman L, Bailen J, et al. Vardenafil in men with stable statin therapy and dyslipidemia. J Sex Med. 2008;5(6):1455–1467. | |
Kang KK, Yu JY, Yoo M, Kwon JW. The effect of DA-8159, a novel PDE5 inhibitor, on erectile function in the rat model of hypercholesterolemic erectile dysfunction. Int J Impot Res. 2005;17(5):409–416. | |
Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, Mulhall JP. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur Urol. 2009;55(2):334–347. | |
Lee CH, Kim HS, Goo MJ, et al. Chronic administration of udenafil, a selective phosphodiesterase type 5 inhibitor, promotes erectile function recovery in an animal model of bilateral cavernous nerve crush injury. J Sex Med. 2011;8(5):1330–1340. | |
Lee CH, Shin JH, Ahn GJ, Kang KK, Ahn BO, Yoo M. Udenafil enhances the recovery of erectile function and ameliorates the pathophysiological consequences of cavernous nerve resection. J Sex Med. 2010;7(7):2564–2571. | |
Kim I, Kim D. Anterior ischemic optic neuropathy associated with udenafil. Korean J Ophthalmol. 2012;26(3):235–238. | |
Bae EK, Ahn JH, Park JJ. Nonaneurysmal subarachnoid hemorrhage after udenafil intake. J Stroke Cerebrovasc Dis. Epub May 2, 2013. | |
Kim MG, Kim JR, Kim BH, et al. The effect of udenafil on the hemodynamics of healthy male volunteers administered tamsulosin. Curr Med Res Opin. 2013;29(6):685–693. | |
Berner MM, Kriston L, Harms A. Efficacy of PDE-5-inhibitors for erectile dysfunction. A comparative meta-analysis of fixed-dose regimen randomized controlled trials administering the International Index of Erectile Function in broad-spectrum populations. Int J Impot Res. 2006;18(3):229–235. | |
Vardi M, Nini A. Phosphodiesterase inhibitors for erectile dysfunction in patients with diabetes mellitus. Cochrane Database Syst Rev. 2007;(1):CD002187. | |
Sáenz de Tejada I, Anglin G, Knight JR, Emmick JT. Effects of tadalafil on erectile dysfunction in men with diabetes. Diabetes Care. 2002;25(12):2159–2164. | |
Goldstein I, Young JM, Fischer J, Bangerter K, Segerson T, Taylor T. Vardenafil, a new phosphodiesterase type 5 inhibitor, in the treatment of erectile dysfunction in men with diabetes: a multicenter double-blind placebo-controlled fixed-dose study. Diabetes Care. 2003;26(3):777–783. | |
Blonde L. Sildenafil citrate for erectile dysfunction in men with diabetes and cardiovascular risk factors: a retrospective analysis of pooled data from placebo-controlled trials. Curr Med Res Opin. 2006;22(11):2111–2120. | |
Kloner RA, Sadovsky R, Johnson EG, Mo D, Ahuja S. Efficacy of tadalafil in the treatment of erectile dysfunction in hypertensive men on concomitant thiazide diuretic therapy. Int J Impot Res. 2005;17(5):450–454. | |
Shabsigh R, Duval S, Shah M, Regan TS, Juhasz M, Veltry LG. Efficacy of vardenafil for the treatment of erectile dysfunction in men with hypertension: a meta-analysis of clinical trial data. Curr Med Res Opin. 2007;23(10):2453–2460. | |
Kloner RA, Brown M, Prisant LM, Collins M. Effect of sildenafil in patients with erectile dysfunction taking antihypertensive therapy. Sildenafil Study Group. Am J Hypertens. 2001;14(1):70–73. | |
van Ahlen H, Wahle K, Kupper W, Yassin A, Reblin T, Neureither M. Safety and efficacy of vardenafil, a selective phosphodiesterase 5 inhibitor, in patients with erectile dysfunction and arterial hypertension treated with multiple antihypertensives. J Sex Med. 2005;2(6):856–864. | |
Rubio-Aurioles E, Porst H, Kim ED, et al. A randomized open-label trial with a crossover comparison of sexual self-confidence and other treatment outcomes following tadalafil once a day vs tadalafil or sildenafil on-demand in men with erectile dysfunction. J Sex Med. 2012;9(5):1418–1429. | |
Rajfer J, Aliotta PJ, Steidle CP, Fitch WP 3rd, Zhao Y, Yu A. Tadalafil dosed once a day in men with erectile dysfunction: a randomized, double-blind, placebo-controlled study in the US. Int J Impot Res. 2007;19(1):95–103. | |
Giuliano F, Vicaut E, Jeanpetit Y. Impact of the pharmacokinetic properties of PDE5 inhibitors on the dose/sexual intercourse interval. Prog Urol. 2008;18:536–542. | |
Eardley I, Ellis P, Boolell M, Wulff M. Onset and duration of action of sildenafil for the treatment of erectile dysfunction. Br J Clin Pharmacol. 2002;53 Suppl 1:61S–65S. | |
Zinner N. Do food and dose timing affect the efficacy of sildenafil? A randomized placebo-controlled study. J Sex Med. 2007;4:137–144. | |
Moncada I, Jara J, Subirá D, Castaño I, Hernández C. Efficacy of sildenafil citrate at 12 hours after dosing: Re-exploring the therapeutic window. Eur Urol. 2004;46:357–360. | |
Porst H, Sharlip ID, Hatzichristou D, et al. Extended duration of efficacy of vardenafil when taken 8 hours before intercourse: a randomized, double-blind, placebo-controlled study. Eur Urol. 2006;50:1086–1095. | |
Meuleman EJ. Review of tadalafil in the treatment of erectile dysfunction. Expert Opin Pharmacother. 2003;4:2049–2056. | |
Corona G, Mondaini N, Ungar A, Razzoli E, Rossi A, Fusco F. Phosphodiesterase type 5 (PDE5) inhibitors in erectile dysfunction: the proper drug for the proper patient. J Sex Med. 2011;8:3418–3432. | |
Mirone V, Fusco F, Rossi A, Sicuteri R, Montorsi F. Tadalafil and vardenafil vs sildenafil: a review of patient-preference studies. BJU Int. 2009;103:1212–1217. | |
Warner Chilcott. Treatment of Erectile Dysfunction II. Available from: http://clinicaltrials.gov/ct2/show/NCT01037218. NLM identifier: NCT01037218. Accessed November 18, 2013. | |
Warner Chilcott. Treatment of Erectile Dysfunction - Long Term Safety and Efficacy. Available from: http://clinicaltrials.gov/ct2/show/NCT01065012. NLM identifier: NCT01065012. Accessed November 18, 2013. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


