Back to Journals » Journal of Asthma and Allergy » Volume 14
Type 2-High Severe Asthma with and without Bronchiectasis: A Prospective Observational Multicentre Study
Authors Crimi C , Campisi R, Nolasco S , Ferri S , Cacopardo G, Impellizzeri P, Pistorio MP, Fagone E, Pelaia C , Heffler E, Crimi N
Received 2 August 2021
Accepted for publication 26 October 2021
Published 30 November 2021 Volume 2021:14 Pages 1441—1452
DOI https://doi.org/10.2147/JAA.S332245
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Claudia Crimi,1 Raffaele Campisi,1 Santi Nolasco,2 Sebastian Ferri,3,4 Giulia Cacopardo,5 Pietro Impellizzeri,2 Maria Provvidenza Pistorio,2 Evelina Fagone,2 Corrado Pelaia,6 Enrico Heffler,3,4 Nunzio Crimi1,2
1Respiratory Medicine Unit, A.O.U. Policlinico “G. Rodolico - San Marco”, Catania, Italy; 2Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy; 3Personalized Medicine, Asthma and Allergy - IRCCS Humanitas Research Hospital, Rozzano, Italy; 4Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, MI, Italy; 5Respiratory Intensive Care Unit, ARNAS Civico General Hospital, Palermo, Italy; 6Department of Medical and Surgical Sciences, University “Magna Graecia”, Catanzaro, Italy
Correspondence: Claudia Crimi
Respiratory Medicine Unit, A.O.U. Policlinico “G. Rodolico - San Marco”, Catania, Italy
Email [email protected]
Introduction: Type 2-high severe asthma (T2-SA) is often associated with several comorbidities. To this extent, the coexistence of T2-SA and bronchiectasis (BE) is considered an emerging phenotype.
Methods: We performed a prospective observational multicentre study, including T2-SA patients. Chest HRCT confirmed the presence of BE. Data on exacerbations, pulmonary function, Asthma Control Test (ACT), chronic mucus hypersecretion (CMH), chronic rhinosinusitis (CRS), oral corticosteroid (OCS) dosage, eosinophils in peripheral blood and FeNO were recorded. The Bhalla score was used for radiological assessment of T2-SA+BE patients and the Bronchiectasis Severity Index (BSI) was calculated.
Results: A total of 113 patients (mean age 55 ± 11 years, 59.3% female) were enrolled. Co-presence of BE was confirmed in 50/113 (44.2%) patients who identified the T2-SA+BE group. CRS and CRSwNP were more prevalent in T2-SA+BE vs T2-SA [respectively, 42/50 (84%) vs 37/63 (58.7%), p = 0.004 and 27/50 (54%) vs 27/63 (42.9%), p = 0.0165]. Furthermore, T2-SA+BE patients reported more CMH compared to T2-SA [29/50 (58%) vs 15/63 (23.8%), p = 0.0004], were more frequently on chronic OCSs intake [28/50 (56%) vs 22/63 (34.9%), p = 0.0357] and experienced more exacerbations/year [10 (4– 12) vs 6 (4– 12), p = 0.0487]. In a multivariate logistic regression model, the presence of CRS, CMH and daily OCS intake were associated with BE presence with a 78% (95% CI: 69– 88) accuracy. Median Bhalla score was 18.3 (16– 20) (Mild radiological severity). Median BSI was 6 (4– 8) and only 6/50 (12%) had a BSI score ≥ 9. Significant inverse linear relationship between BSI and ACT (r = − 0.6095, p < 0.0001), FEV1% (r = − 0.3297, p = 0.0353) and FEV1 mL (r = − 0.4339, p = 0.0046) were found.
Conclusion: Type 2 inflammation could have a causative role in BE development. Chest HRCT is mandatory when a diagnosis of T2-SA is made, especially in presence of CRS, CMH and chronic OCS intake. Early BE detection may be crucial to improve T2-SA patients’ outcomes.
Keywords: type 2 inflammation, severe asthma, bronchiectasis, chest-CT scan, phenotype
Introduction
Severe asthma (SA) is a chronic respiratory disease characterized by bronchial inflammation and airway hyperresponsiveness, inciting daily symptoms and triggering unpredictable acute exacerbations.1,2 The prevalence of SA varies between 3.7% and 10% of the asthma framework, with a high burden on the health-care system.3–5
Recent clinical and translational studies have enabled endotyping, allowing to dichotomize asthma according to levels of type 2 inflammation into “type 2 (T2)-high” and “T2-low” subtypes. Type 2 inflammation, mediated by cytokines such as interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13), occurs in approximately 70% of asthmatic patients.6 Moreover, several comorbidities are associated with type 2-high severe asthma (T2-SA), making their recognition and treatment crucial to improve disease control and outcomes.7,8
Recently, the coexistence of T2-SA and bronchiectasis (BE) has been considered an emerging phenotype of severe and difficult-to-treat asthma9,10 associated with a worse prognosis of the disease,11 representing one of the “treatable traits” highlighted in the latest asthma recommendation.4,12 Indeed, the prevalence of BE is significantly higher in SA (range 24–47%) than in mild-to-moderate asthma (3%).10,11,13
It has been hypothesized that the long-term eosinophils-mediated inflammatory damage, promoted by T2-inflammation, induces tissue changes and airway remodeling, playing a primary role in the pathogenesis of BE.14,15 Nevertheless, this pathogenetic mechanism has not been fully elucidated yet, and the clinical and radiological characteristics of this phenotype have not been clearly defined.
In this study we aimed to:
1) describe the clinical and functional differences of T2-SA patients with and without BE;
2) evaluate the radiological characteristics and clinical severity of BE;
3) specifically assess the clinical impact of BE on T2-SA according to their severity.
Materials and Methods
Study Design and Patient Population
This prospective, observational, multicentre study was conducted in two severe asthma-dedicated outpatient services in Italy: (1) Respiratory Medicine Unit – A.O.U. Policlinico “G. Rodolico – San Marco”, Catania; (2) Personalized Medicine, Asthma and Allergy, Humanitas Research Center IRCCS, Rozzano, Italy between December 2018 and December 2019. Consecutive patients with a T2-SA, defined by a blood eosinophils count ≥150 cells/μL and/or FeNO ≥20 ppb and/or sputum eosinophils ≥2%, and/or asthma clinically allergen-driven and/or need oral corticosteroids (OCS) for maintenance were included.4 We excluded individuals with immunological deficits, allergic bronchopulmonary aspergillosis (ABPA), alpha-1-deficiency, vasculitis, cystic fibrosis, primary ciliary dyskinesia, T2-Low SA, and with previous pneumonia episodes, which could also have the presence of BE.
Asthma diagnosis was assessed and confirmed with a positive bronchial reversibility test (defined as a change in FEV1 of at least 12% and 200 mL from the pre-bronchodilator value, measured 15 minutes after the inhalation of salbutamol 400 mcg).4 SA diagnosis was defined according to the European Respiratory Society/American Thoracic Society (ERS/ATS) guidelines.2 T2-SA assessment criteria were in agreement with Global Initiative for Asthma (GINA) 2019 report.4
As a routine workup, serum IgA-IgG-IgM, IgG subclasses and lymphocyte typing were tested to assess immunological status and testing for allergic bronchopulmonary aspergillosis were performed as suggested by the European Respiratory Society guidelines on bronchiectasis.12 Sweat testing was performed only in patients showing diffuse bronchiectasis at the chest CT scan and with clinical history suspicious for cystic fibrosis.16
This study adhered to the Declaration of Helsinki and was approved by the “Catania 1” Ethics Committee of the A.O.U. Policlinico “G. Rodolico – San Marco” in Catania, Italy (Protocol Number 108/2018/PO). Written informed consent was obtained from all patients.
Data Collection
An established database of pertinent variables was created. Social and demographic data (age, sex, BMI), past medical history, smoking status, and asthmatic profiles [age at onset, sensitization to perennial aeroallergens, pulmonary function, blood analysis for eosinophils count, total IgE level, fractional exhaled nitric oxide (FeNO) value, asthma control test (ACT), sputum characteristic and microbiology, number of asthma exacerbations requiring oral or systemic corticosteroids treatment for at least 3 days and/or emergency visits/hospitalizations in the last 12 months] were collected. Information about the ongoing treatments [inhaled corticosteroids (ICS), long-acting beta-agonists (LABAs), long-acting muscarinic receptor antagonists (LAMAs), leukotriene modifiers and oral corticosteroids] was also reported. Furthermore, available information about other comorbidities (nasal polyps, rhinosinusitis, GERD, obesity) was included. BE diagnosis was documented by HRCT scan. Severity of BE was evaluated from a clinical and radiological point of view, according to the Bronchiectasis Severity Index (BSI) and Bhalla score, respectively.
Measurements
Asthma Exacerbations
Severe asthma exacerbations were defined as disease worsening requiring hospitalization or ≥3 days of treatment with systemic corticosteroids (or a doubling of prednisone equivalent dose if already on OCS).17
Pulmonary Function
Pulmonary function tests were performed according to ERS/ATS guidelines. Forced Vital Capacity (FVC) and Forced Expiratory Volume in 1 second (FEV1) were measured using a spirometer (Sensormedics, Milan, Italy). The best value of three consecutive manoeuvres was expressed as the percentage of the normal value. Bronchodilator reversibility testing was performed.18
Asthma Control Test
Levels of asthma control were assessed using the ACT,19,20 a short, simple five-point scoring system questionnaire eliciting a patient’s perception of symptoms control. An overall score ≥20 means well-controlled asthma, whereas a score ≤19 reflects poor asthma control.
FeNO Measurements
The fraction of exhaled nitric oxide was performed according to ERS/ATS guidelines and was measured using the Niox ® device. FeNO levels were considered normal if FeNO <25 parts per billion (ppb) and elevated if FeNO >50 ppb, as recommended.21
Chronic Mucus Hypersecretion
Symptoms of chronic mucus hypersecretion (CMH), defined as cough and sputum production on most days for at least three months a year for at least two consecutive years, were recorded. Mucus was defined as mucous if its colour was white to light yellow, muco-purulent if yellow to light green and purulent if green or dark green.22
Chronic Rhinosinusitis with Nasal Polyps
The diagnosis of chronic rhinosinusitis (CRS), according to the European position paper on rhinosinusitis and nasal polyps (EPOS) 2020,23 was formulated on symptoms (nasal congestion, nasal discharge, facial pain/pressure, reduction/loss of smell), present for a minimum of 12 weeks. In case of objective confirmation of the presence of polyps with sinus computed tomography scan or nasal endoscopy, the diagnosis of chronic rhinosinusitis with nasal polyps (CRSwNP) was made.23
Radiological Assessment of Bronchiectasis
All patients underwent High-Resolution Chest Tomography (HRCT), performed during stable conditions. Images were obtained during a complete inspiration at 1-mm collimation and 10-mm intervals, from lung apices to bases, in the supine position. Two independent radiologists, blinded to research findings, evaluated the images. The presence of BE was reported according to the following radiological criteria: 1) internal diameter of the bronchus greater than that of the adjacent pulmonary artery; 2) lack of tapering of the bronchial lumen toward the periphery. Bronchiectasis only visible in a single pulmonary segment was not considered.24–26
The radiological severity and extension were assessed with the Bhalla score.27 The Bhalla score range goes from 0 to 25, with a lower score indicating more radiologically severe bronchiectasis. The total was calculated by scoring each of the nine categories and then subtracting from 25. This score was subdivided into mild (16–25), moderate (9–15) and severe (0–8).
We also assessed the number of lobes (the lingula was considered a separate lobe) with bronchiectasis and their prevalent localization. The BE distribution patterns were categorized as central, peripheral or mixed. The type of BE was reported according to the Reid classification.28
Severity Assessment of Bronchiectasis
The Bronchiectasis Severity Index (BSI) is a multidimensional scoring system that has been recently introduced to better understand the disease. The BSI has shown to be an excellent predictor of mortality and hospital admissions and a predictive index of exacerbations. It was calculated in patients affected by bronchiectasis, combining body mass index (BMI), FEV1% predicted, hospital admission in the past two years, number of exacerbations in the previous year, modified Medical Research Council (mMRC) breathlessness score, radiological severity (>3 lobes involved or cystic changes), presence of pseudomonas and chronic bacteria colonization.
Microbiology testing was performed on patients’ spontaneous early morning sputum samples and analysed for bacterial, fungal and mycobacterial cultures. We collected sputum at least on 2 occasions, with a minimum of 3 months for 1 year, to assess the possible presence of chronic colonization.29,30 Subjects unable to provide sputum samples due to the absence of a productive cough were classified as not having chronic infection for analysis purposes. According to the BSI overall score (range from 0 to 26), bronchiectasis was defined as mild (BSI = 0–4 points), moderate (BSI = 5–8 points), or severe (BSI ≥ 9 points).31
Statistical Analysis
Statistical analysis and figures were generated using Prism version 9.0.2 (GraphPad Software Inc). Categorical variables were stated as numbers (n) and percentages (%). Normally distributed continuous variables were expressed as mean ± standard deviation (SD). Median and interquartile range (IQR) were used in case of continuous non-parametric variables. Agreement between the radiologists was estimated using the kappa Intraclass Correlation Coefficient (with 95% CIs). Fisher exact or Chi-Square tests were used for comparisons of categorical variables. The normality of data distribution was checked using the Anderson-Darling and Kolmogorov–Smirnov tests. Mann–Whitney U-test or Student’s t-test were used for continuous data, respectively, when appropriate. Linear regressions analysis with Spearman’s rank coefficient were used to evaluate the correlation between variables. Multivariable analyses were performed using a logistic regression model (with 95% CIs). Variable selection using subsampling methodology was conducted to construct the optimal model. A p-value <0.05 (two-sided) was considered statistically significant.
Results
Characteristics and Differences Between Type-2 High Severe Asthma Patients with and without Bronchiectasis
A total of 195 SA patients were screened. Thirteen did not sign the informed consent. Sixty-seven were excluded after the clinical assessment. One-hundred-fifteen underwent a high-resolution chest CT scan. Two patients were suspected of cystic fibrosis. Five patients had a supportive history of primary cilia dyskinesia and were addressed to centers with expertise in the field to confirm or exclude the diagnosis. One patient resulted positive to the genetic tests for cystic fibrosis and one showed defects in cilia movements compatible with primary cilia dyskinesia (Figure 1). One-hundred thirteen patients met the inclusion criteria and were included in the analysis. The mean age of the total cohort was 55 ± 11 years. Mean age at onset was 32.3 ± 15.5 years. The majority of patients were female (67/113, 59.3%). All patients (113/113, 100%) were prescribed high-dose ICS/LABA and 69% (78/113) were on add-on therapy with LAMA. Eighty-two (72.6%) had positive skin prick tests. Median eosinophil count in the peripheral blood was 500 (340–900) cells/μL, with 82.3% (93/113) showing ≥300 eosinophils/μL. Fifty out of 113 (44.2%) showed co-presence of bronchiectasis at chest HRCT and were identified as T2-SA+BE group. Baseline and clinical characteristics of patients of each group (T2-SA alone and T2-SA+BE) are reported in Table 1. No statistically significant differences in terms of ACT, pre-bronchodilator FEV1%, FEV1 mL, FEV1/FVC%, total IgE, FeNO and blood eosinophil were noted between the groups. Chronic rhinosinusitis (CRS) and chronic rhinosinusitis with nasal polyps (CRSwNP) were significantly more prevalent in T2-SA+BE patients vs T2-SA without BE [respectively, 42/50 (84%) vs 37/63 (58.7%), p = 0.004 and 27/50 (54%) vs 27/63 (42.9%), p = 0.0165] (Figure 2A and B). Twenty-nine out of 50 (58%) in the T2-SA+BE group, compared to 15/63 (23.8%) T2-SA alone patients (p = 0.0004), reported CMH, in particular of muco-purulent [18/50 (36%) vs 10/63 (15.9%), p = 0.0167] and purulent type [8/50 (16%) vs 1/63 (1.6%), p = 0.01] (Figure 2C). A greater proportion of patients with bronchiectasis was on maintenance OCS [28/50 (56%) vs 22/63 (34.9%), p = 0.0357] (Figure 2D). Furthermore, T2-SA+BE patients reported a higher number of asthma exacerbations in the previous year [10 (4–12) vs 6 (4–12), p = 0.0487] (Figure 2E).
 |
Table 1 Clinical and Functional Characteristic of Type-2 High Severe Asthma Patients with and without Bronchiectasis |
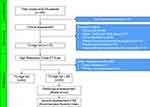 |
Figure 1 Study flow chart. Abbreviations: SA, severe asthma; BE, bronchiectasis. |
A multivariate logistic analysis was conducted based on the differences between the groups, and the optimal model included the following predictors that are strongly associated with comorbid BE in T2-SA: the presence of CRS, chronic sputum production, and daily OCS intake. The resulting accuracy of this model using the area under the curve (AUC) of the receiver operating characteristics (ROC) was 78% (95% CI: 69–88), with a specificity of 76% and a sensibility of 74% (Figure 2F).
Radiological Features of Bronchiectasis in Type-2 High Severe Asthma
HRCT chest features of T2-SA+BE patients are listed in Table 2. The kappa coefficient between the chest radiologists was 0.92 (95% CI: 0.84–0.99) for assessing the Bhalla score [median 18.3 (16–20)]. All the patients had mild (38/50, 76%) or moderate (12/50, 24%) bronchiectasis, in terms of radiological severity, with a median of 3 (2–4) lobes affected and predominantly periphery (72%) and lower fields involvement (Figure 3A and B). Bhalla sub-items prevalence across T2-SA+BE is presented in Figure 4.
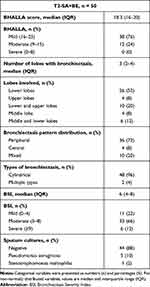 |
Table 2 Radiological and Microbiological Features in Patients with T2-SA+BE |
 |
Figure 3 Bronchiectasis lobes involvement (A) and distribution pattern (B). |
No significant correlation was found between Bhalla score and years of asthma, asthma exacerbations, ACT score, FEV1%, FEV1/FVC%, peripheral blood eosinophil count, total IgE, Vitamin D, Alpha-1 antitrypsin and FeNO.
Clinical Impact of Bronchiectasis in Type-2 High Severe Asthma According to BSI
In T2-SA+BE patients, the median BSI was 6 (4–8) (Table 2). Only 6 out of 50 (12%) had a BSI score ≥9, indicative of clinically severe bronchiectasis. Sputum microbiological examination showed chronic Pseudomonas aeruginosa colonization in 5 patients (10%) and chronic Stenotrophomonas maltophilia colonization in 1 patient (2%) out of 50.
We found a significant inverse linear relationship between BSI and ACT score (Figure 5A; r = −0.6095, p < 0.0001), FEV1% (Figure 5B; r = −0.3297, p = 0.0353) and FEV1 mL (Figure 5C; r = −0.4339, p = 0.0046) respectively. No significant correlation was found between BSI score and duration of asthma, asthma exacerbations, FEV1/FVC%, peripheral blood eosinophil count, total IgE, Vitamin D, alpha-1 antitrypsin and FeNO.
 |
Figure 5 Correlations between BSI, ACT (A), FEV1% (B) and FEV1 mL (C). Abbreviations: BSI, Bronchiectasis Severity Index; ACT, Asthma Control Test; FEV1, forced expiratory volume in 1 second. |
Discussion
In the present prospective observational study, we compared the clinical and functional differences of T2-SA patients with and without BE. We reported novel findings assessing the clinical and radiological characteristics of patients with T2-SA and coexisting BE, using the BSI and Bhalla score.27 The prevalence of BE in our cohort was 44.2%, in line with the already published literature.11,32–35
Our results confirm and expand the current knowledge reporting that T2-SA with coexisting BE was frequently associated with CRS with and without nasal polyposis, chronic sputum production, chronic OCS intake, and a higher exacerbations rate.10
We hypothesized that the post-nasal drip, which is the hallmark symptom of CRS, together with an abundant eosinophilic bronchial inflammation, might play a role in BE development. Discharge of mucus from the upper to the lower airways could act as an irritative stimulus for the bronchial epithelium, promoting the recruitment of neutrophils, and in patients with type 2-high inflammation, may determine an exaggerated recall and activation of the eosinophils via the innate immunity pathway through IL-25, IL-33 and TSLP production and ILC2 stimulation. Several studies have demonstrated that in patients with severe eosinophilic asthma and co-presence of CRS, particularly with nasal polyps, a greater degree of eosinophilic infiltration characterizes the bronchial mucosa; this feature makes the airways of patients affected by this combination of diseases more prone to eosinophilic inflammation.36,37 This pathogenic hypothesis is also corroborated by the already published literature data, which confirm this common association.36–39
Mucus hyperproduction is a clinical characteristic of both T2-SA and BE.40,41 The combination of these diseases may lead to a synergistic effect in sputum production.42,43 To this extent, almost 60% of our T2-SA+BE patients reported CMH, often purulent or muco-purulent. The persistent accumulation of mucus can limit airflow, worsen asthma symptoms, trigger asthma exacerbations, and reduce the asthma control with the standard inhaled high dose ICS+LABA, often making chronic OCS intake necessary. This can expose patients to OCS adverse events and reduce the ability to fight infections due to the suppression of the type 1 immune response.9,24 As we showed, a more significant proportion of T2-SA+BE patients were OCS dependent (56%) than those without BE (35%).
In our multivariable logistic model, all the above-mentioned clinical features predicted almost 80% of T2-SA in association with BE, highlighting the importance of these characteristics in suspecting the co-presence of both diseases to identify the subjects in which performing a chest-CT scan should be mandatory. Therefore, the diagnosis of T2-SA+BE is fundamental in order to adopt the appropriate therapeutic strategies.40,44,45
The high-resolution chest-CT scan allowed us to define the prevalent pattern and the type of bronchiectasis. We reported mostly cylindrical BE (96%), with a median involvement of 3 lobes, predominantly the lower ones and peripheral distribution. Lower lobes predominance of BE is common in the majority of aetiologies of non-cystic fibrosis BE, reflecting the gravity-dependent retention of secretions.46 In terms of radiological severity, according to the Bhalla score, around 90% of patients presented a mild-to-moderate peribronchial thickening, 62% a mild-to-moderate mucus plugging, with almost no sacculations or abscesses, bullae, collapse and consolidation. Dunican et al44 showed that eosinophils via eosinophil peroxidase (EPO) promote mucus plug formation and that mucus plugs are a common finding in HR chest CT scans of SA patients, particularly in those with comorbid BE, as confirmed in our data.
To better evaluate the radiological and clinical findings in relation to the impact of BE on T2-SA, we assessed the BSI. Our results showed 6 as median BSI value, indicating an overall moderate severity (range 5–8), with 22% of subjects classified as mild and 66% moderate. We found an inversely proportional correlation between BSI, ACT and FEV1, confirming that the greater severity of BE resulted in a less stable disease. Therefore, it is essential to establish targeted treatment for both diseases to improve symptoms and other clinical outcomes.47,48
Data obtained from this prospective observational study confirm the hypothesis that a dangerous liaison closely links T2-SA and BE.10 The mechanism underlying the pathogenetic role of type 2 inflammation as a cause of BE has not been fully unveiled yet. Eosinophils in the bronchial tissue induce damage through degranulation and release of enzymes such as eosinophil-derived neurotoxin, eosinophil cationic protein, eosinophil peroxidase, and major basic protein.49,50 The resulting airway injury stimulates the bronchial epithelial cells to produce IL-25, IL-33 and TSLP, a cluster of cytokines known as “alarmins”, which activate the ILC-2 to produce IL-5, key in eosinophil recruitment and activation, IL-4 and IL-13, responsible for mucus production and sub-epithelial fibrosis with airway remodelling.51,52 Recent studies have shown that anti-IL-5 or anti-IL-5Rα monoclonal antibodies could be effective in controlling T2 inflammation in T2-SA+BE patients,53–60 limiting eosinophils tissue survival and reducing mucus stiffening and hypersecretion, with favourable outcomes concerning exacerbations, pulmonary function and asthma control, mainly when bronchiectasis severity was mild, reinforcing the importance of executing an early diagnosis of BE.
Tight junctions, which are the predominant structure that contribute to the epithelial barrier, act as gatekeepers to prevent the entry of microorganisms and foreign antigens. Tight junction breakdown has been suggested to have a causative role in asthma and bronchiectasis development.61 For this reason, T2-SA+BE patients likely present a marked alteration of this structure and consequently of the epithelial barrier and that could nurture a noxious loop of tissue inflammation and damage.
The main strength of this study is the prospective design and the exclusion of patients affected by other diseases often linked to BE. Therefore, we were able to evaluate and report for the first time the prevalence of BE in highly selected patients affected only by type 2-high SA. Furthermore, we used reliable tools, namely Bhalla and BSI, which allowed us to finely describe the radiological features, the clinical severity of BE and their impact on SA. Hence, the data collected are of high quality and harmonized, and findings coming from our study are robust and reliable.
We accurately excluded all the subjects with T2 low SA, cystic fibrosis, ABPA, low alpha-1 antitrypsin, immune deficiency, vasculitis and previous pneumonia episodes during the enrolment process, allowing us to characterize only those affected by genuine type 2-high SA, without other common confounding factors associated with BE.
The main limitations of the study are the absence of induced sputum and data on sputum cytology. Moreover, we did not perform any viral testing or routine sweat testing. Another important limitation, asthma exacerbations were assessed retrospectively, possibly hindering their correlation with BSI. Furthermore, this study could be underpowered to detect differences in outcomes, since its design did not consider a pre-specified sample size. Finally, all the patients were recruited only in two centres, limiting our external validity.
Conclusion
In conclusion, these results support the hypothesis that type-2 inflammation can have a causative role in the development of BE. Performing a HR chest CT scan is highly recommended when a diagnosis of T2-SA is made, especially in patients with CRS who report frequent asthma exacerbations, chronic sputum production and OCS dependency. The early detection of BE can be crucial to provide adequate treatment, necessary to improve and control patients’ respiratory symptoms and quality of life.
Abbreviations
SA, severe asthma; T2-SA, type 2-high severe asthma; BE, bronchiectasis; ACT, asthma control test; ABPA, allergic bronchopulmonary aspergillosis; CRSwNP, chronic rhinosinusitis with nasal polyps; CMH, chronic mucus hypersecretion; BSI, Bronchiectasis Severity Index; BMI, body mass index; FeNO, fractional exhaled nitric oxide; OCS, oral corticosteroids (Prednisone); ICS, inhaled corticosteroids; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; FEV1, forced expiratory volume in the 1st second; FVC, forced vital capacity; GERD, gastroesophageal reflux disease; IgE, immunoglobulin-E; IL-5, interleukin-5; IL-4, interleukin-4; IL-13, interleukin-13; HRCT, high resolution computed tomography.
Acknowledgment
The statistical analysis was designed and performed in collaboration with Filippo Palermo, Professor of Biostatistics at the University of Catania.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, agreed to submit to the current journal, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
Prof. Dr. Enrico Heffler reports personal fees from AstraZeneca, Sanofi, Regeneron, Novartis, GSK, Circassia, Stallergenes-Greer, and Nestlè Purina, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
1. Holguin F, Cardet JC, Chung KF, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55(1):1900588. doi:10.1183/13993003.00588-2019
2. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
3. Hekking PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi:10.1016/j.jaci.2014.08.042
4. Global Initiative for Asthma (GINA). GINA Report, global strategy for asthma management and prevention; 2019. Available from: http://ginasthma.org.
5. Melero Moreno C, Quirce S, Huerta A, Uría E, Cuesta M. Economic impact of severe asthma in Spain: multicentre observational longitudinal study. J Asthma. 2019;56(8):861–871. doi:10.1080/02770903.2018.1499035
6. Kuruvilla ME, Lee FE, Lee GB. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol. 2019;56(2):219–233. doi:10.1007/s12016-018-8712-1
7. Bardin PG, Rangaswamy J, Yo SW. Managing comorbid conditions in severe asthma. Med J Aust. 2018;209(S2):S11–S17. doi:10.5694/mja18.00196
8. Boulet LP, Boulay MÈ. Asthma-related comorbidities. Expert Rev Respir Med. 2011;5(3):377–393. doi:10.1586/ers.11.34
9. Polverino E, Dimakou K, Hurst J, et al. The overlap between bronchiectasis and chronic airway diseases: state of the art and future directions. Eur Respir J. 2018;52(3):1800328. doi:10.1183/13993003.00328-2018
10. Crimi C, Ferri S, Crimi N. Bronchiectasis and asthma: a dangerous liaison? Curr Opin Allergy Clin Immunol. 2019;19(1):46–52. doi:10.1097/ACI.0000000000000492
11. García-Clemente M, Enríquez-Rodríguez AI, Iscar-Urrutia M, et al. Severe asthma and bronchiectasis. J Asthma. 2020;57(5):505–509. doi:10.1080/02770903.2019.1579832
12. Polverino E, Goeminne PC, McDonnell MJ, et al. European respiratory society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. doi:10.1183/13993003.00629-2017
13. Crimi C, Ferri S, Campisi R, Crimi N. The link between asthma and bronchiectasis: state of the art. Respiration. 2020;99(6):463–476. doi:10.1159/000507228
14. Smith SG, Chen R, Kjarsgaard M, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137(1):75–86.e8. doi:10.1016/j.jaci.2015.05.037
15. Choi Y, Sim S, Park HS. Distinct functions of eosinophils in severe asthma with type 2 phenotype: clinical implications. Korean J Intern Med. 2020;35(4):823–833. doi:10.3904/kjim.2020.022
16. Gramegna A, Aliberti S, Seia M, et al. When and how ruling out cystic fibrosis in adult patients with bronchiectasis. Multidiscip Respir Med. 2018;13(Suppl 1):29. doi:10.1186/s40248-018-0142-7
17. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. doi:10.1164/rccm.200801-060ST
18. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
19. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi:10.1016/j.jaci.2003.09.008
20. Crimi C, Campisi R, Noto A, et al. Comparability of asthma control test scores between self and physician-administered test. Respir Med. 2020;170:106015. doi:10.1016/j.rmed.2020.10601
21. Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi:10.1164/rccm.9120-11ST
22. Murray MP, Pentland JL, Turnbull K, MacQuarrie S, Hill AT. Sputum colour: a useful clinical tool in non-cystic fibrosis bronchiectasis. Eur Respir J. 2009;34(2):361–364. doi:10.1183/09031936.00163208
23. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(SupplS29):1–464. doi:10.4193/Rhin20.600
24. Padilla-Galo A, Olveira C, Fernández de Rota-garcia L, et al. Factors associated with bronchiectasis in patients with uncontrolled asthma; the NOPES score: a study in 398 patients. Respir Res. 2018;19(1):43. doi:10.1186/s12931-018-0746-7
25. Lynch DA, Newell JD, Tschomper BA, Cink TM, Newman LS, Bethel R. Uncomplicated asthma in adults: comparison of CT appearance of the lungs in asthmatic and healthy subjects. Radiology. 1993;188(3):829–833. doi:10.1148/radiology.188.3.8351357
26. Naidich DP, McCauley DI, Khouri NF, Stitik FP, Siegelman SS. Computed tomography of bronchiectasis. J Comput Assist Tomogr. 1982;6(3):437–444. doi:10.1097/00004728-198206000-00001
27. Bhalla M, Turcios N, Aponte V, et al. Cystic fibrosis: scoring system with thin-section CT. Radiology. 1991;179(3):783–788. doi:10.1148/radiology.179.3.2027992
28. Lm REID. Reduction in bronchial subdivision in bronchiectasis. Thorax. 1950;5(3):233–247. doi:10.1136/thx.5.3.233
29. Pasteur MC, Helliwell SM, Houghton SJ, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1277–1284. doi:10.1164/ajrccm.162.4.9906120
30. Kwok WC, Ho JCM, Tam TCC, et al. Risk factors for Pseudomonas aeruginosa colonization in non-cystic fibrosis bronchiectasis and clinical implications. Respir Res. 2021;22(132). doi:10.1186/s12931-021-01729-5
31. Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med. 2014;189(5):576–585. doi:10.1164/rccm.201309-1575OC
32. Dimakou K, Gousiou A, Toumbis M, et al. Investigation of bronchiectasis in severe uncontrolled asthma. Clin Respir J. 2018;12(3):1212–1218. doi:10.1111/crj.12653
33. Ferri S, Crimi C, Campisi R, et al. Impact of asthma on bronchiectasis severity and risk of exacerbations. J Asthma. 2020:1–12. doi: 10.1080/02770903.2020.1857395.
34. Perez-Miranda J, Traversi L, Polverino E. Bronchiectasis in severe asthma: a distinct phenotype? Curr Opin Pulm Med. 2019;25(1):71–78. doi:10.1097/MCP.0000000000000542
35. Coman I, Pola-Bibián B, Barranco P, et al. Bronchiectasis in severe asthma: clinical features and outcomes. Ann Allergy Asthma Immunol. 2018;120(4):409–413. doi:10.1016/j.anai.2018.02.016
36. Handley E, Nicolson CH, Hew M, Lee AL. Prevalence and clinical implications of chronic rhinosinusitis in people with bronchiectasis: a systematic review. J Allergy Clin Immunol Pract. 2019;7(6):2004–2012.e1. doi:10.1016/j.jaip.2019.02.026
37. Canonica GW, Malvezzi L, Blasi F, et al. Chronic rhinosinusitis with nasal polyps impact in severe asthma patients: evidences from the Severe Asthma Network Italy (SANI) registry. Respir Med. 2020;166:105947. doi:10.1016/j.rmed.2020.105947
38. Martínez-Rivera C, Crespo A, Pinedo-Sierra C, et al. Mucus hypersecretion in asthma is associated with rhinosinusitis, polyps and exacerbations. Respir Med. 2018;135:22–28. doi:10.1016/j.rmed.2017.12.013
39. Peters AT, Bose S, Guo A, et al. Prevalence of bronchiectasis in patients with chronic rhinosinusitis in a tertiary care center. J Allergy Clin Immunol Pract. 2021;9(8):3188–3195.e2. doi:10.1016/j.jaip.2021.04.054
40. Boucher RC. Muco-Obstructive Lung Diseases. N Engl J Med. 2019;380(20):1941–1953. doi:10.1056/NEJMra1813799
41. Fanta CH. Clinical aspects of mucus and mucous plugging in asthma. J Asthma. 1985;22(6):295–301. doi:10.3109/02770908509087113
42. Hammad H, Lambrecht BN. The basic immunology of asthma. Cell. 2021;184(6):1469–1485. doi:10.1016/j.cell.2021.02.016
43. Crespo-Lessmann A, Bernal S, Del Río E, et al. Association of the CFTR gene with asthma and airway mucus hypersecretion. PLoS One. 2021;16(6):e0251881. doi:10.1371/journal.pone.0251881
44. Dunican EM, Elicker BM, Gierada DS, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. 2018;128(3):997–1009. doi:10.1172/JCI95693
45. Hoshino M, Matsuoka S, Handa H, Miyazawa T, Yagihashi K. Correlation between airflow limitation and airway dimensions assessed by multidetector CT in asthma. Respir Med. 2010;104(6):794–800. doi:10.1016/j.rmed.2009.12.005
46. King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir Med. 2006;100(12):2183–2189. doi:10.1016/j.rmed.2006.03.012
47. Crimi C, Campisi R, Cacopardo G, et al. Real-life effectiveness of mepolizumab in patients with severe refractory eosinophilic asthma and multiple comorbidities. World Allergy Organ J. 2020;13(9):100462. doi:10.1016/j.waojou.2020.100462
48. Crimi C, Campisi R, Nolasco S, et al. Mepolizumab effectiveness in patients with severe eosinophilic asthma and co-presence of bronchiectasis: a real-world retrospective pilot study. Respir Med. 2021;185:106491. doi:10.1016/j.rmed.2021.106491
49. Henderson WR, Chi EY, Klebanoff SJ. Eosinophil peroxidase-induced mast cell secretion. J Exp Med. 1980;152(2):265–279. doi:10.1084/jem.152.2.265
50. Carr TF, Berdnikovs S, Simon HU, Bochner BS, Rosenwasser LJ. Eosinophilic bioactivities in severe asthma. World Allergy Organ J. 2016;9:21. doi:10.1186/s40413-016-0112-5
51. Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007;19(6):676–680. doi:10.1016/j.coi.2007.07.017
52. Cho JY. Recent advances in mechanisms and treatments of airway remodeling in asthma: a message from the bench side to the clinic. Korean J Intern Med. 2011;26(4):367–383. doi:10.3904/kjim.2011.26.4.367
53. Coverstone AM, Seibold MA, Peters MC. Diagnosis and management of T2-high asthma. J Allergy Clin Immunol Pract. 2020;8(2):442–450. doi:10.1016/j.jaip.2019.11.020
54. Kudlaty E, Patel GB, Prickett ML, Yeh C, Peters AT. Efficacy of type 2-targeted biologics in patients with asthma and bronchiectasis. Ann Allergy Asthma Immunol. 2021;126(3):302–304. doi:10.1016/j.anai.2020.11.014
55. Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular Targets for Biological Therapies of Severe Asthma. Front Immunol. 2020;11:603312. doi:10.3389/fimmu.2020.603312
56. Nolasco S, Crimi C, Pelaia C, et al. Benralizumab effectiveness in severe eosinophilic asthma with and without chronic rhinosinusitis with nasal polyps: a Real-World Multicenter Study. J Allergy Clin Immunol Pract. 2021:
57. Carpagnano GE, Scioscia G, Lacedonia D, Curradi G, Foschino Barbaro MP. Severe uncontrolled asthma with bronchiectasis: a pilot study of an emerging phenotype that responds to mepolizumab. J Asthma Allergy. 2019;12:83–90. doi:10.2147/JAA.S196200
58. Ferri S, Crimi C, Heffler E, Campisi R, Noto A, Crimi N. Vitamin D and disease severity in bronchiectasis. Respir Med. 2019;148:1–5. doi:10.1016/j.rmed.2019.01.009
59. Pelaia C, Crimi C, Benfante A, et al. Therapeutic effects of benralizumab assessed in patients with severe eosinophilic asthma: real-life evaluation correlated with allergic and non-allergic phenotype expression. J Asthma Allergy. 2021;14:163–173. doi:10.2147/JAA.S297273
60. Nolasco S, Campisi R, Intravaia R, et al. Case Report: acute effect of benralizumab on asthma exacerbation without concomitant corticosteroid use. F1000Res. 2020;9:637. doi:10.12688/f1000research.24603.2
61. Sugita K, Kabashima K. Tight junctions in the development of asthma, chronic rhinosinusitis, atopic dermatitis, eosinophilic esophagitis, and inflammatory bowel diseases. J Leukoc Biol. 2020;107(5):749–762. doi:10.1002/JLB.5MR0120-230R
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


