Back to Journals » Clinical Ophthalmology » Volume 17
Two Randomized, Double-masked, Placebo-controlled Studies of the Local Anesthetic Effect of Articaine Ophthalmic Solution
Authors Gonzalez VH, Wirta DL, Uram M, Schupp A, Widmann M , Novack GD
Received 4 March 2023
Accepted for publication 4 May 2023
Published 10 May 2023 Volume 2023:17 Pages 1357—1365
DOI https://doi.org/10.2147/OPTH.S409241
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Victor H Gonzalez,1 David L Wirta,2 Martin Uram,3 Audrey Schupp,4 Michelle Widmann,5 Gary D Novack6,7
1Valley Research Institute, McAllen, TX, USA; 2Eye Research Foundation, Newport Beach, CA, USA; 3American Genomics, LLC, Hobe Sound, FL, USA; 4CMC Turnkey Solutions, Bailey, CO, USA; 5Cal Clinical Solutions, Apex, NC, USA; 6PharmaLogic Development, Inc., San Rafael, CA, USA; 7Department of Ophthalmology & Vision Science, University of California, Davis, School of Medicine, Sacramento, CA, USA
Correspondence: Gary D Novack, PharmaLogic Development, Inc., 17 Bridgegate Drive, San Rafael, CA, 94903, USA, Tel +1 415 472-2181, Email [email protected]
Background: We wanted to develop a new topical ocular anesthetic with good bioavailability in anterior segment tissues. Given concerns about contamination and sterility in multi-dose products, we selected a unit-dose, nonpreserved presentation of AG-920 (articaine ophthalmic solution) in blow-fill-seal containers (similar to currently marketed pharmacological therapies for dry eye disease).
Methods: Consistent with US Food and Drug Administration guidance, two pivotal, Phase 3, randomized, placebo-controlled, double-masked, parallel design studies conducted at two US private practices in 240 healthy subjects. A single dose of AG-920 or identical looking placebo into one (study) eye (2 drops 30 s apart). Subjects underwent a conjunctival pinch procedure and assessment of the pain associated with the pinch. The main outcome measure was the proportion of subjects with rating of “No pain at 5 minutes”.
Results: AG-920 provided a rapid onset of local anesthesia (less than one minute) with clinically and statistically significantly greater effect in AG-920 (68% and 83%) than placebo (3% and 18% for Study 1 and Study 2, respectively, P< 0.0001). The most frequent adverse event was instillation site pain (27% vs 3%) followed by conjunctival hyperemia (probably related to the pinch, 9% vs 10%) in the AG-920 and placebo groups, respectively.
Conclusion: AG-920 was found to be have a rapid onset and useful duration of local anesthesia with no major safety issues, and may be useful for the eye-care professional. Registered with clinicaltrials.gov as NCT04513652 and NCT04829344.
Keywords: local anesthesia, articaine, controlled trial
Plain Language Statement
The authors conducted two, large, well-controlled clinical studies to evaluate the efficacy of a novel local anesthetic drug solution for use in diagnostic and therapeutic procedures of the eyes. The authors found the new drug solution was much more effective than a “placebo” solution. The most frequent adverse events were brief stinging and red eyes. This new drug, still investigational, may provide eye care professionals with an additional option for use in patients.
Introduction
Eye-care professionals use topical ocular anesthetics for a myriad of uses in their clinical practice for numerous procedures – diagnosis, external disease surgery, and more recently, as the sole anesthetic for cataract surgery1 and for intravitreal injections. There are numerous local anesthetics available for clinicians.2
Although there are many available topical anesthetics, their intraocular penetration is poor, as expected based upon their design to anesthetize the external surface of the eye and conjunctiva. More current innovations in ophthalmology have demanded the injection of drugs through the eye wall and in particular the internal aspect of the pars plana. Existing anesthetics function less than optimally in this regard, sometimes necessitating multiple applications of drug over variable periods of time to achieve a fair to poor internal anesthetic effect.
Based on the use of articaine in dental procedures to penetrate solid tissues (eg bone),3 its use in peribulbar anesthesia during cataract surgery,4 and the presence of a phenol ring which theoretically conveys its deeper tissue penetration, we selected articaine. We developed a sterile, room-temperature stable ophthalmic formulation of articaine, called AG-920 ophthalmic solution. In preclinical models, AG-920 ophthalmic solution elicited a rapid onset and potentially clinically useful duration of corneal anesthesia (as measured by aesthesiometry), was well tolerated, and did not cause any clinical or histopathological toxicity. In both preclinical and clinical ocular administration, there was limited systemic exposure.5
Based upon this early development, we aimed to further evaluate the clinical anesthetic efficacy of AG-920 ophthalmic solution, as well as its ocular safety and tolerability. Herein, we report the results of two Phase 3, randomized, placebo-controlled, double-masked, parallel design studies. The selection of response to a binary conjunctival pinch efficacy test (Yes/No) and placebo-control was based upon regulatory guidance from the US Food and Drug Administration (FDA).
Materials and Methods
The vehicle-controlled study was performed in accordance with the guidelines outlined in the Declaration of Helsinki and was approved by Institutional Review Boards (WGC/WIRB and Sterling IRB, Study Numbers AG920-CS301 and AG920-CS302), and all individuals provided written informed consent. Subjects were recruited from daily practice patients, previous clinical study subjects, or word of mouth in the community.
Eligible for entry were adults 18 years and older who were able to follow instructions and meet the required study visits. Also required in each eye was a minimum best corrected visual acuity of 20/200, intraocular pressure of 7–30 mmHg, and ability to tolerate bilateral instillation of over-the-counter (OTC) artificial tear product based on investigator judgement.6 Female subjects of childbearing potential were not to be pregnant or nursing and must have had a negative urine pregnancy test at screening (Visit 1).
Excluded were individuals who had recently participated in an investigational study, had contraindications to local anesthetics, had known decreased corneal or conjunctival sensitivity (eg, sequelae of herpetic eye disease, corneal graft), had ocular surgery (intraocular, refractive, extraocular muscles, eyelid) or general surgery in either eye within the past 90 days, had an intravitreal injection in either eye within 14 days of randomization, or a diagnosed corneal pathology which might have led to decreased sensitivity and other relevant ocular disease. Subjects were not allowed to wear contact lenses on the dosing day. Concomitant medications which might have an effect on cornea sensitivity or ocular pain were prohibited (eg, recent systemic opioids or opiate analgesics, topical NSAIDs or over-the-counter (OTC) artificial tear lubricant products).
AG-920 ophthalmic solution 8.0% used in this study was an isotonic, nonpreserved aqueous sterile solution containing Articaine HCl 8% and the standard ophthalmic excipients boric acid, mannitol, sodium acetate trihydrate, glacial acetic acid, and edetate disodium dihydrate. The product formulation is adjusted to pH 4.5 to 5.0 for optimum stability and low impurity profile. The placebo (also referred to as vehicle) was the same formulation, with the exception of the active ingredient, and was formulated to the same pH as AG-920. Both products were packaged in a masked fashion.
These studies were registered with www.clinicaltrials.gov as NCT04513652 and NCT04829344. We conducted two double-masked, (equal) randomized placebo-controlled, parallel Phase 3 trials, AG-920-CS301 (Study 1, oculoplastics sub-specialty private practice in California) and AG-920-CS302 (Study 2, retina sub-specialty private practice in Texas). In each study, 120 healthy subjects were randomized to receive a single dose of AG-920 or identical looking placebo into one (study) eye (2 drops 30 s apart). Subjects underwent a conjunctival pinch procedure and the pain associated with the pinch assessed. A priori, the primary endpoint was “no pain at 5 minutes”.
After a screening ophthalmic examination, subjects were randomized to receive either AG-920 ophthalmic solution 8.0% or its vehicle in one randomly selected eye based upon computer-generated randomization codes (SAS Institute, Cary NC, Version 9.0 or higher). The single dose was administered by the clinic staff as two drops in the study eye 30 s apart.
Consistent with a previous evaluation of a local anesthetic,7 subjects were asked to look up, and the investigators used a 0.3 mm forceps to quickly “pinch” and release the inferior bulbar conjunctiva of the study eye. Clinical staff explained to subjects that the feeling of pressure is not to be judged as pain. Immediately after the release, subjects were asked “Was that painful?”. Subjects were to answer yes or no. All subjects were pinched at 20, 40, 60 s and 5 minutes post dose. If the subject felt no pain at 5 min, the subject was considered “anesthetized”. Pinching of anesthetized subjects continued at 10 min and every 5 min for up to 30 min or until pain resumed. Once pain resumed, pinching stopped. If the subject experienced pain at 5 min, pinching was concluded, and the subject was considered to not have reached anesthesia at 5 min (Figure 1).
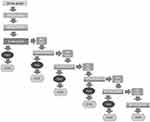 |
Figure 1 Study flow chart. |
Statistics
The primary efficacy endpoint (the proportion of subjects with no pain, ie, anesthetized, at 5 min) was to be performed on the intent-to-treat population (all randomized subjects who received at least one dose of study medication), and then on the per-protocol population to determine robustness of results. However, as there were no subjects excluded from the per-protocol population in either study, this was moot. Additional endpoints included the onset of anesthetic effect, the duration of anesthetic effect, and the proportion of subjects with no pain within 5 min. The proportion of subjects who were anesthetized at each time point was also summarized. Based upon the known safety profile of local anesthetics, temporary stinging or burning upon instillation was listed a priori as an expected risk.
For the pre-specified primary endpoint and secondary endpoint of no pain within 5 min, a Pearson chi-squared test was used in Study 1 for the comparison of the proportions from the two treatment groups. Based upon results of Study 1, and the data in Study 2, a Cochran–Mantel–Haenszel (CMH) chi-squared test stratified by study-eye side (right or left) was used for this between-group comparison. These are essentially the same nonparametric analyses.
In addition, a two-sided 95% confidence interval (CI) for the difference in proportions between the two treatment groups was calculated. If the proportion of subjects (expressed as a percentage) was higher in the AG-920 group and the P-value was statistically significant (P≤0.05), then superiority of AG-920 over placebo was claimed. Descriptive and inferential statistics were performed using SAS, V 9.4 or higher, SAS Institute, Cary NC.
For the secondary endpoints of onset and duration of anesthesia, a two-sample t-test was used in Study 1 to compare the two treatment groups. In Study 2, these endpoints were compared between the two groups using analysis of variance (ANOVA) with treatment and study-eye side (right or left) as factors.
MedDRA version 23.0 and above was used for coding of the adverse events (AE). WHO ATC Index 2020 was used for coding of concomitant and prior medications taken by the subject.
Results
Disposition and Pre-study Characteristics
Between September 2020 and May 2021, each study enrolled 120 subjects, equally randomized into AG-920 and vehicle treatment groups. All 240 subjects received study medication and completed the study. None was excluded from analysis. Within each study, for the most part, there were no statistically or clinically significant differences between treatment groups. Between studies, however, there were more Asians in Study 1 (17%, 20/120) compared to Study 2 (2%, 2/120), and more subjects of Hispanic ethnicity in Study 2 (98%, 118/120) compared to Study 1 (22%, 26/120, Table 1).
 |
Table 1 Demographics |
Efficacy
Primary efficacy endpoint: Study 1: Of the subjects in the AG-920 treatment group, 68.3% (41/60) achieved the primary efficacy endpoint of “no pain at 5 minutes”, compared to 3.3% (2/60) in the vehicle group. This difference of 65% (95%CI: 52.4–77.6%) was statistically significant (P<0.0001). Study 2: Of the subjects in the AG-920 treatment group, 83.3% (50/60) achieved the primary efficacy endpoint of “no pain at 5 minutes”, compared to 18.3% (11/60) in the placebo group. This difference between groups of 65% (95%CI: 47.5–75.3%) was statistically significant (P<0.0001, Table 2, Figure 2).
 |
Table 2 Proportion of Subjects with No Pain at 5 min (ITT Population) |
 |
Figure 2 Anesthetization rate (% of subjects with no pain at 5 min). |
Additional efficacy endpoints: Study 1: Of the subjects in the AG-920 treatment group, 98.3% (59/60) achieved an efficacy endpoint of “no pain within 5 minutes”, compared to 3.3% (2/60) in the vehicle group. This difference of 95% (95%CI: 89.4–100.0%) was statistically significant (P<0.0001). Study 2: Of the subjects in the AG-920 treatment group, 98.3% (59/60) achieved the efficacy endpoint of “no pain within 5 minutes”, compared to 35.0% (21/60) in the placebo group. This difference of 63.3% was statistically significant (P<0.0001).
Study 1: At each time point from 20 s (0.33 min) through 25 min, a substantially greater proportion of subjects in the AG-920 treatment group responded (no pain) than in the vehicle group. Note that everyone was tested at all timepoints through 5 min. At 10 min, subjects who were not anesthetized at 5 min were not tested, and similarly at subsequent time points. Thus, the number of subjects pinched decreased over time. Study 2: At each time point from 20 s (0.33 min) through 15 min, a substantially greater proportion of subjects in the AG-920 treatment group responded no pain than in the vehicle group. At 10 min, subjects who were not anesthetized at 5 min were not tested, and similarly at subsequent time points. Thus, the number of subjects pinched decreased over time (Figure 3).
 |
Figure 3 Time-response (percentage of subjects anesthetized). |
Study 1: The mean onset of anesthesia in the AG-920 treatment group was 0.44±0.6 (mean ±SD) minutes. Five subjects in the vehicle group also experienced anesthesia at the first test (20 s). Study 2: The mean onset time of anesthesia in the AG-920 treatment group was 0.43±0.61 (mean ±SD) minutes. Twenty-one (35%) subjects in the placebo group also experienced anesthesia, with an onset time of 1.0±1.67 min. This finding indicates that for those subjects who experienced anesthesia, subjects treated with AG-920 had an early onset of the anesthetic effect by approximately a half minute.
Study 1: The mean duration of anesthesia in the AG-920 treatment group was 4.8±3.8 (mean ±SD) minutes, compared to the vehicle treatment group which was 0.27±1.4 min (P<0.0001). Study 2: Among the 59 subjects in the AG-920 group and 21.7% subjects in the placebo group who experienced anesthesia, the mean duration of anesthesia was 12.8±8.0 (mean ±SD) min in the AG-920 group and 6.6±10.2 min in the placebo group. The LS-adjusted mean difference from the ANOVA model was 5.68 min (95%CI: 1.30–10.07 min) and this difference was statistically significant (P=0.0117).
Safety
There were no serious AEs in either study. In both studies, AE reports were mild and transient. Study 1: 55 AEs were reported in 47 subjects. The incidence of subjects with at least one AE was 56.7% (34/60) in the AG-920 group and 22% (13/60) in the vehicle group. Most of these AEs were ocular. The proportion of subjects reporting ocular AEs were 53.3% (32/60) and 21.7% (13/60) for the AG-920 and vehicle groups, respectively. The relationship of AE to the study drug was judged as related for all AEs. Study 2: In this study, 14 AE were reported in 14 subjects. The incidence of subjects with at least one AE was 16.7% (10/60) in the AG-920 group and 6.7% (4/60) in the vehicle group. All but one of these AEs were ocular (study eye). The only systemic AE was a single report of headache in the AG-920 group. The relationship of AEs to the study drug was judged as unrelated for all AEs in the AG-920 group, and unrelated for all but one event (eye pain) in the placebo group (Table 3).
 |
Table 3 Adverse Events |
In Study 1, the most frequently reported AE was instillation site pain, seen in 53% (32/60) subjects in the AG-920 group and 7% (4/60) subjects in the vehicle group. Dysgeusia was reported in 7% (4) subjects in the AG-920 treatment group and none of the subjects in the vehicle group. In Study 2, the most frequently reported AE was conjunctival hyperemia, seen in 11.7% (7/60) and 3.3% (2/60) subjects in the AG-920 and placebo groups, respectively.
In both studies, at screening, mean visual acuity in each treatment group was 0.0 logarithm of the minimum angle of resolution (logMAR (20/20 Snellen) in the study eye and non-study eye. Throughout the study, mean changes in visual acuity were less than 0.1 logMAR (1 line). There were no subjects who lost three or more Early Treatment of Diabetic Retinopathy Study (ETDRS) lines of visual acuity.
The incidence of positive changes (ie, findings) in biomicroscopy was tabulated for both study and fellow eye. Study 1: Conjunctival hyperemia, which was absent in all eyes at screening, was noted at Visit 2 (day of instillation) in the study eye in four subjects in the AG-920 group (three mild, one moderate) and nine subjects in the vehicle group (all mild). In Study 2, there were no changes of note in the study or fellow eye.
Discussion
The primary objective of these Phase 3, randomized, placebo-controlled, double-masked, parallel design studies in healthy subjects was to evaluate anesthetic efficacy of AG-920. In both studies, more than two-thirds of subjects were anesthetized at 5 min. The treatment effect (difference from placebo) was 65% in both studies (P<0.0001). In both studies, subjects treated with AG-920 achieved clinically and statistically significant effects on secondary endpoints of anesthetized within 5 min (98.3% in both studies), a rapid mean onset of anesthesia in less than 30 s in both studies, and a mean duration of anesthesia of 4.8 min in Study 1 and 12.8 min in Study 2.
The two studies, each at a geographically different site, differed in the vehicle response rate: 3.3% in Study 1 and 18.3% in Study 2. We found one published paper using this same methodology at a different site 15 years prior (Massachusetts) in which the vehicle response rate was 22%.7 After completion of our work, a new local anesthetic was approved by the US FDA (Iheezo™, chloroprocaine gel). The vehicle response rate in the studies supporting that approval ranged from 12% to 20%.8 Thus, our observations are consistent with others. The difference in placebo response rate in our two studies are probably due to multiple factors including previous treatment experience, subject and investigators expectations, and socioeconomic level.
As noted in the introduction, the design was informed by regulatory precedents and communication with the US FDA. Previous product approvals were based upon a vehicle-controlled study using the conjunctival pinch method used in this study.7,8 This study design supported an indication for a “local anesthetic indicated for ocular surface anesthesia during ophthalmologic procedures”, which includes intravitreal injections. While there was not a positive control in the study, the magnitude of efficacy reported for AG-920 in this controlled study relative to its vehicle, 65%, is similar to that reported with a marketed local anesthetic in a placebo-controlled study.7
The nature of the AE differed in the two studies. In one study, approximately half of the subjects treated with AG-920 reported mild instillation site pain, and 7% (4/60) reported dysgeusia. In the companion study, subjects treated with AG-920, conjunctival hyperemia was seen in 11.7% (7/60) and 3.3% (2/60) subjects in the AG-920 and placebo groups, respectively. As noted for placebo response rate, the nature of these safety reports may have to do with the investigators, the manner in which the staff reported AE related to the pinching, the subject population, and the consideration as to whether patient reports were judged as adverse reactions. Nonetheless, the AEs were mild, and all subjects recovered without any further treatment or sequelae. There were no other safety findings of note. Further, the AEs reported with AG-920 were no worse than, and possibly more limited (eg, no corneal epithelial changes) than a marketed product.9 According to the package insert for a product approved after our work was completed, AEs, including mydriasis, were 26%, versus a vehicle rate of 2%.8
The efficacy results from the two studies in this present report support US regulatory policy. Since 1962, at least two well controlled studies demonstrating the safety and efficacy of the new product have been required for approval. The two studies do not have to be exactly the same in design, nor in results, but they have to be confirmatory. In our report, the design was the same for both studies. While the vehicle response rate was different, 3.3% vs 18.3, the treatment effect was the same, 65%. Thus, we find the two studies confirmatory of each other.
We recognize that the pivotal studies conducted for regulatory approval for novel agents do not always address the wealth of concerns from clinicians. Future studies may compare AG-920 to a marketed product (most likely requiring a larger study for adequate power), using a continuous measure of anesthesia (eg Cochet-Bonnet esthesiometer), and experience in clinical conditions such as intravitreal injection.10
Conclusion
In conclusion, AG-920 was found to be have a rapid onset and useful duration of local anesthesia with no major little or no safety issues, and may be useful for the eye-care professional.
Data Sharing Statement
The authors are pleased to review responsible requests for data sharing after regulatory clearance. (Martin Uram, MD, MPH, [email protected]).
Acknowledgments
The authors wish to thank Linda Wirta and Yesenia Salinas for their conduct of the studies. Portions of this work were previously presented at the Association for Research in Vision and Ophthalmology, May 2022 (Gonzalez VH, Novack GD, Wirta D, Uram M, Schupp A, Widmann MC. Two Randomized, Double-Masked, Placebo-Controlled, Parallel-Group Studies of the Local Anesthetic Effect of Articaine Sterile Topical Ophthalmic Solution 8% (AG-920). Investigative Ophthalmology & Visual Science. 2022;63(7):334).
Funding
This research was sponsored by American Genomics, LLC.
Disclosure
Dr Martin Uram reports Inventor on US Pat. No. 11,096,922. Ms Audrey Schupp reports personal fees from American Genomics, during the conduct of the study. Dr Gary D Novack reports consulting fees paid to employer from American Genomics, during the conduct of the study; and he consults with several medical device and pharmaceutical firms. The authors report no other conflicts of interest in this work.
References
1. Haripriya A, Chang DF. Intracameral antibiotics during cataract surgery: evidence and barriers. Curr Opin Ophthalmol. 2018;29(1):33–39. doi:10.1097/ICU.0000000000000445
2. Han J, Rinella NT, Chao DL. Anesthesia for intravitreal injection: a systematic review. Clin Ophthalmol. 2020;14:543–550. doi:10.2147/OPTH.S223530
3. Martin E, Nimmo A, Lee A, Jennings E. Articaine in dentistry: an overview of the evidence and meta-analysis of the latest randomised controlled trials on articaine safety and efficacy compared to lidocaine for routine dental treatment. BDJ Open. 2021;7(1):27. doi:10.1038/s41405-021-00082-5
4. Fathi AA, Soliman MM. Carticaine versus lidocaine for peribulbar anesthesia in cataract surgery. J Cataract Refract Surg. 2002;28(3):513–516. doi:10.1016/S0886-3350(01)01140-3
5. Verhoeven R, Uram M, Schupp A, Rasmussen S, Widmann M, Novack GD. Early nonclinical and clinical development of AG-920, a repurposed topical ocular anesthetic. J Ocul Pharmacol Ther. 2022;38(7):481–488. doi:10.1089/jop.2022.0026
6. Stone JL, Robin AL, Novack GD, Covert D, Cagle GD. An objective evaluation of eye-drop instillation in glaucoma patients. Arch Ophthalmol. 2009;127(6):732–736. doi:10.1001/archophthalmol.2009.96
7. Busbee BG, Alam A, Reichel E. Lidocaine hydrochloride gel for ocular anesthesia: results of a prospective, randomized study. Ophthalmic Surg Lasers Imaging. 2008;39(5):386–390. doi:10.3928/15428877-20080901-03
8. Anonymous. Iheezo(TM) package insert. Available from: www.accessdata.fda.gov/drugsatfda_docs/label/2022/216227s000lbl.pdf.
9. Anonymous. Akten(R) package insert. Available from: www.accessdata.fda.gov/drugsatfda_docs/label/2014/022221s005lbl.pdf.
10. Novack GD. One more time: generic solutions are the same as branded. Ophthalmol Glaucoma. 2023. doi:10.1016/j.ogla.2023.01.002
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
