Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Two Cases of Immune Drift Phenomena Caused by Biologic Agents for Treating Psoriasis and Atopic Dermatitis
Received 31 October 2023
Accepted for publication 28 November 2023
Published 7 December 2023 Volume 2023:16 Pages 3521—3525
DOI https://doi.org/10.2147/CCID.S445468
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Xiaolei Ma,1 Tianying Li,2 Gangwen Han1
1Department of Dermatology, Peking University International Hospital, Beijing, People’s Republic of China; 2Department of Pathology, Peking University International Hospital, Beijing, People’s Republic of China
Correspondence: Xiaolei Ma, Peking University International Hospital, Life Park Road No. 1 Life Science Park of Zhong Guancun, Chang Ping District, Beijing, People’s Republic of China, Email [email protected]
Abstract: Atopic dermatitis and psoriasis are both common chronic inflammatory skin conditions that can significantly affect the quality of life for individuals affected by them. With the growing use of biologic agents, there have been remarkable clinical advancements in the treatment of these diseases. Interestingly, during biologic therapy for either condition, a phenomenon has emerged where treatment can paradoxically induce a transition to the phenotype of the other condition.We present two cases of immune drift phenomena caused by biologic agents for treating psoriasis and atopic dermatitis.The first one is a case of psoriasis lesion that developed in an old patient with AD who was treated with dupilumab for two months. The second one is a case of eczematoid lesion that developed in an adult patient with ankylosing spondylitis who was treated with Secukinumab for 1 year.
Keywords: immune drift phenomena, secukinumab, dupilumab, psoriasis and atopic dermatitis
Introduction
Atopic dermatitis and psoriasis are both common chronic inflammatory skin conditions that can significantly affect the quality of life for individuals affected by them. With the growing use of biologic agents, there have been remarkable clinical advancements in the treatment of these diseases.1–3 Interestingly, during biologic therapy for either condition, a phenomenon has emerged where treatment can paradoxically induce a transition to the phenotype of the other condition.
Case Description
The first patient was a 87-year-old Chinese woman who complained of severe itching and rashes on her forehead, scalp, shoulders, and waist for the past 2 years. Previous treatments with oral antihistamine drugs such as cetirizine (10mg/d) or ebastine (10mg/d), combined with topical corticosteroids (hydrocortisone butyrate) and 0.1% tacrolimus ointment for several weeks, had not reduced the disease activity. Upon dermatological examination, erythema, papules, scales, and excoriation were observed on her shoulders and waist (Figure 1). She had a medical history of urticaria, seasonal allergic rhinitis, hypertension, and coronary heart disease. Her father had a history of allergic rhinitis, and her son and granddaughter had eczema. Laboratory tests revealed an increased absolute and percentage of blood eosinophils (0.8×109/L) and an elevated total IgE level (300 IU/mL). She was screened for allergens, and pollen was graded at level 3 (6.5 kU/L). According to the Chinese Zhang’s criteria,4 the patient was diagnosed with atopic dermatitis (AD). The EASI score was 19.6, and the NRS score was 9. Given the lack of significant relief from conventional treatments (allergen avoidance and optimization of local treatment), biological agents were considered.
 |
Figure 1 Erythema, papules, scales and excoriation on the waist. |
Dupilumab was administered subcutaneously with a loading dose of 600mg, followed by 300mg every other week. After eight weeks, the patient’s symptoms and signs were significantly relieved. However, the erythema and papules on her waist had evolved into well-demarcated scaly erythematous lesions (Figure 2). A 3-mm puncture biopsy was performed in this area, and histopathology revealed psoriasiform acanthosis with a diminished granular layer and lymphocyte infiltration in the superficial dermis, suggesting the development of dupilumab-induced psoriasiform dermatitis (Figure 3). Initially, we discontinued dupilumab and applied topical calcipotriol and betamethasone ointment to the psoriasis-like lesions. This local treatment improved the skin lesions (Figure 4), but the pruritus remained significant, affecting her sleep. Due to the beneficial effect of calcipotriol/betamethasone, we decided to reintroduce dupilumab for continuous treatment. This patient’s psoriasiform rash did not recur during the four months of following-up.
 |
Figure 2 Psoriasis- like lesions: demarcated scaly erythematous lesions. |
 |
Figure 3 Histopathology showed psoriasiform acanthosis with a diminished granular layer, lymphocyte infiltration in the superficial dermis. (HE stain, magnification 4*10). |
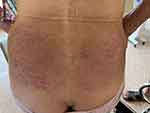 |
Figure 4 Topical calcipotriol and betamethasone ointment was applied to the psoriasis-like lesions. |
The second patient was a 30-year-old Chinese man who complained of a rash with pruritus on his extremities and trunk lasting for more than one month. Dermatological examination revealed erythema, papules, and scales on the limbs and back of the patient (Figure 5A–D). He had a history of ankylosing spondylitis for seven years, and the Department of Rheumatology and Immunology had prescribed Secukinumab for over a year. There was no history of eczema, allergic rhinitis, asthma, etc. Laboratory tests showed no obvious abnormalities.
 |
Figure 5 (A–D) Erythema, papules, scales on the shoulder (A), back (B), lower limbs (C) and popliteal fossa (D). |
Considering the temporal relationship between the initiation of secukinumab treatment and the onset of the eczematous eruption, it suggests that secukinumab might have contributed to its development. We prescribed oral cetirizine in combination with topical hydrocortisone butyrate ointment, and the patient’s skin lesions significantly improved. He stopped secukinumab and switched to Adalimumab, the eczematous rash did not recur.
Discussion
As the utilization of biologic agents continues to rise, an emerging phenomenon is the potential for biologic therapy for either condition to induce a paradoxical phenotypic switch to the other. We have compiled and summarized the relevant reports from the past 5 years5–10 in Table 1.
 |
Table 1 The Relevant Reports from the Past 5 Years |
Atopic dermatitis (AD) and psoriasis are both common immune-induced inflammatory skin diseases mediated by Th cells. On one hand, the activation of the Th1 cascade, along with related cytokines and cell subsets such as Th17 and Th22, plays a central role in the pathogenesis of psoriasis (PSO). Psoriasis is primarily driven by Th17 cells, resulting in elevated levels of IL-17A, IL-22, and IL-23. On the other hand, the Th2 cascade and its associated cytokines are implicated in the pathogenesis of AD. We now understand that IL-4 and IL-13 are key players in AD inflammation, spanning acute, childhood, chronic, and adult stages. The primary cytokines secreted by Th2 cells include IL-4, IL-13, and IL-31. IL-31 is primarily associated with pruritus and is not directly involved in skin barrier dysfunction. IL-13, however, can directly affect skin epithelial cells, leading to skin barrier dysfunction. Additionally, they can directly influence B cells, prompting them to produce IgE antibodies against allergens, and some antibodies may also target self-antigens. The combined effects of allergens and self-antigens contribute to skin barrier dysfunction.
In terms of the immune mechanism, psoriasis is characterized by tissue infiltration by numerous myeloid dendritic cells that produce TNF and iNOS (TIP-DCs), along with IL-23. The activation and survival of Th17 T-cells in psoriasis lesions are highly dependent on IL-23, as evidenced by the efficacy of specific IL-23 antibodies in treatment. At this mechanistic level, psoriasis differs from AD due to ① the absence of Th2 T-cells producing IL-4, IL-5, and IL-13, ②the absence of IgE antibodies, which are strongly associated with activated Th2 T-cells, and ③differences in the CD11c+ dermal dendritic cell populations, which favor Th2 T-cell activation in AD.11 As a result, the T cells responsible for psoriasis and atopic dermatitis exhibit antagonistic behavior toward each other.12
Currently, we have highly specific biological treatments for inflammatory skin diseases that utilize biological agents. However, due to the antagonistic nature of basic immunity responses, new therapies that specifically target Th17 or Th2 immunity may potentially disrupt the delicate balance between these two immune response patterns. In some instances, this disruption could lead to the development of antagonistic diseases, wherein the roles of these two immune responses are reversed.
It’s important to note that there is currently no universally accepted standard for treatment. Treatment strategies encompass a range of options, including no treatment, the use of topical corticosteroids, the administration of broad-acting systemic agents, and the consideration of discontinuing or switching biologic therapy.
Ethics and Consent Statements
Both the patients informed consent for publication of their case details include publication of the images.
Institutional approval was not required to publish the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mastorino L, Viola R, Panzone M, et al. Dupilumab induces a rapid decrease of pruritus in adolescents: a pilot real-life study. Dermatol Ther. 2021;34(6):e15115. doi:10.1111/dth.15115
2. Mastorino L, Cantafio Duò VL, Vecco C, et al. Impact of comorbidities in the response of atopic patients treated with dupilumab: a real-life study up to 36 weeks. J Eur Acad Dermatol Venereol. 2022;36(12):e1021–e3. doi:10.1111/jdv.18427
3. Miniotti M, Ribero S, Mastorino L, et al. Long-term psychological outcome of patients with moderate-to-severe atopic dermatitis continuously treated with Dupilumab: data up to 3 years. Exp Dermatol. 2023;32(6):852–858. doi:10.1111/exd.14786
4. Liu P, Zhao Y, Mu ZL, et al. Clinical features of adult/adolescent atopic dermatitis and Chinese criteria for atopic dermatitis. Chinese Med J. 2016;129(7):757–762. doi:10.4103/0366-6999.178960
5. Al-Janabi A, Foulkes AC, Griffiths CEM, Warren RB. Paradoxical eczema in patients with psoriasis receiving biologics: a case series. Clin Exp Dermatol. 2022;47(6):1174–1178. doi:10.1111/ced.15130
6. Napolitano M, Megna M, Fabbrocini G, et al. Eczematous eruption during anti-interleukin 17 treatment of psoriasis: an emerging condition. Br J Dermatol. 2019;181(3):604–606. doi:10.1111/bjd.17779
7. Varma A, Levitt J. Dupilumab-induced phenotype switching from atopic dermatitis to psoriasis. AAD Case Rep. 2020;6(3):217–218. doi:10.1016/j.jdcr.2020.01.012
8. Stout M, Guitart J, Tan T, Silverberg JI. Psoriasis-like dermatitis developing in a patient with atopic dermatitis treated with dupilumab. Dermatitis. 2019;30(6):376–378. doi:10.1097/DER.0000000000000509
9. Napolitano M, Scalvenzi M, Fabbrocini G, Cinelli E, Patruno C. Occurrence of psoriasiform eruption during dupilumab therapy for adult atopic dermatitis: a case series. Dermatol Ther. 2019;32(6):e13142. doi:10.1111/dth.13142
10. Kurihara K, Fujiyama T, Tokura Y, Honda T. Two cases of psoriasiform dermatitis arising during dupilumab therapy and successfully treated with delgocitinib ointment. Eur J Dermatol. 2021;31(5):658–660. doi:10.1684/ejd.2021.4140
11. Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68–73. doi:10.1016/j.coi.2017.08.008
12. Schäbitz A, Eyerich K, Garzorz-Stark N. So close, and yet so far away: the dichotomy of the specific immune response and inflammation in psoriasis and atopic dermatitis. J Intern Med. 2021;290(1):27–39. doi:10.1111/joim.13235
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
