Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Two Case Reports of Elastolytic Giant Cell Granuloma on the Palms
Authors Promsena P , Triyangkulsri K , Rutnin S
Received 23 May 2023
Accepted for publication 5 September 2023
Published 13 September 2023 Volume 2023:16 Pages 2497—2502
DOI https://doi.org/10.2147/CCID.S422554
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Pichamon Promsena, Korn Triyangkulsri, Suthinee Rutnin
Division of Dermatology, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Suthinee Rutnin, Division of Dermatology, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Ratchathewi, Bangkok, 10400, Thailand, Tel +66-2-2011141, Fax +66-2-201-1211, Email [email protected]
Abstract: Elastolytic giant cell granuloma (EGCG) is a rare granulomatous reaction hypothesized to occur because of the altered antigenicity of elastic fibers, resulting in cellular immunological reactions. The hallmarks of EGCG include elastolysis, elastophagocytosis, and multinucleated giant cell infiltrations. EGCG was first described as an actinic granuloma or annular elastolytic giant cell granuloma that classically presents as centrifugally expanding annular plaques on sun-exposed areas. It was recently named EGCG due to reports of non-annular lesions in various sun-protected areas. Hand involvement has been described but is almost exclusively confined to the dorsal and lateral aspects of the hands. Herein, we report two cases of EGCG on the palms, an unusual site of presentation, that were successfully treated with topical, high-potency corticosteroids. EGCG should be included in the differential diagnosis in cases of annular skin lesions located on the marginal area of the palms.
Keywords: actinic granuloma, collagenous and elastotic marginal plaques of the hands, elastophagocytosis, granuloma annulare, paraneoplastic syndrome, autoimmunity
Introduction
Elastolytic giant cell granuloma (EGCG) is a rare granulomatous skin disorder of unknown etiology.1 It was first described as actinic granuloma or annular elastolytic giant cell granuloma (AEGCG), characterized by annular erythematous plaques on sun-exposed areas.2,3 Hallmark histologic findings of EGCG include elastolysis and elastophagocytosis with multinucleated giant cell infiltration.4 Over the years, many cases of non-annular lesions, including papular, giant, reticular, generalized, and mixed types, have been reported. Many of these cases occurred in sun-protected areas, thus they were later referred to as EGCG or giant cell elastolytic granuloma.1 Two cases of unusual EGCG of the palms are presented in this report.
Case Report
Case 1
A 75-year-old male, with diabetes mellitus (DM) with a hemoglobin A1C of 6.58%, dyslipidemia, and osteoporosis visited our dermatology clinic for the evaluation of an asymptomatic rash along the margins of both palms for 2 months. He denied any history of trauma, sun, or heat exposure. Dermatological examination revealed multiple erythematous papules and plaques with subtle scales on the margins of both palms (Figure 1). The clinical differential diagnosis includes granuloma annulare (GA), annular leukocytoclastic vasculitis, sarcoidosis, and secondary syphilis.
 |
Figure 1 (A and B) A 75-year-old male with multiple erythematous papules and plaques on the margins of both hands. |
A skin biopsy of the lesion on his right palm was done to confirm the diagnosis, which revealed nodular infiltration of lymphocytes, histiocytes, and multinucleated giant cells with elastophagocytosis (Figure 2A and B). Conspicuous degenerated and fragmented elastic fibers stained by Verhoeff-Van Gieson (VVG) were observed in the upper dermis, as opposed to diminished elastic fibers within the granulomatous area (Figure 2C and D). Alcian blue pH 2.5 showed negative staining correlated with the absence of mucin deposition. In addition, haphazardly arranged thickened collagen bundles, some perpendicular to the epidermis, were also observed. A significant improvement was observed after 1 month of treatment with 0.05% clobetasol propionate cream twice daily, and clinical remission was observed for 1 year after cessation.
Case 2
A 68-year-old male with dyslipidemia, pituitary adenoma, and benign prostatic hyperplasia (BPH) visited the dermatology clinic for an asymptomatic red-brown rash on his palms for 2 weeks. Dermatological examination revealed multiple erythematous to brownish papules and plaques on the margins of both palms (Figure 3). Histological examination and VVG staining showed findings similar to those in case 1, consisting of granulomatous inflammation, elastolysis, elastophagocytosis, and thickened degenerate collagen (Figure 4A–D). Mucin staining was negative with Alcian blue pH 2.5. Improvements in color and texture were observed after the application of topical 0.05% clobetasol propionate twice daily for 2 weeks, and clinical remission was observed for 11 months after cessation.
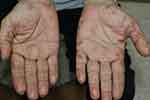 |
Figure 3 A 68-year-old male with erythematous to brownish annular papules and plaques on the margins of both hands. |
Discussion
EGCG, or actinic granuloma, was first reported by O’Brien in 1975 as annular erythematous papules and plaques with a raised border and a slightly atrophic, hypopigmented center. Sun-exposed areas, such as the face, neck, upper trunk, and arms are frequently involved.2 It occurs mainly in fair-skinned middle-aged females and is mostly asymptomatic.1 The involvement of sun-protected areas has also been observed in several studies.5,6 Qian et al recently published 105 cases of EGCG, 72.4% of these cases had lesions limited to the sun-exposed areas. Some of them (20.9%) had lesions on both sun-exposed and sun-protected areas, while a minority (6.7%) of patients exhibited lesions exclusively on the sun-protected areas.1 In these studies, hand involvement was also documented but entirely occurred on the dorsal sun-exposed surface.
The etiology and pathogenesis of EGCG are not well characterized. Current findings indicate that elastic fibers are essential in the pathogenesis of EGCG, and studies have shown that areas devoid of elastic fibers, such as burn scars or striae distensae, are not involved in EGCG.7,8 Environmental factors, such as ultraviolet radiation and heat, or host factors, such as vascular damage, systemic diseases, or internal malignancies, are all hypothesized to alter the antigenicity of elastic fibers. This shift in antigenicity may trigger a cascade of cellular immune responses, which ultimately results in a granulomatous reaction, elastolysis, and elastophagocytosis.9 Both of our cases had lesions occurring in the sun-protected area without obvious environmental triggers. In terms of host factors, both our cases had underlying dyslipidemia, which was also found in a few cases of EGCG.1,6,10 Although the correlation and pathomechanism of dyslipidemia and EGCG have not been elucidated, several systemic disorders, such as DM, alcoholic liver diseases, systemic amyloidosis, temporal arteritis, and protoporphyria, have been reported to be associated with EGCG.1 It was hypothesized that unstable metabolic status and vasculopathy may contribute to elastic fiber’s architectural damage.6 Apart from dyslipidemia, case 1 has also had DM for 10 years, which has been postulated to be a possible causative factor of EGCG. Aso et al highlighted that 37% of Japanese patients with EGCG have definite or latent DM. The proposed underlying mechanism includes the high serum glucose concentration inciting elastic fiber structural damage and inflammation.11 However, our patient’s diabetes condition was relatively well controlled, with a hemoglobin A1C of 6.58%. In fact, if DM were the cause of the patient’s EGCG, a high serum glucose concentration might not be the only possible explanation for the pathomechanism of EGCG. The idea of EGCG as a paraneoplastic condition has also been widely discussed. EGCG has been reported in patients with both hematogenous malignancies, including adult T-cell leukemia, acute myelogenous leukemia, primary cutaneous T-cell lymphoma, and solid malignancies such as prostate cancer, squamous cell carcinoma of the tonsil, esophageal carcinoma, and bronchial carcinoma.1,12–16 Among these cases, one reported EGCG having disease activity parallel with the patients’ underlying malignancy.14 Case 2 had biopsy-proven BPH with a normalized level of prostate-specific antigen for 6 years before the occurrence of EGCG. Reevaluation of the prostate after diagnosis with EGCG showed no evidence of malignant potential. Although there have been no previous reports of pituitary gland tumors in EGCG and the patient’s pituitary adenoma was completely removed 8 years prior to the onset of EGCG, long-term follow-up is essential to determine the association between the pituitary tumor and EGCG in this patient.
The histopathology of both cases was consistent with that of previous cases of EGCG.1 Interestingly, thickened, degenerated collagen bundles were observed in our cases. Qian et al recently described EGCG as exhibiting several histological patterns. With regard to diseases that occur on the lateral margins of the hands, elastorrhexis and degenerate collagen bundles are also found in collagenous and elastotic marginal plaques of the hands (CEMPH), a rare entity that often presents as a linear depressed band appearing symmetrically on the hands of the elderly.17,18 However, CEMPH was reported to contain a relatively acellular dermis without giant cells or any other significant inflammation. Elastophagocytosis was also not a feature of CEMPH.19 The overlapping clinical and histological features of these two entities in both of our patients raise the possibility that EGCG may occur as a secondary process to CEMPH. As the elastic fibers undergo the degenerative processes observed in CEMPH, their antigenicity may also undergo alteration and trigger the inflammatory reactions observed in EGCG. Another entity that can present clinically as papules and plaques on the hands and histologically as granulomas with degenerated collagen is GA. However, mucin deposition is an important hallmark of GA.20 The absence of mucin in both cases makes a GA diagnosis unlikely. A comparison of the clinicopathological characteristics of GA, CEMPH, and EGCG is shown in Table 1.10,18,19
 |
Table 1 Comparison of Clinicopathological Characteristics of Granuloma Annulare, Collagenous and Elastotic Marginal Plaques of the Hands, and Elastolytic Giant Cell Granuloma |
Treatment with EGCG is often not required because the lesions are benign and asymptomatic. Spontaneous remission over months to years has been documented.1,21 Nevertheless, several forms of treatment have been reported with varying results, including hydroxychloroquine, cyclosporine, topical, intralesional, and systemic corticosteroids, cryotherapy, methotrexate, isotretinoin, acitretin, pentoxifylline, retinoid with psoralen plus ultraviolet A, pulsed dye laser, and fractionated carbon dioxide laser.1,21–23 Both of our cases responded well to topical high-potency corticosteroids without any additional treatment. However, this deduction of the efficacy of the corticosteroids in these patients has some limitations due to the possibility of spontaneous resolution, which has also been characterized in EGCG.1,21 Additionally, this study lacks long-term follow-up to assess the possibility of recurrence and the possible disease association, which may occur much later in the disease course.
Conclusion
We report two cases of unusual Elastolytic giant cell granuloma (EGCG) with palmar involvement that were readily responsive to topical high-potency corticosteroids. This information broadens our understanding of EGCG and helps clinicians better characterize this rare disease. In cases without obvious external factors, investigation for underlying metabolic, autoimmune, and inflammatory conditions may be warranted.
Ethics Approval and Informed Consent
The patients provided written informed consent for case details and accompanying images to be published. Institutional approval was not required to publish the case details.
Funding
The authors received no financial support for this research.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Qian YT, Liu JW, Liu W, Chen T, Tan Y, Ma DL. A retrospective study of 105 patients with elastolytic giant cell granuloma and a proposal for a new clinical classification. Acta Derm Venereol. 2022;102:adv00684. doi:10.2340/actadv.v102.1985
2. O’Brien JP. Actinic granuloma. An annular connective tissue disorder affecting sun- and heat-damaged (elastotic) skin. Arch Dermatol. 1975;111(4):460–466. doi:10.1001/archderm.1975.01630160050003
3. Ma DL, Vano-Galvan S. Actinic granuloma. N Engl J Med. 2017;376(5):475. doi:10.1056/NEJMicm1600384
4. Gutiérrez-González E, Pereiro M, Toribio J. Elastolytic actinic giant cell granuloma. Dermatol Clin. 2015;33(3):331–341. doi:10.1016/j.det.2015.03.002
5. Caldas R, Guimarães MJ, Rodrigues AP, Araújo C. Generalised papular variant of elastolytic giant cell granuloma. BMJ Case Rep. 2019;12(12). doi:10.1136/bcr-2019-231580
6. Chen WT, Hsiao PF, Wu YH. Spectrum and clinical variants of giant cell elastolytic granuloma. Int J Dermatol. 2017;56(7):738–745. doi:10.1111/ijd.13502
7. Ozkaya-Bayazit E, Büyükbabani N, Baykal C, Oztürk A, Okçu M, Soyer HP. Annular elastolytic giant cell granuloma: sparing of a burn scar and successful treatment with chloroquine. Br J Dermatol. 1999;140(3):525–530. doi:10.1046/j.1365-2133.1999.02723.x
8. Oka M, Kunisada M, Nishigori C. Generalized annular elastolytic giant cell granuloma with sparing of striae distensae. J Dermatol. 2013;40(3):220–222. doi:10.1111/1346-8138.12048
9. Szabo SR, Fernelius C, Arora N. Annular elastolytic giant cell granuloma: mysterious enlarging scarring lesions. Cutis. 2019;103(1):E5–E7.
10. Burlando M, Herzum A, Cozzani E, Paudice M, Parodi A. Can Methotrexate be a successful treatment for unresponsive generalize annular elastolytic giant cell granuloma? Case report and review of the literature. Dermatol Ther. 2021;34(1):e14705. doi:10.1111/dth.14705
11. Aso Y, Izaki S, Teraki Y. Annular elastolytic giant cell granuloma associated with diabetes mellitus: a case report and review of the Japanese literature. Clin Exp Dermatol. 2011;36(8):917–919. doi:10.1111/j.1365-2230.2011.04094.x
12. Asahina A, Shirai A, Horita A, Saito I. Annular elastolytic giant cell granuloma associated with prostate carcinoma: demonstration of human metalloelastase (MMP-12) expression. Clin Exp Dermatol. 2012;37(1):70–72. doi:10.1111/j.1365-2230.2011.04110.x
13. Gyldenløve M, Faurschou A, Nielsen SL, Thyssen JP. Annular elastolytic giant cell granuloma in a patient with squamous cell carcinoma of the tonsil. JAAD Case Rep. 2015;1(1):34–35. doi:10.1016/j.jdcr.2014.11.001
14. Garg A, Kundu RV, Plotkin O, Aronson IK. Annular elastolytic giant cell granuloma heralding onset and recurrence of acute myelogenous leukemia. Arch Dermatol. 2006;142(4):532–533. doi:10.1001/archderm.142.4.532
15. Kuramoto Y, Watanabe M, Tagami H. Adult T cell leukemia accompanied by annular elastolytic giant cell granuloma. Acta Derm Venereol. 1990;70(2):164–167. doi:10.2340/0001555570164167
16. Michaelis TC, Woodcock JL, Basic KK. Actinic granuloma presenting as tender, linear plaques on the lateral fingers in a patient with newly diagnosed esophageal cancer. JAAD Case Rep. 2017;3(1):55–57. doi:10.1016/j.jdcr.2016.11.010
17. young PA, Rangel J, Pettey AA. Degenerative collagenous plaques of the hands in an elderly woman. Dermatol Online J. 2022;28(2). doi:10.5070/D328257403
18. Good AJ, Snodgrass AL, De Benedetto A, Motaparthi K. Collagenous and elastotic marginal plaques of the hand: a potential clue to the diagnosis of alkaptonuria. J Cutan Pathol. 2019;46(1):74–79. doi:10.1111/cup.13368
19. Mortimore RJ, Conrad RJ. Collagenous and elastotic marginal plaques of the hands. Australas J Dermatol. 2001;42(3):211–213. doi:10.1046/j.1440-0960.2001.00520.x
20. Joshi TP, Duvic M. Granuloma annulare: an updated review of epidemiology, pathogenesis, and treatment options. Am J Clin Dermatol. 2022;23(1):37–50. doi:10.1007/s40257-021-00636-1
21. Lazzarini R, Rotter A, Farias DC, Muller H. O’Brien’s actinic granuloma: an unusually extensive presentation. An Bras Dermatol. 2011;86(2):339–342. doi:10.1590/S0365-05962011000200019
22. Stefanaki C, Panagiotopoulos A, Kostakis P, Stefanaki K, Petridis A. Actinic granuloma successfully treated with Acitretin. Int J Dermatol. 2005;44(2):163–166. doi:10.1111/j.1365-4632.2005.02043.x
23. Mamalis A, Ho D, Parsi KK, Jagdeo J. Successful treatment of actinic granuloma with pulsed-dye laser and fractionated carbon dioxide laser. Dermatol Surg. 2018;44(3):452–454. doi:10.1097/DSS.0000000000001227
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


