Back to Journals » Journal of Pain Research » Volume 13
Tumor Necrosis Factor-α Regulates the TRPA1 Expression in Human Odontoblast-Like Cells
Authors Liu J , Que K, Liu Y, Zang C, Wen J
Received 25 March 2020
Accepted for publication 19 June 2020
Published 6 July 2020 Volume 2020:13 Pages 1655—1664
DOI https://doi.org/10.2147/JPR.S255288
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Jie Liu,1,* Kehua Que,1,* Yangqiu Liu,1 Chengcheng Zang,1 Jing Wen1,2
1Department of Endodontics, College of Stomatology, Tianjin Medical University, Tianjin, People’s Republic of China; 2Lotus Dental Clinic, Guangzhou, Guangdong Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jing Wen Tel +86 13682031869
Email [email protected]
Purpose: Transient receptor potential cation channel, subfamily A, member 1 (TRPA1) is a promiscuous chemical nociceptor involved in the perception of cold hypersensitivity, mechanical hyperalgesia and inflammatory pain in human odontoblasts (HODs). Here, we aimed to study the underlying mechanism in which inflammatory cytokine tumor necrosis factor (TNF)-α regulated the expression of TRPA1 channel at both cellular and subcellular levels.
Materials and Methods: Immunohistochemistry was used to confirm the expression of TRPA1 channel in HODs. Dental pulp cells were induced and differentiated to HOD-like cells and used in succedent experiments. Real-time quantitative polymerase chain reaction assay and Western blotting were used to examine the expression changes of TRPA1 channel with the presence and absence of TNF-α and TNF receptor (TNFR) inhibitor, R 7050. Finally, immunoelectron microscopy (IEM) and quantitative analysis were performed to directly display the TNF-α-regulated distribution change of TRPA1 channel in HOD-like cells.
Results: TRPA1 channel was positively expressed in the cell bodies and processes of HODs. The expression TRPA1 channel was significantly up-regulated by high concentration of TNF-α, which could be suppressed by R 7050. Under IEM, TNF-α treatment could increase the expression of TRPA1 in the ER membrane, cytoplasm and mitochondria.
Conclusion: Our study demonstrated that TRPA1 expression in HOD-like cells was evidently upregulated by TNF-α, presumably via TNFR1. TNF-α induced significant increasement in the intracellular distributions of TRPA1 proteins, with increases in the cytoplasm, ER membrane, and mitochondria, to actively participate in noxious external stimuli perception and transduction of hyperalgesia.
Keywords: human odontoblasts, transient receptor potential ankyrin 1, tumor necrosis factor-α, immunoelectron microscopy
Introduction
Transient receptor potential cation channel, subfamily A, member 1 (TRPA1) is the only mammalian ankyrin and is abundantly expressed in primary sensory neurons.1 As a promiscuous chemical nociceptor, TRPA1 can be activated by irritant chemicals and noxious cold (<17°C) to stimulate mechanical allodynia and hyperalgesia to produce pain and neurogenic inflammation.2–4
Human odontoblasts (HODs) form the outermost layer of dental pulp, and each possesses a long monopolar process extending into a dentinal tubule.5 Various studies have described similar features in HODs and sensory cells, such as the expression levels of mechanosensitive calcium (Ca2+) and TRP channels,6–8 suggesting that HODs can detect external stimuli and transmit the signals. The TRPA1 has been found in human dental pulp tissues, and the proportion of axons expressing TRPA1 is remarkably increased in painful pulp.9,10 Activated TRPA1 promotes adenosine triphosphate (ATP) release to activate adjacent sensory neurons and contribute to sensory transduction.11
In decayed teeth, bacteria can destroy hard dental tissues and release inflammatory cytokines such as tumor necrosis factor (TNF)-α.12,13 Several studies have reported that TNF-α expression is significantly upregulated in decayed teeth and tooth defects and is positively associated with clinical symptom severity.13,14 Therefore, with the development of caries, the deep layer of dentine will be exposed to an increasing amount of TNF-α, and TRP channels such as TRPA1, TRPV2 and TRPV4 in dental pulp tissues, can be activated to promote and maintain inflammation-related pain.10,15,16
The underlying mechanism by which TNF-α induces alters TRPA1 expression and localization in HODs remains to be elucidated. In this study, we used immunoelectron microscopy (IEM) to directly assess TRPA1 levels in the cellular compartments of HOD-like cells with the presence or absence of TNF-α at the subcellular level to investigate the reason for enhanced sensitivity to external noxious irritation in dental caries or other tooth defects.
Materials and Methods
Cell Culture and Treatments
Intact third molar teeth were extracted and collected from patients aged between 16 and 18 years, and written informed consent was obtained from parents or the patients’ legal guardians. This study was approved by the Local Ethics Committee (approval number: TMUSHh-MEC2014010, Stomatological Hospital of Tianjin Medical University) and performed in accordance with the committee guidelines. HOD-like cells were cultured as previously described by About et al.17 The expression levels of dentin sialophosphoprotein (DSPP) and nestin were detected to confirm the HOD phenotype.18 Cells derived from dental pulp explants were cultured in an incubator with 5% CO2 at constant temperature of 37°C. HOD-like cells were cultured in minimum essential medium supplemented with 2 mmol/L L-glutamine, 100 mg/mL streptomycin, 100 UI/mL penicillin, 0.25 mg/mL amphotericin B, and 2 mmol/L β-glycerophosphate with 10% fetal bovine serum. Cells from passages 4 to 6 were used in experiments. For experimental groups, HOD-like cells were incubated with 1 or 10 ng/mL TNF-α for 24 h with or without 5 μmol/mL R 7050, a TNF-α receptor (TNFR) inhibitor.
Immunohistochemistry (IHC)
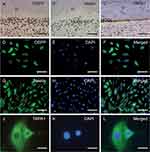
Healthy and intact teeth were collected and fixed. Routine processing was performed as previously described.19 Briefly, slices were incubated with the following primary antibodies: mouse polyclonal antibody against human DSPP (1:100; Santa Cruz Biotechnology, Dallas, TX, USA), rabbit polyclonal antibody against human nestin (1:50; Proteintech Group, Chicago, IL, USA), and rabbit polyclonal antibody against human TRPA1 (1:1000; Santa Cruz Biotech) overnight at 4°C. Then, the samples were incubated with the appropriate secondary antibody for 30 min at 37°C: goat polyclonal anti-rabbit immunoglobulin G (IgG) Alexa 594 (Thermo Fisher Scientific, Waltham, MA, USA) or goat polyclonal antimouse IgG-fluorescein isothiocyanate (ZSGB-BIO, Beijing, China). The localizations of DSPP, nestin, and TRPA1 were revealed with 3,3′-diaminobenzidine and counterstained with hematoxylin. Images were captured with an Olympus DP72 microscope (Olympus, Tokyo, Japan).
Immunofluorescence (IF)
The cultured HOD-like cells were seeded on coverslips and fixed in 4% paraformaldehyde at room temperature for 15 min. After permeabilization with 1% Triton X-100 in 1× phosphate-buffered saline (PBS) for 5 min, nonspecific binding sites were blocked with 10% complete serum at 37°C for 2 h. Then, the cell coverslips were incubated with the primary antibodies of DSPP, nestin, and TRPA1 overnight at 4°C and their secondary antibodies for 30 min at 37°C. After tyramide signal amplification, the samples were microwave heat-treated. The nuclei were counterstained with 4′-6-diamidino-2-phenylindole (Thermo Fisher Scientific). IF images were observed through upright fluorescence microscopy.
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
TRIzol reagent (Cwbio, Beijing, China) was used to extract the total RNA of HOD-like cells in accordance with the manufacturer’s instructions. The purified total RNA was then reverse transcribed into first-strand complementary DNA using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA). Relative mRNAs were evaluated through RT-qPCR with SYBR Green Master mix (Thermo Fisher Scientific) on Applied Biosystems7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). PCR amplification was performed with the following conditions: initial denaturation of 3 min at 95°C, followed by 30 cycles of 20 s at 95°C, 20 s at 58°C, 20 s at 72°C, and a final step of 10 min at 72°C. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as housekeeping gene to normalize the relative expression levels. The primer sequences were as follows: TRPA1, forward 5′-ACAAGAAGTACCAAACATGACACA-3′ and reverse 5′-TTAACTGCGTTTAAGACAAAATTCC-3′; GAPDH, forward 5′-CGTGACATTAAGGAGAAGCTG-3′ and reverse 5′-CTAGAAGCATTTGCGGTGGAC-3′.
Western Blot (WB)
HOD-like cells were washed with ice-cold PBS and lysed in lysis buffer containing 50 mmol/L Tris-HCl (pH 7.4), 0.1% sodium dodecyl sulfate, 1 mmol/L phenylmethanesulfonyl fluoride, 150 mmol/L NaCl, and 1% NP-40 (Beyotime, Shanghai, China). Total proteins were fractionated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred to a polyvinylidene fluoride membrane, which was blocked with 5% nonfat milk for 1 h at room temperature, sequentially probed with rabbit polyclonal antibody against human TRPA1 (1:1000; Santa Cruz Biotech) and horseradish peroxidase-conjugated secondary antibodies, and visualized on an electrochemiluminescence system (Solarbio, Beijing, China).
IEM
HOD-like cells were fixed and collected with 4% paraformaldehyde fixation fluid with 0.1% glutaraldehyde. The samples were treated as previously described.19 Ultrathin sections were made and mounted on nickel grids. The grids were incubated in Tris-buffered saline solution (TBS, pH 7.4) for 5 min, sealed with TBS (pH 7.4) supplemented with 2% bovine serum albumin for 10 min, and were incubated with rabbit polyclonal antibody against human TRPA1 (1:1000; Santa Cruz Biotech) at 4°C overnight. After washing, the grids were floated on drops of goat anti-rabbit antibody (1:40, Laboratory Aurion, Wageningen, Netherlands), and conjugated with 10-nm gold particles for 2 h at room temperature. Finally, the grids were dried, stained with uranium acetate, air-dried, and viewed under an electron microscope.
Quantitative IEM Distribution Analysis
The data were analyzed in accordance with the method described by Mayhew et al.19–21 Four compartments (nucleus, cytoplasm, mitochondria, and endoplasmic reticulum [ER] membrane) were systematically and randomly selected to calculate the intracellular distribution of TRPA1 with or without 10 ng/mL TNF-α treatment. The expected distribution was generated by superimposing a lattice of test point and count points (P) in selected compartments. Gold particles on all selected fields were counted and named as observed gold particles (Go). For each compartment, the observed Go was compared with the expected number (Ge, derived from the observed frequencies of point P). Labeling density (LD) was calculated as the number of gold particles per test intersection (LD = Go/P), which represents the number of particles per stereological point count (organelles). For each compartment, the relative labeling index (RLI) = LDcomp/LDcell = Go/Ge. RLI = 1 indicates random labeling, and RLI > 1 denotes that compartments are preferentially labeled.22 Using two-sample Chi-squared (χ2) analysis with two columns (Go and Ge) and c compartments, two distributions were compared, and the total and partial χ2 values were calculated to determine whether to accept or reject the null hypothesis (no difference between distributions) for c−1 degrees of freedom. Partial χ2 was calculated as (Go−Ge)2/Ge. Total χ2 is the sum of partial χ2, representing whether the gold labeling distributions are different, and the partial χ2 values identify the main responsible compartments for that difference. A convenient arbitrary cut-off is a partial χ2 value accounting for ≥10% of total χ2.23
For between-group comparisons, counting gold particles on all randomly sampled fields is the first step according to Mayhew.20 The Go in each group was then statistically compared with the corresponding “expected” distributions (Ge) using contingency table analysis. Sets of partial χ2 values were generated for each compartment and group. Examination of the total χ2 values revealed whether or not the observed gold labeling distributions in the experimental groups were different. Partial χ2 values will identify the largely responsible compartments. A convenient arbitrary cut-off is a partial χ2 value accounting for ≥10% of the total.
Statistical Analysis
SPSS for Windows (version 15.0; IBM SPSS, Chicago, IL, USA) was used to perform data analysis. Data are presented as means ± SEM. The t-test and one-way analysis of variance were used with the non-parametric Mann–Whitney and Kruskal–Wallis tests (where the data were not normally distributed). The level of significance was set at P < 0.05.
Results
Identification of HOD-Like Cells and TRPA1 Expression
IHC staining of tooth sections showed that DSPP (Figure 1A), nestin (Figure 1B), and TRPA1 (Figure 1C) were more abundant in the cell bodies and processes of HODs compared to inner pulp tissues. IF staining confirmed the expression of DSPP (Figure 1D–F) and nestin (Figure 1G–I) in HOD-like cells that were used in follow-up experiments. TRPA1 expression was evident in these cultured cells (Figure 1J–L).
TNF-α Enhanced TRPA1 Expression via TNFR1 in HOD-Like Cells
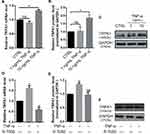
The RT-qPCR and WB results revealed that the gene and protein expression of TRPA1 significantly increased after 10 ng/mL TNF-α treatment, whereas no evident change was found in cells treated with 1 ng/mL TNF-α (Figure 2A–C, *P < 0.05).
The TNFR inhibitor R 7050 significantly suppressed the TNF-α-induced upregulation of TRPA1 gene and protein expression; indeed, TRPA1 mRNA levels in r 7050-treated cells were even lower than that in the control group (Figure 2D–F, *P < 0.05).
Increased TRPA1 in HOD-Like Cells on IEM After TNF-α Treatment
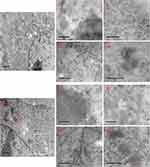
Under the high magnification of IEM, TRPA1-labeled colloidal gold particles were sparse in the entire cell (Figure 3A1) and disparate organelles, including the nuclei (Figure 3A2), cytoplasm (Figure 3A3), ER membrane (Figure 3A4), and mitochondria (Figure 3A5). After 24-h TNF-α treatment, the total number of colloidal gold particles evidently increased in the cytoplasm, ER membrane, and mitochondria (Figure 3B1–5).
The IEM quantitative analysis indicated that the intracellular distribution of TRPA1-conjugated colloidal gold particles was preferentially in the cytoplasm and mitochondria (Table 1). After TNF-α treatment, these gold particles increased and were preferentially in the cytoplasm, ER membrane, and mitochondria (Table 2).
 |
Table 1 Labeling Distributions of TRPA1 Antigen in Organelle Compartments of HOD-Like Cells Under IEM |
 |
Table 2 Labeling Distributions of TRPA1 Antigen in Organelle Compartments of HOD-Like Cells Treated with TNF-α Under IEM |
The numbers of colloidal gold particles were counted as follows. In the control group (no TNF-α), the total points of TRPA1 antigen-labeled slices of HOD-like cells (P = 463) and total number of Go (Go = 142) were used to calculate Ge for each region. The Ge for the ER membrane region was equal to 122 × 142/463 = 37.42. The RLI for the ER membrane was approximately calculated as RLI = Go/Ge = 31/37.42 = 0.83. The RLIs for the nuclei, cytoplasm, and mitochondria were 0.57, 1.13, and 4.77, respectively. The corresponding partial χ2 for the ER membrane region approximately equates to χ2 = (Go − Ge)2/Ge = (31−37.42)2/37.42 = 1.10. Using the same method, the partial χ2 values for the nuclei, cytoplasm, and mitochondria were7.17, 1.05, and 56.53, and the total χ2 was 65.84 (Table 1). With 3° of freedom (given by 2-1 group × 4-1 compartments), the total χ2 yielded a probability level of P < 0.001. Thus, the null hypothesis of no difference from random labeling must be rejected. That is, the observed and expected distributions were different; the TRPA1 antigen preferentially labeled the cytoplasm and mitochondria (Table 1). With the same assumption, after TNF-α treatment, the TRPA1 antigen preferentially labeled the ER membrane, cytoplasm, and mitochondria (Table 2).
The count results of gold label particles and partial and total χ2 values in the two groups (with and without TNF-α treatment) are provided in Table 3. The total χ2 for 3° of freedom (2-1 group × 4-1 compartments) amounted to 204.91 (P < 0.001). Consequently, the null hypothesis of no difference between groups was rejected. As shown in Table 3, the partial χ2 values revealed that the ER membrane, cytoplasm, and mitochondria were mainly responsible for the group differences. After 24-h TNF-α treatment, many TRPA1-conjugated colloidal gold particles shifted toward the ER membrane, cytoplasm, and mitochondria.
Discussion
This study investigated the regulatory mechanism of TNF-α on TRPA1 in HOD-like cells at the cellular and subcellular levels. The results suggested that the high concentration TNF-α treatment evidently upregulated TRPA1 expression in HOD-like cells and that TNFR1, a key receptor of TNF-α, was involved in this process. Profound differential intracellular distributions and changes in TRPA1 protein levels in TNF-α-treated HOD-like cells were revealed through IEM, and expression increased in the ER membrane, cytoplasm and mitochondria. In this study, cultured cells were identified through the expression levels of odontogenic-specific markers and had similar morphology and functions to HODs.17
In decayed teeth, increases in inflammatory gene expression primarily arises in the odontoblast layer rather than other pulp tissues and can produce antimicrobial peptides to increase the defensive capacity, thereby protecting teeth.12 TRPA1 plays an important role in dentin hypersensitivity induced by mechanical stimulation and early inflammatory sensitization.24 Although the mechanism of dentin hypersensitivity remains unclear, the protein expression levels of many TRP channels in HODs are significantly higher than in other pulp tissues,19 suggesting that HODs have an important sensory function and play a vital role in dentin hypersensitivity pathogenesis. TRP channels in HODs and HOD-like cells can be activated by the corresponding stimuli and then participate in the process of sensing and responding to different stimuli and induce hyperalgesia. Once the dentin is exposed, dental pulp tissue can be affected by bacterial toxins. As the outermost cells of dental pulp tissue, HODs are susceptible to external stimuli, which in turn lead to enhanced dental pulp sensation and defensive effects. As a common inflammatory mediator in dental caries, a high concentration of TNF-α significantly upregulated TRPA1 expression. The activated HODs were sensitive to external stimuli and released ATP to activate the surrounding nerve fibers for pain transduction. This is in accordance with the hyperalgesia to external irritation in teeth with deep caries or noncarious cervical defects, implying a vital role of HODs in blocking pulp pain. TNF-α induces cytotoxicity and pain-associated behavior by stimulating TNFR1.25 In this study, the inhibitory effect of R 7050 suggested that TNFR1 was involved in this regulation via association with intracellular adaptor molecules.26
After combination with colloidal gold particles, high-resolution immunolocalization of identified antigens in different subcellular and cellular compartments was clearly defined with IEM. Image analysis revealed that TNF-α upregulated TRPA1 expression in the cytoplasm, ER membrane, and mitochondria but downregulated it in nuclei. This might involve the production and transport of TRPA1 protein in HODs after upregulation by inflammatory cytokines. On the basis of the exocytotic trafficking mechanism of membrane receptors to the plasma membrane in response to stimuli,27 TRPA1 protein expression can be upregulated and aggregate in the cytoplasm, assembled in the ER membrane, sorted into vesicles, and transported toward the plasma membrane as mature proteins. During inflammation, they interact with signaling molecules and then actively participate in the perception and transmission of noxious stimuli. Increased TRPA1 expression in the mitochondria might explain the TRPA1-mediated increase in ATP release. Excessive TRPA1-mediated Ca2+ entry can induce inflammatory cytokine production,28 mitochondrial depolarization,29 and cell apoptosis.30 A widespread increase in intracellular TRPA1 expression as described here might promote and aggravate inflammatory responses through the same mechanism, mediating the apoptosis of HODs in teeth with deep caries and hard tissue defects.
Conclusion
This study demonstrated that TRPA1 expression in HOD-like cells was evidently upregulated by TNF-α, presumably via TNFR1. Exogenous TNF-α induced significant alterations in the intracellular distributions of TRPA1 proteins, with increases in the cytoplasm, ER membrane, and mitochondria, to actively participate in noxious external stimuli perception and transduction of hyperalgesia.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bhattacharya MR, Bautista DM, Wu K, Haeberle H, Lumpkin EA, Julius D. Radial stretch reveals distinct populations of mechanosensitive mammalian somatosensory neurons. Proc Natl Acad Sci U S A. 2008;105:20015–20020. doi:10.1073/pnas.0810801105
2. Bandell M, Story GM, Hwang SW, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi:10.1016/S0896-6273(04)00150-3
3. Zygmunt PM, Hogestatt ED. TRPA1. Handb Exp Pharmacol. 2014;222:583–630.
4. Nassini R, Materazzi S, Benemei S, Geppetti P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev Physiol Biochem Pharmacol. 2014;167:1–43.
5. Khatibi Shahidi M, Krivanek J, Kaukua N, et al. Three-dimensional imaging reveals new compartments and structural adaptations in odontoblasts. J Dent Res. 2015;94:945–954. doi:10.1177/0022034515580796
6. Allard B, Couble ML, Magloire H, Bleicher F. Characterization and gene expression of high conductance calcium-activated potassium channels displaying mechanosensitivity in human odontoblasts. J Biol Chem. 2000;275:25556–25561. doi:10.1074/jbc.M002327200
7. El Karim IA, Linden GJ, Curtis TM, et al. Human odontoblasts express functional thermo-sensitive TRP channels: implications for dentin sensitivity. Pain. 2011;152:2211–2223. doi:10.1016/j.pain.2010.10.016
8. Allard B, Magloire H, Couble ML, Maurin JC, Bleicher F. Voltage-gated sodium channels confer excitability to human odontoblasts: possible role in tooth pain transmission. J Biol Chem. 2006;281:29002–29010. doi:10.1074/jbc.M601020200
9. Kim YS, Jung HK, Kwon TK, et al. Expression of transient receptor potential ankyrin 1 in human dental pulp. J Endod. 2012;38:1087–1092. doi:10.1016/j.joen.2012.04.024
10. El Karim I, McCrudden MT, Linden GJ, et al. TNF-alpha-induced p38MAPK activation regulates TRPA1 and TRPV4 activity in odontoblast-like cells. Am J Pathol. 2015;185:2994–3002. doi:10.1016/j.ajpath.2015.07.020
11. Egbuniwe O, Grover S, Duggal AK, et al. TRPA1 and TRPV4 activation in human odontoblasts stimulates ATP release. J Dent Res. 2014;93:911–917. doi:10.1177/0022034514544507
12. Horst OV, Horst JA, Samudrala R, Dale BA. Caries induced cytokine network in the odontoblast layer of human teeth. BMC Immunol. 2011;12:9. doi:10.1186/1471-2172-12-9
13. Kokkas AB, Goulas A, Varsamidis K, Mirtsou V, Tziafas D. Irreversible but not reversible pulpitis is associated with up-regulation of tumour necrosis factor-alpha gene expression in human pulp. Int Endod J. 2007;40:198–203. doi:10.1111/j.1365-2591.2007.01215.x
14. Hall BE, Zhang L, Sun ZJ, et al. Conditional TNF-alpha overexpression in the tooth and alveolar bone results in painful pulpitis and osteitis. J Dent Res. 2016;95:188–195. doi:10.1177/0022034515612022
15. Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain. 2000;85(1–2):145–151. doi:10.1016/S0304-3959(99)00262-6
16. Liu J, Zhao Z, Wen J, et al. TNF-alpha differently regulates TRPV2 and TRPV4 channels in human dental pulp cells. Int Endod J. 2019;52:1617–1628. doi:10.1111/iej.13174
17. About I, Bottero MJ, de Denato P, Camps J, Franquin JC, Mitsiadis TA. Human dentin production in vitro. Exp Cell Res. 2000;258:33–41. doi:10.1006/excr.2000.4909
18. Lee HK, Park JW, Seo YM, et al. Odontoblastic inductive potential of epithelial cells derived from human deciduous dental pulp. J Mol Histol. 2016;47:345–351. doi:10.1007/s10735-016-9676-1
19. Wen W, Que K, Zang C, et al. Expression and distribution of three transient receptor potential vanilloid (TRPV) channel proteins in human odontoblast-like cells. J Mol Histol. 2017;48:367–377. doi:10.1007/s10735-017-9735-2
20. Mayhew TM. Quantifying immunogold localization patterns on electron microscopic thin sections of placenta: recent developments. Placenta. 2009;30:565–570. doi:10.1016/j.placenta.2009.04.013
21. Mayhew T, Griffiths G, Habermann A, Lucocq J, Emre N, Webster P. A simpler way of comparing the labelling densities of cellular compartments illustrated using data from VPARP and LAMP-1 immunogold labelling experiments. Histochem Cell Biol. 2003;119:333–341. doi:10.1007/s00418-003-0523-6
22. Mayhew TM, Lucocq JM. Multiple-labelling immunoEM using different sizes of colloidal gold: alternative approaches to test for differential distribution and colocalization in subcellular structures. Histochem Cell Biol. 2011;135:317–326. doi:10.1007/s00418-011-0788-0
23. Mayhew TM, Lucocq JM. Developments in cell biology for quantitative immunoelectron microscopy based on thin sections: a review. Histochem Cell Biol. 2008;130:299–313. doi:10.1007/s00418-008-0451-6
24. Magloire H. Odontoblast and dentin thermal sensitivity. Pain. 2011;152:2191–2192. doi:10.1016/j.pain.2011.02.042
25. McFarlane SM, Pashmi G, Connell MC, et al. Differential activation of nuclear factor-kappaB by tumour necrosis factor receptor subtypes. TNFR1 predominates whereas TNFR2 activates transcription poorly. FEBS Lett. 2002;515:119–126. doi:10.1016/S0014-5793(02)02450-X
26. Gururaja TL, Yung S, Ding R, et al. A class of small molecules that inhibit TNFalpha-induced survival and death pathways via prevention of interactions between TNFalphaRI, TRADD, and RIP1. Chem Biol. 2007;14:1105–1118. doi:10.1016/j.chembiol.2007.08.012
27. Ferrandiz-Huertas C, Mathivanan S, Wolf CJ, Devesa I, Ferrer-Montiel A. Trafficking of ThermoTRP channels. Membranes (Basel). 2014;4:525–564. doi:10.3390/membranes4030525
28. Deveci HA, Akyuva Y, Nur G, Naziroglu M. Alpha lipoic acid attenuates hypoxia-induced apoptosis, inflammation and mitochondrial oxidative stress via inhibition of TRPA1 channel in human glioblastoma cell line. Biomed Pharmacother. 2019;111:292–304. doi:10.1016/j.biopha.2018.12.077
29. Llorente-Folch I, Rueda CB, Pardo B, Szabadkai G, Duchen MR, Satrustegui J. The regulation of neuronal mitochondrial metabolism by calcium. J Physiol. 2015;593:3447–3462. doi:10.1113/JP270254
30. Kim JY, Yu SJ, Oh HJ, Lee JY, Kim Y, Sohn J. Panaxydol induces apoptosis through an increased intracellular calcium level, activation of JNK and p38 MAPK and NADPH oxidase-dependent generation of reactive oxygen species. Apoptosis. 2011;16:347–358. doi:10.1007/s10495-010-0567-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.