Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Tuberculosis Surveillance in Romania Among Vulnerable Risk Groups Between 2015 and 2017
Authors Munteanu I , Cioran N, van Hest R, Abubakar I, Story A, Chiotan D, de Vries G, Mahler B
Received 18 January 2022
Accepted for publication 10 April 2022
Published 20 April 2022 Volume 2022:18 Pages 439—446
DOI https://doi.org/10.2147/TCRM.S347748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Ioana Munteanu,1 Nicoleta Cioran,1,2 Rob van Hest,3,4 Ibrahim Abubakar,5 Alistair Story,6,7 Domnica Chiotan,1 Gerard de Vries,8,9 Beatrice Mahler1,10
1Department of Pneumology, Marius Nasta Institute of Pneumology, Bucharest, Romania; 2 3rd Department – Complementary Sciences, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania; 3Department of Tuberculosis Control, Regional Public Health Service Groningen, Groningen, The Netherlands; 4Department of Lung Diseases and Tuberculosis, University Medical Centre Groningen (UMCG), Groningen, The Netherlands; 5Institute for Global Health, University College London, London, UK; 6Institute of Health Informatics, University College London, London, UK; 7Find and Treat, University College Hospitals NHS Foundation Trust, London, UK; 8KNCV Tuberculosis Foundation, The Hague, The Netherlands; 9Centre for Infectious Disease Control, National Institute for Public Health and the Environment, Bilthoven, The Netherlands; 10 4th Department – Cardio-Thoracic Pathology, “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
Correspondence: Nicoleta Cioran, Department of Pneumology, Marius Nasta Institute of Pneumology, Soseaua Viilor nr.90, Sector 5, Bucharest, Romania, Tel +40 745 419 994, Fax +40 213 373 801, Email [email protected]
Purpose: Romania has the highest tuberculosis (TB) burden in the European Union/European Economic Area (EU/EEA) comprising almost a quarter (23.4%) of the reported patients in 2017, and a TB notification rate six times higher than the EU/EEA average. Although the overall TB notification rate in Romania declined from 154/100.000 individuals to 66/100.000 individuals in the general population between 2002 and 2017, TB notification rates remain high in certain vulnerable populations groups such as prisoners, the homeless population and among drug users.
Patients and Methods: We conducted a descriptive study regarding TB monitoring data in Romania, including the aforementioned TB risk groups.
Results: Analysis regarding notified TB cases among these risk groups indicates that TB rates are 7 to 18 times higher than in the general population. One of the most alarming aspects regards the exceedingly high proportion of HIV-seropositivity among drug users and the high mortality rates among the homeless population and among drug users with TB.
Conclusion: This data underlines the importance of early identification among social risk groups using outreach active case-finding (ACF) activities, possibly combining TB screening with screening for other common, possibly life-threatening, co-morbidities for which an effective treatment is available. ACF could have a decisive role in TB control and eradication in Romania, when aimed at these high-risk groups.
Keywords: Romania, tuberculosis, notification rate, risk groups
Introduction
Tuberculosis (TB) is a public health issue in the European Union (EU) and European Economic Area (EEA), disproportionately affecting certain risk groups that oftentimes are socially excluded.1 Vulnerable and hard-to-reach populations at risk for TB include the incarcerated population or those in enforced segregation, the homeless population or people with a history of homelessness, those with high-risk drug abuse, individuals struggling with alcohol addiction, some vulnerable migrant populations who are excluded from health and social care services, and other marginalized, poor and remote groups.2 According to the European Centre for Disease Prevention and Control (ECDC), interventions in vulnerable groups is key to the eradication of TB in Europe.3
Romania has the highest TB burden in the EU/EEA comprising almost a quarter (23.4%) of the reported patients in 2017, and a TB notification rate six times higher than the EU/EEA average.3 The overall TB notification rates in Romania declined substantially from 154/100.000 individuals in 2002 to 66/100.000 individuals in 2017 (Figure 1), in absolute numbers from 33.595 patients in 2002 to 13.005 patients in 2017.3,4 Multi-drug resistant (MDR) cases have declined between 2012 and 2015 from 634 to 538 patients and extensively drug-resistant (XDR) TB cases have increased in the same timeframe from 41 to 71.4 Despite the steady decline in TB incidence rates in the general population (71.7 in 2015, 64.8 in 2016 and 62.8 in 2017), they remain high in certain vulnerable populations groups such as the incarcerated population, the homeless population, and those suffering from drug addiction. Their increased risk of TB is a consequence of specific conditions favouring transmission and progression of the disease, such as poor living conditions and overcrowding, HIV-infection and other co-morbidities, and they often have limited access to health care services.5
 |
Figure 1 Trend of TB notification rates in Romania, 2002–2017. |
The control of TB is founded on early identification of cases, initiation and completion of treatment, and impeding transmission. In order to address the high TB burden in vulnerable EU populations, in 2016 the Early DETECTion of tuberculosis consortium (E-DETECT TB) was formed and received funding from the European Commission.6 One of the work packages (WP) of E-DETECT TB is focusing on active case finding (ACF) in vulnerable groups in Romania including inmates, the homeless population, and those suffering from drug addiction, and applying innovative diagnostic tools such as a mobile digital chest X-ray unit (MXU) equipped with a computer-aided reading device and rapid bacteriological tests such as Xpert MTB/RIF.
In this study, we describe the analysis of TB surveillance data in Romania, with specific focus on the incidence of TB and the characteristics of vulnerable populations, in order to inform current and future targeting of ACF.
Materials and Methods
Study Period and Data
The data for all patients with TB and those belonging to the TB risk groups were obtained from the National Tuberculosis Programme (NTP) register of Romania, found at the Institute for Lung Diseases Marius Nasta in Bucharest. The data was collected for all notified TB cases from January 2015 to December 2017. The database is dynamic, ie, the annual data for specific years uploaded to The European Surveillance System (TESSy) at ECDC are continuously updated and may change again in subsequent years. Therefore, data published in the World Health Organization (WHO)/ECDC Tuberculosis surveillance and monitoring in Europe reports for 2017 may slightly differ from the updated numbers used in this manuscript.
Demographic variables from the NTP register (sex, age group, country of birth and urban residence) were collected for all notified TB patients and those belonging to the TB risk groups under study, alongside co-morbidities (recorded HIV infection), history of previous TB disease and bacteriological variables (pulmonary TB, sputum smear-positive TB, culture-positive TB, and rifampicin-resistance (RR-TB)). The results of treatment outcome monitoring were classified as “successful” (cured/completed); “deceased”; and “other” (treatment failed; lost to follow-up; transferred out).
Risk Group Definitions
In the Romanian NTP register the following definitions for inmates, homeless people and drug user are applied:
A homeless person is a person who has no accommodation, cannot document accommodation, or occupies accommodation without the legal consent of the owner.
A drug user is a person who consumes large and regular doses of (possibly different) drugs, resulting in addiction, ie, the loss of capability to refrain from using the drug(s), progressively affecting social life, material status and existence in general.
An inmate is a person who is reported to be incarcerated at the time of the notification of the TB diagnosis.
Until February 2017, only one social risk group category could be entered in the register, eg, either individuals without health insurance or homeless individuals. Since February 2017, multiple entries are possible (or more categories). By default, an incarcerated person cannot be classified as homeless.
TB Risk Group Population Size Estimates
In order to calculate incidence rates, the size of the population groups needs to be known or estimated. Samusocial, a non-governmental organisation (NGO) working with the homeless populations, estimated that the number of homeless individuals in Bucharest was approximately 5.000 and for the whole of Romania was approximately 15,000. ARAS, an NGO working with drug users, estimated that their numbers were around 10.000 (7356 drug users who received assistance at the national level in 2019). The National Prison Authorities report the number of inmates in Romania on an annual basis, the number of inmates being 28.354 in 2015, 27.114 in 2016 and 22.532 in 2017.
Statistical Methods
We analysed data with SPSS version 24 (SPSS, Inc., Chicago, IL) and calculated the odds ratio, including 95% confidence intervals, of demographic and disease-related characteristics of TB cases among inmates, homeless individuals, and drug users by comparing TB patients with these three risk profiles and those without. Multivariable logistic regression was performed, considering factors significant in univariable analysis. Variables with p < 0.25 were considered for inclusion in multivariable modelling. By backward elimination, the most parsimonious model was selected through −2 log likelihood testing. P < 0.05 was considered statistically significant.
Ethics Statement
The data protection committee of the Institute for Lung Diseases Marius Nasta approved the use of the surveillance data for this study. No informed consent was sought from patients, as secondary data were extracted in aggregate from international reports – ECDC (Tessy) and WHO (TME) – by the National Tuberculosis Prevention, Surveillance and Control Program from Romania.
Results
The total number of notified TB cases in Romania during the study period (2015–2017) was 41.789 (15.183, 13.601 and 13.005 cases in 2015, 2016 and 2017 respectively), with 441 registered as inmates, 405 patients as homeless individuals and 189 as drug users. The annual number of notified TB patients among inmates, homeless individuals, and drug users in Romania between 2006 and 2017 is shown in Figure 2. Figure 3 shows the overlapping profiles of patients belonging to the three risk groups. In total, 972 patients (2.3%) belonged to one or two of these risk groups between 2015 and 2017.
 |
Figure 2 Annual number of notified tuberculosis patients among inmates, homeless individuals and drug users in Romania, 2006–2017. |
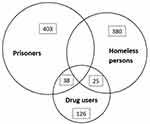 |
Figure 3 Overlap of tuberculosis risk groups (inmates, homeless individuals and drug users) in Romania, 2015–2017. |
The characteristics for notified TB patients belonging to the three risk groups and all other notified TB patients are shown in Table 1. Almost all TB patients (99.8%) were born in Romania. Compared to TB patients without the three risk profiles, inmates with TB were significantly more often male (aOR 10.8) and within the age group of 20 to 39 years old. They had pulmonary TB more frequently (aOR 4.9), smear-positive TB less frequently (aOR 0.3) and were more often co-infected with HIV (aOR 7.8). Homeless individuals with TB were significantly more often male (aOR 2.4), within the age group of 40 to 59 years old and lived in urban areas (aOR 5.1). They had pulmonary TB more frequently (aOR 1.7), RR TB (aOR 1.6), previous history of TB (aOR 2.1) and HIV co-infection (aOR 5.4). Drug users with TB were significantly more often male (aOR 4.0), within the age group of 20 to 39 years old and lived in urban areas (aOR 6.4). Drug users with TB very frequently have an HIV co-infection (86.8%, aOR 247).
 |
Table 1 Demographic and Disease Characteristics of TB Patients in Romania, 2015–2017, by Risk Group (None, Inmates, Homeless Individuals and Drug Users).* |
Since 2006, the number of inmates registered with TB has reduced from 281 to 110 per year (Figure 2), resulting in an average annual TB notification rate of 542/100.000 prisoners between 2015 and 2017 (taking the number of prisoners in 2016 −27.114- as the denominator). In contrast, the number of homeless individuals registered with TB gradually increased over the years, resulting in an overall TB notification rate of 1.220/100.000 homeless individuals (183/15.000) in 2017. Of these, 42 homeless persons were registered in Bucharest, resulting in a TB incidence of 840/100.000 homeless individuals (42/5000). The number of drug users notified with TB increased five-fold, from only 12 in 2006 to 57 in 2017, translating into a TB notification rate of 570/100.000 drug users.
The treatment outcome data for the 2015, 2016 and 2017 cohorts are shown in Table 2A (for non-RR TB) and Table 2B (for RR TB). With 60.3% and 62.7% successful treatment outcomes respectively, homeless individuals and drug users with non-RR TB have a much less favourable outcome compared to all TB patients (84.3%). Successful treatment outcomes are, as expected, much lower for all TB patients with RR TB, also among inmates, homeless individuals and drug users, when compared to those with non-RR TB, although the numbers are small.
 |
Table 2 Treatment Outcome for the 2015–2017 Cohorts for Non-Rifampicin-Resistant and Rifampicin-Resistant Tuberculosis (After 12 and 24 Months Respectively) |
Discussion
Despite the considerable decline in TB notification rates in the general population of Romania from 154/100.000 individuals to 66/100.000 individuals between 2002 and 2017, in certain vulnerable TB risk groups these rates are higher, and TB remains a major public health concern.4 Analysis of notified TB cases among inmates, homeless people, and drug users in the NTP indicates that TB notification rates are 7 to 18 times higher among these risk groups compared to the general population. Individuals belonging to the TB risk groups are predominantly male; homeless people tend to be older (40–59 years) compared to inmates and drug users (predominantly 20–39 years old). Although TB in Romania is more common in rural areas, the TB high-risk groups of homeless individuals and drug users are over-represented in urban areas. Inmates tend to have more pulmonary TB but are less often infectious compared to homeless individuals and drug users, probably as a result of the screening process (which consists in a pulmonary X-ray) performed for intrathoracic TB upon incarceration. Most alarming is the exceedingly high proportion of HIV-seropositivity among drug users and the high mortality rates among both homeless people and drug users with TB.
Not surprisingly, inmates have a very high chance of a successful outcome of their TB treatment (91.8%), with a low mortality rate (3,3%). The mortality among homeless individuals (15.9%) and drug users (19.3%) is almost twice as high compared to all TB cases (8%). Also, “other” treatment outcomes (treatment failed; lost to follow-up; transferred out) is more common among homeless individuals and drug users with non-RR TB.
The strength of this study is that national surveillance data of the NTP was used, portraying a more complete picture, but at the same time introducing various possible limitations. Foremost, registration and classification bias cannot be excluded when risk group information is not appreciated or recorded, or when risk group definitions are not ubiquitously applied. Since the national TB register in Romania allows for recording more variables on vulnerable risk groups for TB, under-registration might be reduced. Consistent use of identical social risk group definitions could further improve the quality of the NTR.7 Apart from inmates, risk group denominators for homeless individuals and drug users were estimated in the absence of an existing and consistent national register, possibly resulting in under-estimation of population size, translating in over-estimation of specific TB notification rates. On the other hand, the number of homeless people and drug users notified with TB seems relatively low, and therefore under-registration cannot be excluded, possibly resulting into under-estimation of the respective TB notification rates; both biases could (partly) balance-out. An alternative to estimate the size of “hard-to-count” populations, such as homeless people or drug users, could be represented by inventory studies and capture-recapture analysis, as applied earlier in Rotterdam.8–10
In most EU/EEA countries, when TB incidence drops in the general population, the disease becomes more concentrated among those who are at the lower end of the socio-economic scale, especially among vulnerable and socially excluded populations, often found in urban areas.11,12 Tailored approaches are needed to facilitate effective prevention and control of TB among these groups, whose socio-economic conditions or lifestyle make it difficult to recognise TB symptoms, access health services, self-administer treatment and attend regular healthcare appointments.13,14 Vulnerable populations not only experience a higher risk of developing TB but also, once the disease is manifest, face a higher risk for sputum smear-positivity, drug resistant TB and poor treatment outcomes than that of TB patients not belonging to a social risk group, as previously described in London and Rotterdam.15,16 Also, socially vulnerable TB risk groups often face a higher risk of co-morbidities, such as hepatitis or HIV infection.17
For early case-finding among social risk groups ACF activities are indispensable.13 The effectiveness, and in some cases, cost-effectiveness of ACF among social risk groups, particularly among homeless individuals, have been demonstrated, including evidence of transmission interruption and outbreak control.15,18 Integrating outreach services to include both case-detection and case-management interventions that share a resource infrastructure may allow cost-effectiveness to be maximised. The remarkably high proportion of HIV-seropositivity among drug users in Romania calls for intensive cooperation with services for HIV-seropositive individuals, such as the Romanian NGO ARAS, as this could also be the case for hepatitis C co-infection.19 Integrating screening and treatment for other diseases that are prevalent among targeted risk groups into TB outreach interventions may further improve cost-effectiveness.13 As an example, Find&Treat in London are a specialist outreach team dedicated to the early detection and management of TB and blood-borne viral diseases among inmates, homeless individuals, drug users and other vulnerable populations, guided by epidemiological analyses and has shown to be cost-effective.14 Find&Treat operates a mobile health unit equipped with digital radiography, XpertMTB/RIF, and services for blood-borne viruses, such a HIV and hepatitis B and hepatitis C.17,19,20 For case-management, compared to medication supervision though directly observed therapy (DOT), Find&Treat demonstrated that video-observed therapy (VOT) supervision appears to be a promising tool for the future, in line with the use of social media.21
This study demonstrates that vulnerable populations in Romania, such as inmates, homeless individuals and drugs users are at increased risk for TB compared to the general population without these social risk factors. It also shows that, at the same time, the treatment outcome among homeless individuals and drug users is considerably less successful compared to the general population, and with high mortality rates. Inherently, inmates with TB are identified early and have a high treatment success rate, with low mortality. An alarmingly high proportion of drug users with TB are also HIV seropositive. This study has not examined hepatitis C infection, but high co-morbidity has been described among homeless individuals elsewhere.19
Conclusion
Our results underpin the need for ACF through outreach services in Romania to tackle TB and related, possibly life-threatening, co-morbidities, for which an effective treatment is available, among vulnerable risk groups, especially homeless people and drug users, as inmates can be screened upon (and on indication during) incarceration. Early case-finding, treatment initiation and treatment completion, resulting in interruption of TB transmission, reduction of mortality and addressing possible co-morbidities, through dedicated and multidisciplinary outreach services are key to eliminate TB among social risk groups in Romania, and indirectly reduce TB incidence among the general population, as well as tackling blood-borne virus diseases among the risk groups.22
Disclosure
The authors report no conflicts of interest in this work.
References
1. (13) Tuberculosis control in homeless persons in European Union: more than words alone | request PDF. Available from: https://www.researchgate.net/publication/26700749_Tuberculosis_control_in_homeless_persons_in_European_Union_more_than_words_alone.
2. Story A, van Hest R, Hayward A. Tuberculosis and social exclusion. BMJ. 2006;333(7558):57–58. doi:10.1136/BMJ.333.7558.57
3. Interventions in vulnerable groups are the key to eliminating tuberculosis in Europe. Available from: https://www.ecdc.europa.eu/en/publications-data/interventions-vulnerable-groups-are-key-eliminating-tuberculosis-europe.
4. Golli A-L, Niţu MF, Turcu F, Popescu M, Ciobanu-Mitrache L, Olteanu M. Tuberculosis remains a public health problem in Romania. Int J Tuberc Lung Dis. 2019;23(2):226–231. doi:10.5588/IJTLD.18.0270
5. Feral-Pierssens A-L, Aubry A, Truchot J. Emergency Care for Homeless Patients: a French Multicenter Cohort Study. Am J Public Health. 2016;106(5):893–898. doi:10.2105/AJPH.2015.303038
6. Abubakar I, Matteelli A. Towards tackling tuberculosis in vulnerable groups in the European Union: the E-DETECT TB consortium. Eur Respir J. 2018;51(5):332. doi:10.1183/13993003.02604-2017
7. EMCDDA | European Drug Report 2015: data and statistics: methods and definitions for drug-related deaths and mortality. Available from: https://www.emcdda.europa.eu/data/stats2015/methods-drd.
8. Van Hest N. Capture-recapture Methods in Surveillance of Tuberculosis and Other Infectious Diseases. Univ Med Cent Rotterdam Repos. 2007;1:65.
9. World Health Organization. Assessing Tuberculosis Under-Reporting Through Inventory Studies. Assess Tuberc Under Rep Through Invent Stud. 2012;1(May):129.
10. Van Hest N, De VRIES G, Smit F, Grant A, RICHARDUS J. Estimating the coverage of a targeted mobile tuberculosis screening programme among illicit drug users and homeless persons with truncated models. Epidemiol Infect. 2008;136(5):628–635. doi:10.1017/S0950268807009235
11. Van Hest NA, Aldridge RW, De Vries G. Tuberculosis control in big cities and urban risk groups in the European Union: a consensus statement. Euro Surveill. 2014;19(9). doi:10.2807/1560-7917.ES2014.19.9.20728
12. Vries G, Aldridge RW, Cayla JA. Epidemiology of tuberculosis in big cities of the European Union and European Economic Area countries. Euro Surveill. 2014;19(9). doi:10.2807/1560-7917.ES2014.19.9.20726
13. Gupta RK, Lipman M, Story A. Active case finding and treatment adherence in risk groups in the tuberculosis pre-elimination era. Int J Tuberc Lung Dis. 2018;22(5):479–487. doi:10.5588/IJTLD.17.0767
14. Jit M, Stagg HR, Aldridge RW, White PJ, Abubakar I. Dedicated outreach service for hard to reach patients with tuberculosis in London: observational study and economic evaluation. BMJ. 2011;343:7826. doi:10.1136/BMJ.D5376
15. de Vries G, van Hest RAH, Richardus JH. Impact of mobile radiographic screening on tuberculosis among drug users and homeless persons. Am J Respir Crit Care Med. 2007;176(2):201–207. doi:10.1164/RCCM.200612-1877OC
16. Story A, Murad S, Roberts W, Verheyen M, Hayward AC. Tuberculosis in London: the importance of homelessness, problem drug use and prison. Thorax. 2007;62(8):667–671. doi:10.1136/THX.2006.065409
17. Aldridge RW, Hayward AC, Hemming S, et al. Original article: high prevalence of latent tuberculosis and bloodborne virus infection in a homeless population. Thorax. 2018;73(6):557. doi:10.1136/THORAXJNL-2016-209579
18. van Hest R, de Vries G. Active tuberculosis case-finding among drug users and homeless persons: after the outbreak. Eur Respir J. 2016;48(1):269–271. doi:10.1183/13993003.00284-2016
19. Aldridge RW, Hayward AC, Hemming S. High prevalence of latent tuberculosis and bloodborne virus infection in a homeless population. Thorax. 2018;73(6):557–564. doi:10.1136/THORAXJNL-2016-209579
20. Lee Y, Raviglione MC, Flahault A. Use of Digital Technology to Enhance Tuberculosis Control: scoping Review. J Med Internet Res. 2020;22:2. doi:10.2196/15727
21. Story A, Aldridge RW, Smith CM. Smartphone-enabled video-observed versus directly observed treatment for tuberculosis: a multicentre, analyst-blinded, randomised, controlled superiority trial. Lancet. 2019;393(10177):1216–1224. doi:10.1016/S0140-6736(18
22. Mahler B, Croitoru A. Pulmonary rehabilitation and tuberculosis: a new approach for an old disease. Pneumologia. 2019;68(3):107–113. doi:10.2478/PNEUM-2019-0024
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
