Back to Journals » Clinical Ophthalmology » Volume 14
Tube Revision Outcomes for Exposure with Different Repair Techniques
Authors Alawi A, AlBeshri A, Schargel K , Ahmad K , Malik R
Received 15 May 2020
Accepted for publication 17 July 2020
Published 2 October 2020 Volume 2020:14 Pages 3001—3008
DOI https://doi.org/10.2147/OPTH.S261957
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Abeer Alawi,1 Ali AlBeshri,1 Konrad Schargel,1 Khabir Ahmad,2 Rizwan Malik1
1Glaucoma Division, King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia; 2Research Department, King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia
Correspondence: Rizwan Malik
King Khaled Eye Specialist Hospital, Al Urubah Branch Road, Umm Al Hamam Al Gharbi, Riyadh 12329, Saudi Arabia
Tel +966 11 482 1234
Fax +966 11 4829311
Email [email protected]
Purpose: The aim of this study was to report our experience with eyes that presented with an initial GDD exposure and their subsequent outcome in terms of re-exposure.
Methods: A retrospective review of charts of 42 patients (43 eyes) who presented with a GDD exposure during the period 2008– 2015 in a tertiary eye care center was performed. Demographic data, past ocular history, pre-operative and post-operative information including the surgical technique of GDD surgery and exposure repair were recorded. The patients were followed for further exposure to the date of the last follow-up clinic visit. For each type of repair technique, details were collected on risk and timing of GDD exposure. The baseline features of eyes that had further exposure after initial exposure were compared to eyes without further exposure.
Results: Forty-three eyes were identified which had repair after an initial exposure. The mean ± SD age was 54 ± 27 years. Of the GDDs, Ahmed FP7 was performed in 31 eyes, Ahmed FP8 in two eyes, Ahmed S2 in five eyes, Krupin valve in two eyes and Baerveldt 350 GDD in three eyes. The methods of repair and the relative risk [95% CI] of re-exposure were: conjunctival closure only (n=4; RR=2.10 [0.84– 5.23]); repair with patch graft and conjunctival repair (n=18), RR=1.24 [0.51– 3.01]; tube repositioning, use of patch graft and conjunctival repair (n=14), RR=1.0; tube removal with replacement in a different quadrant, patch graft and conjunctival repair (n=3), RR=1.87 [0.64– 5.48]. After the first exposure, 18 eyes had a second re-exposure, four eyes had a third re-exposure, and 1 eye had a fourth exposure.
Conclusions: The GDD exposure rates at our institution are consistent with other reports. Lack of a patch graft for repair is associated with a two-fold risk of subsequent re-exposure.
Keywords: glaucoma drainage device, tube exposure, surgical repair
Introduction
Glaucoma drainage devices (GDD) are conventionally used to control intraocular pressure (IOP) in patients with glaucoma refractory to filtering surgery and create a non-physiologic pathway in which the aqueous is diverted from the anterior chamber to the subconjunctival space.
Whilst GDDs are associated with high surgical success, they can be associated with intraoperative and post-operative complications. The intraoperative complications include hyphema, scleral perforation, and suprachoroidal hemorrhage. The early post-operative complications include flat chamber, hypotony, tube blockage, retinal detachment and choroidal detachment and late post-operative complications include encapsulation, cataract, corneal decompensation, strabismus, exposure of the device and endophthalmitis.1,2
Exposure of the GDD is one of the major complications of this procedure. Once the exposure has been identified, timely revision is important in order to prevent possible vision-threatening complications such as endophthalmitis.3
While many repair techniques have been developed and modified, the published literature comparing different techniques for repair in the same institution are limited.4,5 The aim of the current study was to report our experience with eyes that presented with an initial GDD exposure, and their subsequent outcome in terms of re-exposure.
Methods
Study Design
A retrospective review of charts of 42 patients (43 eyes) identified with GDD exposure during the period 2008–2015 in a previous study (Risk Factors for Glaucoma Drainage Device Exposure in a Middle-Eastern population) was performed.6 The preceding study included 53 GDD exposures that occurred in 45 eyes. Of these, two eyes of two patients were excluded, one patient lost follow-up at our institution and one patient had exposure repair elsewhere with no details on surgical repair available. So, for the present study, 43 eyes that were identified with the first exposure from the previous study were included.
Ethical Considerations
The study was approved by the Institutional Review Board (IRB) at King Khaled Eye Specialist Hospital (IRB number RP1834-R, December 2018). Due to the retrospective nature of the study, written informed patient consent was waived by the IRB. All data were anonymized for collection and analysis and this report contains no identifiable information. The study followed the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act regulations.
Surgical Technique
GDD surgery was done for patients with refractory glaucoma, after the failure of trabeculectomy or in eyes considered to be at high risk of trabeculectomy failure. The type of GDD depended on the surgeon preference, surgical history and type of glaucoma, with available implants including Ahmed Glaucoma Valve [New World Medical, Inc, Rancho Cucamonga, CA] (Ahmed S2, Ahmed FP7, Ahmed FP8), Krupin Eye Valve [Eagle Vision, Inc, Memphis, TN] or Baerveldt Glaucoma implant 250 and 350 [Abbott Medical Optics, Santa Ana, CA]. The surgical technique of implantation of a GDD has been described in detail elsewhere in the literature,2 but is summarized here.
A corneal traction suture was placed to expose the quadrant of choice (most frequently the superotemporal quadrant). A fornix-based conjunctival incision was created with a creation of a posterior pocket for the insertion of the plate. The GDD implant was placed 8 to 10 mm posterior to the limbus (Ahmed valve at 8 mm and Baerveldt implant at 9–10 mm). Then, the plate of the implant was fixed to the globe with two non-absorbable sutures either 9–0 nylon or 9–0 prolene sutures which were passed through the plate’s eyelets and then passed through the sclera. The tube was introduced through a needle track into the anterior chamber or the ciliary sulcus. The tube was then secured to the sclera with 9–0 nylon or 9–0 prolene sutures and covered with a patch graft which was either pericardium, sclera, dura, or cornea. The patch graft was sutured to the globe with interrupted sutures at the corners by using either 7–0 vicryl or 10–0 nylon sutures. The conjunctiva was closed to cover the plate, tube, and the patch graft with 9–0 vicryl suture in an interrupted or continuous manner. Mitomycin C was not routinely used.
The method of GDD exposure repair depending on the extent of exposure and perceived risk of re-exposure. For small conjunctival erosions, necrotic conjunctival tissue around the tube was debrided, and the conjunctiva closed (usually after placement of a patch graft over the tube), with the use of a conjunctival flap if needed. If the risk of re-exposure was deemed to be high, the tube was removed, and the patient put back on topical antiglaucoma medication, treatment with transscleral cyclophotocoagulation undertaken or a tube placed in a different quadrant (either at the same or subsequent time). Buccal mucosa was not used in our practice.
Data Collection
Patient information was collected from both electronic and paper charts using IRB-approved data collection sheets. Since we studied a cohort of patients that were included in a previous study and since we wanted to have decent follow up of repair outcome, the chart review included exposures between 2008 and 2015.6 The Data collected included demographic information, including age, gender, as well as the presence of any comorbid conditions (hypertension, diabetes). We did not specifically record ethnicity or race because our patients are of homogeneous race, all being Saudi Arabs. The past ocular history was documented including topical medications and ocular surgeries. Pre-operative data prior to presentation with GDD exposure and re-exposure were documented. Details of the implant surgery including the eye with the implant, type of implant used, date of surgery, location of the implant (quadrant), any associated procedures, type of patch graft used to cover the tube, and types of suture used to fix the plate and the tube to the sclera were collected.
Details of GDD exposure, including date of diagnosis of initial exposure, type and location of exposure, surgical technique for repair, type of patch graft used to cover the tube if used. Complications such as endophthalmitis were noted. Details of re-exposure included specifying whether re-exposure occurred or not, time to re-exposure after the initial repair, type and location of re-exposure, surgeries done before re-exposure, ocular medications used before re-exposure, surgical technique used for re-exposure repair and associated complications. For the ease of analysis, the repair technique was categorised into one of the following: conjunctival repair only; patch graft (with conjunctival repair); tube repositioning (leaving the GDD in the same quadrant) and patch graft; tube removal and placement in a different quadrant or tube removal (without another tube).
Similar data were collected for all subsequent re-exposures. Post-operative data were collected up to the final available follow-up date. The best-corrected visual acuity and intraocular pressure were collected at three intervals: Baseline (1st appointment in glaucoma clinic before GDD implant surgery), after the first exposure, and the final visit available. The data were then entered into a computer spreadsheet.
Data Analysis
Patient demographics and baseline characteristics were analyzed using descriptive statistics. Continuous variables such as age, IOP and number of glaucoma drops were reported using mean and standard deviation. Qualitative measurements such as gender and diagnosis were reported using frequencies and percentages.
Frequency was calculated for the number of patients with GDD exposures for the first, second, third and fourth time. The median time to exposure was calculated for the first, second and third exposure. Bivariate analysis involved a comparison of baseline parameters between eyes that developed second exposure with eyes that did not. A p value of 0.05 or less was considered statistically significant. Frequency and percentage of eyes with re-exposure and the risk ratio for each exposure repair technique were calculated. Eyes that had tubes removed, without the insertion of a new tube were excluded from this calculation.
Survival (exposure-free period) after primary exposure repair was estimated using the Kaplan-Meier survival curve.
Results
The baseline demographics of the whole group (43 eyes) are shown in Table 1. The mean ± SD age was 54 ± 27 years. The mean (SD) duration of follow-up was 9.0 years (6.3 years) with a range of 1.6 to 26.1 years. The systemic diagnoses in these patients were: 13 patients had type 2 Diabetes Mellitus; 17 patients had systemic hypertension; seven patients had ischemic heart disease; three had hypothyroidism; two had renal disease; one patient had bronchial asthma; one had sickle cell trait and another one had eczema. None of our patients had autoimmune disease or collagen vascular disorder. The glaucoma diagnosis was fairly heterogenous. The mean ± SD number of surgeries was 2.5 ± 2.4, with trabeculectomy and AGV being the most frequent prior surgery. Most of the patients were pseudophakic, with approximately a third having undergone a corneal graft previously.
 |
Table 1 Baseline Characteristics of 42 Patients (43 Eyes) |
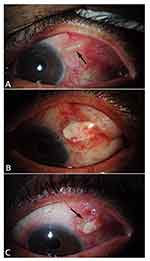
AGV was the most frequent type of GDD performed, with an Ahmed FP7 in 31 eyes, Ahmed FP8 in 2 eyes, Ahmed S2 in 5 eyes, Krupin valve in two eyes and Baerveldt 350 GDD in three eyes. Most GDDs were implanted in the superotemporal quadrant (38); followed by three in the inferotemporal, two in the superonasal and one in the inferonasal quadrant. A pericardial patch graft was used in 35 eyes, dura in five eyes, sclera in one eye and no patch graft in two eyes. Figure 1 shows one patient in our series, who after initial repair with conjunctival debridement, scleral patch graft and conjunctival repair, developed re-exposure of the tube near the limbus 4 months after the repair.
A variety of methods were employed for primary GDD exposure repair of the 43 eyes, by several different surgeons (Figure 2). In four eyes, the tube exposure was repaired without any patch graft. In 18 eyes, a patch graft was placed over the exposed tube before the conjunctiva was repaired (pericardium in 14 eyes, sclera in three eyes and cornea in one eye). In another 14 eyes, the tube was repositioned through a new sclerostomy and a new patch graft used (pericardium in 12 eyes, sclera in one eye and cornea in one eye). In four eyes, the tube was removed completely; in three eyes, the exposed tube was removed, and another tube was placed in another quadrant with a pericardial patch graft.
 |
Figure 2 Methods of repair identified and re-exposures. |
The re-exposure rate for eyes based on different methods of primary repair is shown in Table 2. Out of the four eyes that had just conjunctival closure, there was GDD re-exposure in three eyes again. Eight out of 18 eyes that had a patch graft and another five of 14 that had a patch graft with tube repositioning became exposed a second time. The relative risk of future exposure was greater than 2.0 for conjunctival suturing only after exposure and less than 2.0 for other methods of repair. For three tubes that were removed and placed in a different quadrant, two still got re-exposed.
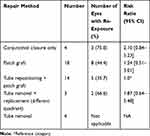 |
Table 2 Exposure Repair Method and Subsequent Exposure |
Of the 43 eyes that had a primary repair, 18 eyes re-exposed for a first time, four for a second time and one eye re-exposed for a third time. The median (IQR) time to the first exposure from the time the GDD surgery was done (prior to repair) was 24.0 (41.8) months; time to the second exposure (first exposure after repair was 15.5 (32.8) months; time to third exposure was 37.5 (39.8) months); the one eye that exposed a fourth time exposed at 8 months after the previous repair. The probability of exposure with time is shown in Figure 3. The cumulative probability of remaining exposure-free at 5 years was 60.5% (95% CI: 47.5–77.0).
 |
Figure 3 Survival probability (of remaining exposure-free) after initial repair with time. |
Regarding the location of exposure, 39 out of 43 eyes had the exposure at the tube. Three out of 43 eyes had the exposure at the plate with one of them having the tube also exposed. Both the tube and the patch were exposed in one eye. The re-exposure occurred at the tube in almost all cases. The exposure and re-exposure did not necessarily occur at the same location. The mean distance from the limbus at first exposure was 2.0 ± 0.7 mm and for second exposure was 3.5 ± 2.1 mm from the limbus.
The characteristics, in terms of the patient characteristics, number of topical medications, number of prior surgeries and pre-operative IOP, of eyes that had one exposure were similar to those that had more than one exposure (Table 3). There was no demonstrable difference, in terms of baseline characteristics, in eyes that had an exposure after primary repair compared to those that did not.
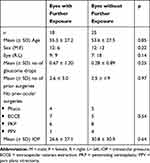 |
Table 3 Comparison of Baseline Parameters Between Eyes That Developed Second Exposure with Eyes That Did Not |
Discussion
The main purpose of our study was to report our experience of managing eyes which have already had a GDD exposure, in terms of repair outcomes and re-exposure rates. As a secondary aim, we compared the re-exposure rates for different techniques of repair. Existing such studies are few as GDD exposure is itself a rare complication, occurring at a rate of 6.3% in a study by Al-Beishri et al, which was conducted at our institution and was a precursor to this study.6 Other studies had similar rates of exposure. Muir et al reported a 6% rate of exposure.7 Trubnik et al reported 8.3%.8
Many surgical techniques for GDD exposure repair have been described in the literature. At our institute surgical technique for GDD exposure and re-exposure repair was tailored to each case depending on exposure site, the extent of exposure and the surgeon’s preference. The repair mainly depended on the health of the conjunctiva. Almost all cases required undermining of the conjunctiva, using a patch graft to cover the tube (with or without repositioning first) and finally closing the conjunctiva and tenon. Many materials have been used as a patch graft to cover the exposed GDD. Some of those materials are the same used to cover the initial GDD. They include ocular tissues like conjunctiva,9 cornea,4 and sclera or extraocular tissues like buccal mucosa,5 dura mater, pericardium,10 amniotic membrane,11 or Synthetic biodegradable materials.12–14
Einan et al used corneal lamellar patch graft covered by a buccal mucous membrane graft for repair and reported no re-exposure.5 Rosentreter et al used biodegradable collagen-glycosaminoglycan matrix (CGM) as a patch in eight patients with GDD exposure with favorable results.13
The results of our study show that re-exposure can occur with sclera or pericardial patch graft. Although the small numbers do not allow an adequate comparison of the relative risk of re-exposure for each, sclera is likely to be protective compared to pericardium for primary exposure.6 In the absence of sclera, current literature suggests that a double pericardial patch graft may be more resilient. A retrospective comparative case series by Lankaranian et al compared the use of single-thickness pericardium patch graft versus double thickness patch graft. Sixteen percent of eyes in the single-thickness pericardium patch group developed conjunctival erosion and none of the eyes in the double thickness pericardium patch group developed conjunctival erosion.10
Merrill et al reported three cases of tube exposure repair with the use of a commercially available tube extender (New World Medical Tube Extender [NWMTE]; New World Medical, Rancho Cucamonga, California, USA) to reroute the GDD tube to an area with healthier conjunctiva with long-term success.15 In some cases, the whole implant needs to be removed in spite of the initial trial to repair the exposure with or without reimplantation of another GDD in another quadrant in the future.16
In our study, 18 out of 43 eyes (41.8%) experienced re-exposure (2nd exposure). Of these, four eyes experienced a subsequent re-exposure (3rd exposure). Previous studies have noted re-exposure (after initial repair) in 40–75% of the eyes studied. In a retrospective study of aqueous shunt exposure repair outcome by Huddleston et al, 40 eyes with aqueous shunt exposure repair were followed for evidence of recurrent aqueous shunt exposure with 17 eyes requiring additional surgical interventions.17 Thompson et al reported 44% re-exposure rate.18 Singh et al reported a higher exposure rate of 75%. Six out of eight eyes which underwent one or more GDD exposure repairs using direct closure of conjunctival defect over the GDD or using conjunctival autograft or donor pericardium or sclera. They reported no further exposure after using corneal patch grafts for repair in those six eyes with a follow-up period of 36.3 6 28.4 months (range 11–83 months). The remaining two eyes were repaired using corneal patch graft initially and had no re-exposure upon follow-up, attributing this to the high tensile strength and rigidity of the corneal tissue.4
Around 60% of eyes in our study remained exposure free at 5 years (Figure 3). In a study by Einan et al, 95% of eyes remained exposure free at almost 80 months (6 years) using corneal lamellar patch graft covered by a buccal mucous membrane graft which is sutured to surrounding conjunctiva. This suggests that firstly, most eyes remain free of re-exposure after the initial repair.5 Secondly, corneal lamellar patch graft covered by buccal membrane, which was not performed in any of our patients, may be the most successful method for repair. In the study by Thompson et al, more than 60% of eyes with scleral patch graft remained exposure-free long term compared with around 40% of eyes with a non-scleral patch graft.18
From our clinical intuition, we had expected that subsequent exposures after the first exposure occurred sooner after the repair. However, we did not find a significant difference between the time to first and subsequent exposures. The first GDD exposure occurred at a median (IQR) time of 24 (41.8) months, whilst the second exposure occurred at a median time of 15.5 (32.8) and the third at 37.5 (39.8) months. The mean time to first exposure was 21.5 months in Huddleston study with a range of 0 to 118 months. The mean time to re-exposure was 78 months.17 In the study by Thompson et al, the average time to first exposure was 110 weeks (SD 109, median 74.2). The average time between first GDD exposure and second exposure was 40.7 weeks.18
In our series, we encountered a variety of repair techniques after initial exposure. Thompson et al reported 44% re-exposure rate and attributed this to the use of non-scleral patch grafts in the initial exposure revision.18 An observational case series by Low et al reported (15%) failure after GDD repair (requiring additional repair procedure) after a mean follow-up of 1.7 years.19 They used buccal mucous membrane grafts in combination with a lamellar corneal patch graft. The study was extended in a retrospective longitudinal study by Einan-Lifshitz et al, including 23 GDD tube exposures. The exposure rate was (13.1%) after a follow-up duration of 53.8 ± 38.3 months.5
Since GDD exposure and re-exposure are rare complications, sample size, and the resulting large confidence intervals around risk ratios of different repair, was an important limitation of our study. As such, we cannot give definitive conclusions about the “ideal” repair method from this study. Also, we did not examine the risk factors for re-exposure. However, baseline factors were similar between eyes that developed GDD re-exposure to those that did not, suggesting that there may not be specific factors associated with failure of repair. Huddleston et al examined the factors associated with successful or unsuccessful shunt exposure repair. Black race, diabetes mellitus, a high number of glaucoma medications before initial implantation, a history of multiple glaucoma laser procedures, combining the initial aqueous shunt implantation with another surgical procedure was associated with a worse outcome after exposure repair.17 Thompson et al looked at risk factors for re-exposure. They found that the Caucasian race and use of a non-scleral patch graft during revision surgery doubled the risk of experiencing a sooner re-exposure of the GDD. The majority of these patches were made of pericardium.18 They also suggest that the use of a scleral patch graft during revision for initial erosion is protective against re-exposure and will delay re-exposure compared to the use of graft made of non-scleral material or tissue such as cornea, pericardium, amniotic membrane, or MEDPOR porous polyethylene.18
In one study, double-layer pericardium in repair prevented re-exposure when compared with single-layer pericardium.10 Another important limitation was the retrospective design of the study. As such, follow-up of patients was variable and different repair techniques were used by different surgeons.
In conclusion, our GDD exposure rates are consistent with previous literature. Around 60% of eyes remain exposure-free at 5 years after initial repair. Use of a patch graft is associated with less risk of subsequent exposure.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Giovingo M. Complications of glaucoma drainage device surgery: a review. Semin Ophthalmol. 2014;29(5–6):397–402. doi:10.3109/08820538.2014.959199
2. Riva I, Roberti G, Oddone F, Konstas AGP, Quaranta L. Ahmed glaucoma valve implant: surgical technique and complications. Clin Ophthalmol. 2017;11:357–367. doi:10.2147/OPTH.S104220
3. Al-Torbak AA, Al-Shahwan S, Al-Jadaan I, Al-Hommadi A, Edward DP. Endophthalmitis associated with the Ahmed glaucoma valve implant. Br J Ophthalmol. 2005;89(4):454–458. doi:10.1136/bjo.2004.049015
4. Singh M, Chew PTK, Tan D. Corneal patch graft repair of exposed glaucoma drainage implants. Cornea. 2008;27(10):1171–1173. doi:10.1097/ICO.0b013e3181814d15
5. Einan-Lifshitz A, Belkin A, Mathew D, et al. Repair of exposed Ahmed glaucoma valve tubes. J Glaucoma. 2018:1. doi:10.1097/IJG.0000000000000951
6. Al-Beishri AS, Malik R, Freidi, Ahmad S. Risk factors for glaucoma drainage device exposure in a middle-eastern population. J Glaucoma. 2019;28(6):529–534. doi:10.1097/IJG.0000000000001220
7. Muir KW, Lim A, Stinnett S, Kuo A, Tseng H, Walsh MM. Risk factors for exposure of glaucoma drainage devices: a retrospective observational study. BMJ Open. 2014;4(5):1–6. doi:10.1136/bmjopen-2013-004560
8. Trubnik V, Zangalli C, Moster MR, et al. Evaluation of risk factors for glaucoma drainage device-related erosions: a retrospective case-control study. J Glaucoma. 2015;24(7):498–502. doi:10.1097/IJG.0000000000000034
9. Godfrey DG, Merritt JH, Fellman RL, Starita RJ. Interpolated conjunctival pedicle flaps for the treatment of exposed glaucoma drainage devices. Arch Ophthalmol. 2003;121(12):1772–1775. doi:10.1001/archopht.121.12.1772
10. Lankaranian D, Reis R, Henderer JD, Choe S, Moster MR. Comparison of single thickness and double thickness processed pericardium patch graft in glaucoma drainage device surgery: a single surgeon comparison of outcome. J Glaucoma. 2008;17(1):48–51. doi:10.1097/IJG.0b013e318133fc49
11. Ainsworth G, Rotchford A, Dua HS, King AJ. A novel use of amniotic membrane in the management of tube exposure following glaucoma tube shunt surgery. Br J Ophthalmol. 2006;90(4):417–419. doi:10.1136/bjo.2005.084905
12. Oana S, Vila J. Tube exposure repair. J Curr Glaucoma Pract. 2012;6(3):139–142. doi:10.5005/jp-journals-10008-1121
13. Rosentreter A, Lappas A, Widder RA, Alnawaiseh M, Dietlein TS. Conjunctival repair after glaucoma drainage device exposure using collagen-glycosaminoglycan matrices. BMC Ophthalmol. 2018;18(1):1–5. doi:10.1186/s12886-018-0721-6
14. Tanito M, Sano I, Ikeda Y, Fujihara E. Patch grafting using an ologen collagen matrix to manage tubal exposure in glaucoma tube shunt surgery. Case Rep Ophthalmol. 2018;9(1):9–16. doi:10.1159/000485549
15. Merrill KD, Suhr AW, Lim MC. Long-term success in the correction of exposed glaucoma drainage tubes with a tube extender. Am J Ophthalmol. 2007;144(1):136–137. doi:10.1016/j.ajo.2007.03.017
16. Roy AK, Senthil S. Management of implant plate exposure of silicone Ahmed glaucoma valve: a review of six cases. GMS Ophthalmol Cases. 2016;6(12x15mm):Doc10. doi:10.3205/oc000047
17. Huddleston SM, Feldman RM, Budenz DL, et al. Aqueous shunt exposure. J Glaucoma. 2013;22(6):433–438. doi:10.1097/ijg.0b013e3181f3e5b4
18. Thompson AC, Manjunath V, Muir KW. Risk factors for earlier reexposure of glaucoma drainage devices. J Glaucoma. 2017;26(12):1155–1160. doi:10.1097/IJG.0000000000000821
19. Low SAW, Rootman DB, Rootman DS, Trope GE. Repair of eroded glaucoma drainage devices: mid-term outcomes. J Glaucoma. 2012;21(9):619–622. doi:10.1097/IJG.0b013e3182447d83
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.