Back to Journals » Clinical Interventions in Aging » Volume 18
TT3, a More Practical Indicator for Evaluating the Relationship Between Sarcopenia and Thyroid Hormone in the Euthyroid Elderly Compared with FT3
Authors Chen J, Wei L, Zhu X, Xu W, Zou Y, Qi X, Fang J, Wang X, Shi X, Sheng Y, Ding G, Ouyang X, Duan Y
Received 10 May 2023
Accepted for publication 22 July 2023
Published 4 August 2023 Volume 2023:18 Pages 1285—1293
DOI https://doi.org/10.2147/CIA.S420558
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Jihai Chen,1,2 Lijun Wei,2 Xiaoxia Zhu,2 Wenli Xu,2 Yuxin Zou,2 Xinyu Qi,2 Jia Fang,2 Xiaodong Wang,2 Xiaolan Shi,1 Yunlu Sheng,2 Guoxian Ding,2 Xiaojun Ouyang,1 Yu Duan2
1Department of Geriatric, Geriatric Hospital of Nanjing Medical University, Nanjing, 210024, People’s Republic of China; 2Department of Geriatric Endocrinology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, 210029, People’s Republic of China
Correspondence: Yu Duan, Department of Geriatric Endocrinology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, People’s Republic of China, Email [email protected] Xiaojun Ouyang, Department of Geriatric, Geriatric Hospital of Nanjing Medical University, Luojia Road 30, Nanjing, 210024, People’s Republic of China, Email [email protected]
Background and Aims: Sarcopenia is a common disease in the elderly, and the thyroid hormone (TH) might participate in the pathogenesis of sarcopenia. However, the results of previous studies were not completely consistent. We performed this study to investigate the association between THs and sarcopenia in a Chinese elderly euthyroid population.
Subjects and Methods: A total of 309 Chinese elderly euthyroid subjects with an average age of 85.19 ± 7.8 years were enrolled. Participants were divided into four groups (non-sarcopenia, possible sarcopenia, sarcopenia and serve sarcopenia) according to the consensus update of AWGS in 2019. Serum levels of TT3, FT3, TT4, FT4, TSH, rT3 and TBG were measured. Muscle mass was measured by multifrequency bioelectrical impedance analysis, hand grip (HG) was represented by spring-type dynamometer, and gait speed (GS) was determined by 6-metre walk test. The FRAIL scale was used to assess frailty.
Results: Compared to the non-sarcopenia group, the sarcopenia group showed a significant increase in age and FRIAL score, while FT3 and TT3 levels decreased significantly. Partial correlation analysis (adjusted by age, gender and the scores of FRIAL scale) indicated that FT3, TT3 and TSH had significant positive correlations with HG, and there also was a significant positive correlation between TT3 and GS. In addition, after adjusting for age, gender, BMI, ALT, sCr, and score on the FRAIL scale, the multivariate linear regression analysis showed that TT3 was positively associated with muscle strength and negatively associated with sarcopenia risk.
Conclusion: There is an association between the low TT3 level and sarcopenia. Therefore, maintaining higher T3 concentrations within the normal range appears to be beneficial for sarcopenia in the elderly. In addition, due to the fluctuation of FT3, TT3 is a more stable and practical indicator to evaluate the relationship between sarcopenia and thyroid hormone in the elderly euthyroid population.
Keywords: sarcopenia, thyroid hormone, elderly, muscle strength
A Letter to the Editor has been published for this article.
Graphical Abstract:
Introduction
Sarcopenia is a common disease in the elderly, which is defined as “age-related loss of skeletal muscle (SM) mass plus loss of muscle strength and/or reduced physical performance”.1,2 According to the consensus update of Asian Working Group for Sarcopenia (AWGS) in 2019, the prevalence of sarcopenia is about 7.3–12%.3–5 Sarcopenia increases the risk of falling,6 recurrent fall,7 fracture8 and mortality.9 On the other hand, it was also significantly associated with cardiometabolic disease10 and cognitive impairment.11 However, the pathogenesis of sarcopenia remains unclear and there are no effective medications.
Previous studies have suggested that age,12 neuromuscular dysfunction,13 mitochondrial dysfunction,14 proinflammatory cytokines,15 myocyte apoptosis,16 and heredity13 play a role in the pathogenesis of sarcopenia. Notably, recent studies demonstrated thyroid dysfunctions reduced the skeletal muscle mass or strength of mice,17,18 suggesting that thyroid hormone (TH) disorder had an association with sarcopenia. However, there were very few studies that mention euthyroid and sarcopenia. In addition, the results were not fully consistent due to differences in the aging population and region-dependent diagnostic criteria.19,20 Moreover, there were more limitations in these studies. Only free triiodothyronine (FT3), free thyroxine (FT4), thyroid stimulating hormone (TSH) were selected to evaluate thyroid function in the previous research. However, the activity of deiodinase decreases with age, as well as sexual hormones change and the risk of frailty increases, which all affect the levels of reverse triiodothyronine (rT3) and thyroxine-binding globulin (TBG).21,22 Therefore, in the elderly population, in addition to FT3, FT4, and TSH, total triiodothyronine (TT3), total thyroxine (TT4), rT3, and TBG also need to be included to comprehensively evaluate thyroid function. On the other hand, the appendicular lean mass (ALM) or ALM adjusted by Body mass index (BMI) was used to assess muscle mass in the previous studies,19,20 instead of the skeletal muscle mass index (SMI) recommended by the European Working Group on Sarcopenia in Older People (EWGSOP)1 and AWGS.2
In this study, we detected more thorough thyroid-related indicators (FT3, TT3, FT4, TT4, TSH, rT3 and TBG), adopted more recognized indicators for evaluating sarcopenia (SMI, hand grip (HG) and gait speed (GS)), and included frailty assessment to evaluate the relationship between sarcopenia and thyroid hormones in the elderly population, thus providing better understanding effect of THs in sarcopenia pathogenesis.
Subjects and Methods
Study Population
A total of 309 elderly euthyroid subjects (age range 60–97 years, mean age 85.19±7.84 years) were included in this study, and all of them were stable outpatients followed up by the Geriatric Hospital of Nanjing Medical University. Most of the participants were over 80 years old (n=233, 75.46%), with 34.30% (n=106) of them being over 90 years old. The proportion of females was 39.48% (female: n=122, male: n=187). All recruited subjects were asked to complete a self-assessment questionnaire that included demographic data, height, weight, disease history, medication history and frailty assessment (FRAIL scale). BMI was calculated as: BMI (kg/m2) = weight (kg)/height2 (m2).
Patients taking glucocorticoids, androgens, thyroxine and other drugs that could affect thyroid function and muscle were excluded. Subjects with frailty, thyroid dysfunction, acute infection, acute liver dysfunction, acute kidney dysfunction, stroke sequelae, Parkinson’s disease and other conditions that may affect muscle function or TH levels were also excluded.
This study complied with the guidelines of the Helsinki Declaration. The Ethics Committee of the Geriatric Hospital of Nanjing Medical University approved this study protocol (No: 2021029). All participants provided written informed consent and patient anonymity will be preserved.
Blood Sampling
A venous blood sample was obtained after an overnight fast, and were tested immediately by the laboratory of the Geriatric Hospital of Nanjing Medical University (HITACHI 7600 automatic analyzer). The indicators and their normal reference range are as follows: Alanine aminotransferase (ALT, 0–55 U/L), albumin (ALB, 35–55 g/L), prealbumin (PA, 0.2–0.4 g/L), serum creatinine (sCr, 55–109 µmol/L), glucose (GLU, 3.9–6.1 mmol/L) determined.
THs and Related Indicators
Blood samples were collected from all subjects followed an overnight fast, and THs were detected by electrochemiluminescence assay (Roche electrochemiluminescence instrument E170). The THs and their normal reference range are as follows: TT3 (0.64–1.52 ng/mL), FT3 (2.43–6.01pmol/L), TT4 (62.68–150.8 nmol/L), FT4 (9.01–19.05 pmol/L), TSH (0.35–0.012 uIU/mL), rT3 (0.67–1.87 nmol/L), and TBG (2.4–8.7 ng/mL).
Assessments of Sarcopenia
According to the consensus of AWGS 2019, SMI, hand grip (HG) and gait speed (GS) were used to represent the skeletal muscle mass, muscle strength and muscle function.
The bioelectrical impedance analysis (BIA) (InBody S10) was used to measure appendicular skeletal muscle mass (ASM). The appendicular skeletal muscle mass index (SMI) was calculated as: 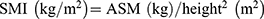 . Low SMI is defined as <7.0 kg/m2 in men and <5.7 kg/m2 in women.2
. Low SMI is defined as <7.0 kg/m2 in men and <5.7 kg/m2 in women.2
The hand dynamometer ((Jamar®, Los Angeles, CA, USA) was used to measure the HG of the dominant hand. All hands were naturally drooped and tested three times in 1 minute using dynamometer to record the maximum. Low muscle strength is defined as <28.0 kg for men and <18.0 kg for women.2
GS was measured using the 6-meter (6 m) walk test. The examiner measured the time taken to walk 6 m at a normal pace from a moving start without deceleration, and took the average result of at least 2 trials as the recorded speed. According to AWGS 2019 consensus, the cutoff for slow GS is <1 m/s2.
All the assessments were performed by a single examiner in a dedicated Comprehensive Geriatric Assessment room to avoid distractions during the procedure.
Diagnosis of Sarcopenia
According to the AWGS 2019 consensus, the definition of sarcopenia includes low SMI, combined with low HG or slow GS. Severe sarcopenia is defined as low SMI, combined with low HG and slow GS. Possible sarcopenia is defined as normal SMI but low HG, with or without slow GS.
Statistical Analysis
Descriptive data were presented as means (M) ± standard deviation (SD). Before statistical analysis, the data should be tested for normal distribution. The One-Way ANOVA and t-test analyses were performed when the data were normally distributed, otherwise Rank-sum tests were used. The Chi-square test was chosen to compare the proportion of females. In the correlation analysis, Pearson’s partial correlation analysis was used. To investigate the association of THs with sarcopenia and its defining components (low muscle mass, low muscle strength, and low muscle function), we fitted two logistic regression models. For model 1, we adjusted for age, male, and FRIAL scale score, which differed between groups in the baseline. For model 2, age, sex, BMI, FRIAL scale score, PA, ALT, and sCr were adjusted to be potentially causal for sarcopenia or contribute to its development.
Results
Baseline Data, THs, TBG, SMI, HG and GS Levels for Participants
All patients were euthyroid and divided into non-sarcopenia group, possible sarcopenia group, sarcopenia group and severe sarcopenia group according to the AWGS 2019 consensus. Among all 309 euthyroid subjects, 103 were diagnosed with sarcopenia (sarcopenia and severe sarcopenia) and the prevalence rate was 33.33%. In the <80 age group, 80–90 age group and ≥90 age group, the prevalence of sarcopenia was 28.94% (22/76), 34.65% (44/127) and 34.91% (37/106), respectively. In addition, 102 subjects were diagnosed with possible sarcopenia and the prevalence rate was 33.01%, while the prevalence of possible sarcopenia was respectively 17.11% (13/76), 37.79% (48/127) and 38.68% (41/106) in three age groups.
The age (F=16.53, P<0.001), the proportion of female (χ2=17.65, P=0.007), SMI (F=67.19, P<0.001), HG (F=53.74, P<0.001), GS (F=21.80, P<0.001), the scores of FRIAL scale (χ2=14.14, P=0.003) were significantly different among four groups. While, the BMI, ALT, ALB, sCr and GLU had no significant differences. The proportion of female (χ2=27.36, P<0.001), the score of FRAIL scale (Z=2.09, P=0.036) in serve sarcopenia had significant differences compared with non-sarcopenia group, while the proportion of female (χ2=30.68, P<0.001) and the score of FRAIL scale (Z=3.14, P=0.002) in sarcopenia group were also lower than that in non-sarcopenia group. The baseline data for the four groups is shown in Table 1.
 |
Table 1 Baseline Characteristics for the Participants |
Levels of THs and TBG in Different Groups
According to Figure 1, the levels of FT3 (F=5.70, P=0.001) and TT3 (F=6.067, P=0.001) had significant differences among the four groups. The level of FT3 (t=0.161, P=0.020) and TT3 (t=2.675, P=0.008) in the severe sarcopenia group were significantly lower than that in the non-sarcopenia groups. On the other hand, the levels of FT4, TT4, TSH, rT3 and TBG did not show significant differences between the four groups.
Correlation Between THs and Sarcopenia Elements
Based on the results of the baseline data, we chose partial correlation analysis to analyze the correlation between thyroid hormones (FT3, TT3 and TSH) and sarcopenia elements (SMI, HG and GS) in order to avoid the influence of gender, age and frailty status. After adjusting for age, gender and the score of FRAIL scale, HG had positive correlations with FT3 (r=0.151, P=0.009), TT3 (OR=0.175, P=0.002) and TSH (r=0.149, P=0.009), while GS had a positive correlation with TT3 (r=0.183, P=0.001). The correlation between THs and sarcopenia elements is shown in Figure 2.
The Effect of THs on Sarcopenia
Binary logistic regression analysis was used to reveal the relationship between sarcopenia and TH. In the model 1, the level of FT3 (OR=0.618, 95% CI: 0.399–0.957, P=0.031) and TT3 (OR=0.060, 95% CI: 0.011–0.346, P=0.002) reduced the risk of sarcopenia, and TT3 (OR=0.123, 95% CI: 0.026–0.593, P=0.009) also reduce the risk of low HG. In the model 2, TT3, but not FT3, still had a significant negative correlation with the risk of sarcopenia (OR=0.115, 95% CI: 0.016–0.821, P=0.031) and low HG (OR=0.165, 95% CI: 0.028–0.972, P=0.046). The results are presented in Table 2.
 |
Table 2 The Correlation Between TH, Sarcopenia, Low SMI, Low HG and Low GS |
Discussions
As a common muscle disease in the elderly, sarcopenia is strongly associated with adverse events such as falls and fractures.7,8 It is necessary to explore the pathogenesis of sarcopenia and find better intervention opportunities and strategies. TH is closely related to the regeneration, differentiation, and mitochondrial energy metabolism of SM cells,23,24 and also acts as a regulator of muscle fibers.25 Thus, THs retain their crucial effect on the SM, yet the relationship between THs and sarcopenia is still under debated. In this study, we focused on the association between TH and sarcopenia using a Chinese elderly population of 309 euthyroid individuals, most of whom were the old-old. We found that serum levels of FT3 and TT3 were positively associated with muscle strength and TT3 had a negative association with the risk of sarcopenia, suggested that T3 is closely related to sarcopenia in the euthyroid elderly population, and maintaining a moderate T3 concentration within the normal range, especially TT3, would reduce the decline of muscle strength and the occurrence of sarcopenia.
SM is an important target organ for TH. TH enters the skeletal muscle cell through the monocarboxylate transporter, converted by deiodinase and binds with the thyroid hormone receptor (THR) in the nucleus to play its physiological functions. There are two kinds of deiodinase in SM cells, deiodinase type 2 (DIO2) and type 3 (DIO3). The function of DIO2 is to facilitate the conversion of T4 to T3, while DIO3 accelerates the degradation of T4 to the inactive rT3.23 As age increases, the expression of DIO 3 gene increases and the amount of THR decreases, leading to the decline of the effect of T3 on SM23 and the development of sarcopenia.24 In our study, it was also shown that FT3 was strongly associated with sarcopenia and HG, similar to previous studies.19,20 However, after correcting for age, gender, BMI, PA, scores of FRIAL, ALT and sCr, it was found that TT3, but not FT3, had a significant correlation with sarcopenia and HG. This suggests that TT3 appears to be more closely related to sarcopenia than FT3. One of the reasons for these specificities is that the subjects in this study were older than the others, which increases the volatility of FT3. TH is easily influenced by age, season and temperature, especially for FT3,26–29 and the levels of deiodinase and TBG are also affected by age, which directly affects FT3 levels. The above factors contribute to the instability of FT3, and it is not a good indicator for evaluating T3 levels in the elderly population. On the contrary, as the main component of T3, although TT3 does not directly play a physiological role, it fluctuates less and it is better suited to represent the relationship between T3 and sarcopenia. Besides, we adopted the more appropriate diagnostic criteria of the AWGS due to the Asian subjects in our study. Another important possibility was more thyroid indicators to be included to evaluate the association between the TH and sarcopenia, while SMI, a more recognized diagnostic indicator, was used to assess the muscle mass. In addition, we have chosen the FRIAL scale to evaluate frailty status, which was poorly covered by studies. The FRAIL scale scores were found to differ significantly between the four groups. Frailty may increase the prevalence of low T3 syndrome,22,30 affected TBG synthesis,31 thus increasing FT3 volatility.
The fibers in SM are mainly divided into two main types, the slow and fast muscle fibers, and the reduction of the fast muscle fibers was considered to be one of the main characteristics of sarcopenia.12,32 Slow muscle fibers primarily mediate low-intensity activities or posture maintenance, while fast muscle fibers mediate fast or intense muscle-contracting movements such as jumping and kicking.33 TH could promote the conversion of muscle fibers to fast muscle fibers by inhibiting the effects of TEA domain family member 1 (TEAD1)34 and μ-Crystallin (Crym).35 Thus, TH is also an important regulator of muscle fibers. As T3 decline and THR decrease in older adults, the regulatory effect of TH on fast muscle fibers also decays. Our study found T3 was closely related to HG and GS, both of which are associated with the reduction of fast muscle fibers. After adjusting for the correction factors, TT3 showed a positive correlation with grip strength and a significant protective effect on muscle strength. This suggested that maintaining an appropriate TT3 level within the normal range was beneficial to muscle strength, which may be the main way for T3 to play a protective role against sarcopenia.
In addition, in this study, we also included the possible sarcopenia group as an independent group, which was not involved in other studies. The results showed that the T3 level in the possible sarcopenia group decreased than non-sarcopenia group. Compared to the non-sarcopenia group, the levels of FT3 and TT3 were reduced in the possible sarcopenia group. Although there was no significant difference about this decline of FT3 and TT3, we found that the HG and GS of the possible sarcopenia group were significantly decreased compared to the non-sarcopenia group. This suggested that the decline in T3 might have caused changes in muscle strength and function in the stage of pre-sarcopenia, and this may be a key point for further research.
Conclusions
Our study showed there is an association between low TT3 level and sarcopenia. Therefore, maintaining higher TT3 concentrations within the normal range appeared to be beneficial to reduce the risk of sarcopenia, and prevent the decline of grip strength. Moreover, compared to FT3, TT3 is a more practical indicator for evaluating the relationship of sarcopenia and thyroid hormone in the elderly.
Funding
This work was supported by National Natural Science Foundation of China (No. 81670724, 82071582) and Jiangsu Health Commission Foundation of China (No. BJ19029, LKM2022011).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601. doi:10.1093/ageing/afz046
2. Chen LK, Woo J, Assantachai P, et al. Asian Working Group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307 e302. doi:10.1016/j.jamda.2019.12.012
3. Yoshimura N, Muraki S, Oka H, et al. Is osteoporosis a predictor for future sarcopenia or vice versa? Four-year observations between the second and third ROAD study surveys. Osteoporos Int. 2017;28(1):189–199. doi:10.1007/s00198-016-3823-0
4. Yu R, Leung J, Woo J. Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the Osteoporotic Fractures in Men (MrOs) Study. J Am Med Dir Assoc. 2014;15(8):551–558. doi:10.1016/j.jamda.2014.02.005
5. Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc. 2015;16(3):247–252. doi:10.1016/j.jamda.2014.11.013
6. Kinoshita K, Satake S, Matsui Y, Arai H. Association between sarcopenia and fall risk according to the muscle mass adjustment method in Japanese older outpatients. J Nutr Health Aging. 2021;25(6):762–766. doi:10.1007/s12603-021-1620-8
7. Schaap LA, van Schoor NM, Lips P, Visser M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: the Longitudinal Aging Study Amsterdam. J Gerontol a Biol Sci Med Sci. 2018;73(9):1199–1204. doi:10.1093/gerona/glx245
8. Yu R, Leung J, Woo J. Sarcopenia combined with FRAX probabilities improves fracture risk prediction in older Chinese men. J Am Med Dir Assoc. 2014;15(12):918–923. doi:10.1016/j.jamda.2014.07.011
9. De Buyser SL, Petrovic M, Taes YE, et al. Validation of the FNIH sarcopenia criteria and SOF frailty index as predictors of long-term mortality in ambulatory older men. Age Ageing. 2016;45(5):602–608. doi:10.1093/ageing/afw071
10. Tamura Y, Ishikawa J, Fujiwara Y, et al. Prevalence of frailty, cognitive impairment, and sarcopenia in outpatients with cardiometabolic disease in a frailty clinic. BMC Geriatr. 2018;18(1):264. doi:10.1186/s12877-018-0955-4
11. Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Association between sarcopenia and cognitive impairment: a systematic review and meta-analysis. J Am Med Dir Assoc. 2016;17(12):1164 e1167–1164 e1115. doi:10.1016/j.jamda.2016.09.013
12. Goljanek-Whysall K, Iwanejko LA, Vasilaki A, Pekovic-Vaughan V, McDonagh B. Ageing in relation to skeletal muscle dysfunction: redox homoeostasis to regulation of gene expression. Mamm Genome. 2016;27(7–8):341–357. doi:10.1007/s00335-016-9643-x
13. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–2646. doi:10.1016/S0140-6736(19)31138-9
14. Picca A, Calvani R, Bossola M, et al. Update on mitochondria and muscle aging: all wrong roads lead to sarcopenia. Biol Chem. 2018;399(5):421–436. doi:10.1515/hsz-2017-0331
15. Jo E, Lee SR, Park BS, Kim JS. Potential mechanisms underlying the role of chronic inflammation in age-related muscle wasting. Aging Clin Exp Res. 2012;24(5):412–422. doi:10.3275/8464
16. Budui SL, Rossi AP, Zamboni M. The pathogenetic bases of sarcopenia. Clin Cases Miner Bone Metab. 2015;12(1):22–26. doi:10.11138/ccmbm/2015.12.1.022
17. Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid. 2006;16(4):375–380. doi:10.1089/thy.2006.16.375
18. Butler-Browne GS, Herlicoviez D, Whalen RG. Effects of hypothyroidism on myosin isozyme transitions in developing rat muscle. FEBS Lett. 1984;166(1):71–75. doi:10.1016/0014-5793(84)80047-2
19. Szlejf C, Suemoto CK, Janovsky C, et al. Thyroid function and sarcopenia: results from the ELSA-Brasil Study. J Am Geriatr Soc. 2020;68(7):1545–1553. doi:10.1111/jgs.16416
20. Sheng Y, Ma D, Zhou Q, et al. Association of thyroid function with sarcopenia in elderly Chinese euthyroid subjects. Aging Clin Exp Res. 2019;31(8):1113–1120. doi:10.1007/s40520-018-1057-z
21. Chaker L, Cappola AR, Mooijaart SP, Peeters RP. Clinical aspects of thyroid function during ageing. Lancet Diabetes Endocrinol. 2018;6(9):733–742. doi:10.1016/S2213-8587(18)30028-7
22. Muller NA, Kaegi-Braun N, Durmisi M, et al. Low T3 syndrome upon admission and response to nutritional support in malnourished medical inpatients. J Clin Endocrinol Metab. 2022. doi:10.1210/clinem/dgac743
23. Wang L, Sheng Y, Xu W, et al. Mechanism of thyroid hormone signaling in skeletal muscle of aging mice. Endocrine. 2021;72(1):132–139. doi:10.1007/s12020-020-02428-9
24. Salvatore D, Simonides WS, Dentice M, Zavacki AM, Larsen PR. Thyroid hormones and skeletal muscle--new insights and potential implications. Nat Rev Endocrinol. 2014;10(4):206–214. doi:10.1038/nrendo.2013.238
25. Simonides WS, van Hardeveld C. Thyroid hormone as a determinant of metabolic and contractile phenotype of skeletal muscle. Thyroid. 2008;18(2):205–216. doi:10.1089/thy.2007.0256
26. Mammen JSR. Thyroid and aging. Endocrinol Metab Clin North Am. 2023;52(2):229–243. doi:10.1016/j.ecl.2022.10.008
27. Peeters RP. Thyroid hormones and aging. Hormones. 2008;7(1):28–35. doi:10.14310/horm.2002.1111035
28. Kim MJ, Choi S, Kim S, et al. Sex, menopause, and age differences in the associations of persistent organic pollutants with thyroid hormones, thyroxine-binding globulin, and peripheral deiodinase activity: a cross-sectional study of the general Korean adult population. Environ Res. 2022;212(Pt A):113143. doi:10.1016/j.envres.2022.113143
29. Watts HE. Seasonal regulation of behaviour: what role do hormone receptors play? Proc Biol Sci. 2020;287(1930):20200722. doi:10.1098/rspb.2020.0722
30. Liu Y, Sun Q, Zhang M, Ren M, Chen P, Liang T. Association between thyroid hormone levels and frailty in an older inpatient cohort: a cross-sectional study. Ann Palliat Med. 2021;10(6):6678–6686. doi:10.21037/apm-21-1102
31. Ekins R. Effect of thyroid hormone-binding proteins and fatty acids on modified analog assays of FT4 and FT3 in serum. Clin Chem. 1989;35(4):708–710. doi:10.1093/clinchem/35.4.708
32. Nilwik R, Snijders T, Leenders M, et al. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol. 2013;48(5):492–498. doi:10.1016/j.exger.2013.02.012
33. Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports. 2003;13(1):40–47. doi:10.1034/j.1600-0838.2003.00299.x
34. Zhang D, Wang X, Li Y, et al. Thyroid hormone regulates muscle fiber type conversion via miR-133a1. J Cell Biol. 2014;207(6):753–766. doi:10.1083/jcb.201406068
35. Seko D, Ogawa S, Li TS, Taimura A, Ono Y. mu-crystallin controls muscle function through thyroid hormone action. FASEB J. 2016;30(5):1733–1740. doi:10.1096/fj.15-280933
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


