Back to Journals » Journal of Inflammation Research » Volume 16
TSG6 Plays a Role in Improving Orbital Inflammatory Infiltration and Extracellular Matrix Accumulation in TAO Model Mice
Authors He X, Chen S , Wang X, Kong M , Shi F, Qi X , Xu Y
Received 20 February 2023
Accepted for publication 21 April 2023
Published 4 May 2023 Volume 2023:16 Pages 1937—1948
DOI https://doi.org/10.2147/JIR.S409286
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xiuhui He, Siya Chen, Xiaohui Wang, Min Kong, Fangzheng Shi, Xiaoxuan Qi, Yuxin Xu
Department of Ophthalmology, Second Affiliated Hospital of Anhui Medical University, Hefei, People’s Republic of China
Correspondence: Yuxin Xu, Department of Ophthalmology, Second Affiliated Hospital of Anhui Medical University, No. 678 Furong Road, Economic and Technological Development Zone, Hefei, People’s Republic of China, Tel +86-13866752297, Email [email protected]
Introduction: TSG-6 plays a wide anti-inflammatory and therapeutic role in a variety of autoimmune diseases as the key mediator of mesenchymal stem cells (MSCs).
Purpose: We aimed to test whether TSG-6 could exert similar effects as MSCS in TAO via establishing TAO animal model immunized by hTSHR-A subunit plasmid.
Material and Methods: We tested the expression level of TSG-6 on intraconal orbital fat from controls and patients with TAO. We established a stable thyroid-associated ophthalmopathy animal model by immunizing 6-week-old female Balb/c mice with recombination hTSHR-A subunit plasmid. After four immunizations, TSG-6 or dexamethasone was injected through the tail vein. The effects of the drugs on body weight, thyroid function, orbital inflammation, fibrosis and lipogenesis were observed.
Results: The expression of TSG-6 in the orbital tissues of TAO patient is lower than that of normal people. In our animal model, mice showed weight loss, higher TT4 and TSHR antibody levels, and ocular symptoms such as inflammation and proptosis. TSG-6 can reduce ocular fibrosis and lipogenesis by inhibiting the infiltration of CD3+ T lymphocytes and macrophages in the mouse model of thyroid associated ophthalmopathy. Compared with dexamethasone, TSG-6 showed comparable anti-inflammatory effect, moreover, it has given a better performance in inhibiting adipogenesis.
Conclusion: It was demonstrated that TSG-6 has a considerable positive impact on improving eye symptoms of TAO mice, which could be a novel candidate for the early treatment of TAO.
Keywords: TAO, TSG-6, hTSHR-A subunit plasmid, anti-inflammatory, anti-fibrosis, lipogenesis
Introduction
Thyroid associated ophthalmopathy (TAO) is an autoimmune disease caused by the thyrotropin receptor (TSHR) over-expressed, which is disfiguring, vision-threatening and has a substantial negative impact on patients’ mental and physical health. The occurrence of TAO is usually accompanied by Graves’ disease, also known as Graves orbitopathy.1,2 TAO is mainly manifested as proptosis, conjunctival edema and upper eyelid retraction. Orbital fibroblasts (OFs) act as both target and effector cells during the pathogenesis, over-expressing TSHR and insulin-like growth factor I receptor (IGF-IR), which synergically lead to T and B lymphocyte infiltration.3,4 Initially, inflammation, congestion and edema occur in the orbital tissue due to immune responses, which stimulate OFs hyperproliferation and adipogenicity, further lead to overproduction of the extracellular matrix, tissue remodeling and fibrosis.5,6 In the last decade, researchers have successfully built mature and stable TAO animal modes after many explorations, the mainstream method is to immunize Balb/c mice with recombinant plasmid or adenovirus.7,8 However, the therapeutic options for TAO are limited and unsatisfactory due to the pathogenesis is not exhaustive, it is necessary to explore novel targets for the treatment of TAO.9
Tumor necrosis factor α stimulated gene 6(TSG-6) is a well-known anti-inflammatory factor, which plays a positive role in mediating mesenchymal stem cell (MSC) immunoregulatory function and inhibiting the adhesion and migration of inflammatory cells.10 Previous findings suggest that human OFs display an array of characteristics of MSCs, which play a role in proliferation and differentiation, but in terms of immunosuppression, TAO-OFs perform poorly in reducing T cell infiltration and secretion of inflammatory factors.11,12 The dysfunction may be closely related to endogenous TSG-6. MSC has been demonstrated as an excellent treatment in TAO. For instance, placenta-derived mesenchymal stem cells (PD-MSCs) inhibit TAO mice orbital fat production through exogenous orbital injection,13 which decreased a lipid accumulation by downregulating the phosphorylated PI3K/AKT/mTOR expression in OFs from TAO patients.14 In addition, it was reported that exogenous administration of TSG-6 alone can ameliorate liver fibrosis in male C57BL/6J mice, while TSG-6 knocked MSCs (Lv-TSG-6 MSCs) lost antifibrotic effect, and TSG-6 injection alone was as effective as MSCs transplantation.15 Hence, we reckon that TSG-6 may have similar functions as PD-MSCs in the treatment of TAO mice models.
The purpose of this study is to observe curative effect of TSG-6 for mice with TAO. In this study, we established TAO mice model by intramuscular (i.m) injection of recombinant plasmid expressing hTSHR A subunit, after four doses of immunization, mice in the model group were selectively treated with TSG6 or dexamethasone. The therapeutic effect of TSG-6 on TAO mice were evaluated by observing the morbidity and disease severity of mice in each group. The study suggests that TSG6 may be a novel therapeutic strategy for TAO.
Materials and Methods
Acquisition and Western Blot Testing of Patient Specimens
Orbital adipose/connective tissue specimens were obtained in the course of orbital decompression surgery for TAO patients (n=5: three women and two men, aged 25–58 years). Normal orbital adipose/connective tissue specimens were collected in the course of orbital surgery for other noninflammatory problems from patients with no history of thyroid disease and with no clinical evidence of TAO (n = 4; two women and two men, aged 22–60 years). The TAO patients had not received steroid medication for at least 3 months before surgery, and were euthyroid during the time of surgery. The clinical activity scores at the time of tissue harvest were below 4 in all patients. None of the patients had been treated previously with orbital radiotherapy. The research followed the tenets of the Declaration of Helsinki and was approved by the ethics Committee of the Second Affiliated Hospital of Anhui Medical University (YX2022-125), and all study participants gave their written informed consent.
All specimens were homogenized by using RIPA Lysis Buffer (P0013B, Beyotime) and the tissue homogenate fractions were centrifuged for 10 minutes at 12,000g, then the supernatant was stored at −80°C before use. Protein concentrations were determined by the Bradford assay. Equal amounts of protein (20μg) were separated by 10% SDS polyacrylamide gel electrophoresis. The resolved proteins were transferred to nitrocellulose membranes and incubated overnight with primary antibodies (polyclonal mouse anti-GAPDH antibody: Abmart#20006, polyclonal rabbit anti-TSG-6 antibody: Abcam#ab267469) at 4°C, and followed by 1 hour of incubation at room temperature with the peroxidase-conjugated secondary antibody. Revelation was performed by enhanced chemiluminescence (ECL) on CL-Xposure film. Densitometric quantification of each immunoreactive band was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA); values were normalized to GAPDH levels in the same sample.
Quantitative Reverse Transcription Polymerase Chain Reaction (q RT-PCR) Analyses
Total RNA was extracted using TaKaRa MiniBEST Universal RNA Extraction Kit (Takara 9767) according to the manufacturer’s protocol. To analyze the expression of TSG-6 and IL-6 in orbital tissues, GAPDH was used as an internal control, PCR amplification was performed with specific primers (GAPDH: primer forward: 5’-CGGAGTCAACGGATTTGGTCGTA-3’ and primer reverse: 5’-AGCCTTCTCCATGGTGGTGAAGAC-3’; TSG-6: primer forward: 5’-CTCCATATGGCTTGAACGAGCA-3’ and primer reverse: 5’-GCCCTTAGCCATCCATCCAG-3’; IL-6: primer forward: 5’-GGTACATCCTCGACGGCATC-3’ and primer reverse: 5’-ACCAGGCAAGTCTCCTCATT-3’). Q-PCR reactions were performed via LightCycler 480 II machine (Roche) and the gene expression was quantified by the delta CT method. All reactions were performed in triplicate.
Construction of Human TSHR a Subunit Expressing Recombinant Plasmid
The TSHR gene sequence was obtained from the NCBI website, the TSHR A subunit (1–289 bits) was inserted into the expression vector pcDNA3.1. The recombinant plasmid pcDNA3.1-hTSHR A-subunit (otherwise known as hTSHR289) and the control pcDNA3.1-Gal plasmid were expressed by Ecoli. α, respectively. The plasmids were extracted using the Qiagen plasmid maxi kit and verified by restriction enzyme digestion, obtained fragment is confirmed by agarose gel electrophoresis and DNA sequencing.
Animal Study
Six-week-old female BALB/C mice were purchased from Anhui medical university animals experiment center (Hefei, China) and were housed under specific pathogen-free conditions. Mice were allowed free access to food and water, and all animal care procedures and treatments were performed according to the standard of Animal Research Ethics Committee of Anhui Medical University. Twenty-four mice were randomly divided into four groups: n (Control) = 6; and n (TSHR) = 6; n (TSHR+TSG6) = 6; n (TSHR+Dexamethasone) = 6. For immunization, the mice were injected with 50uL TSHR-A plasmid (1 mg/mL) or pcDNA3.1-Gal plasmid (for the control group) into each bicep femoris (thigh) muscle. Injection was performed 4 times at 3-week intervals, and weights of mice in each group were recorded weekly. After four immunizations, the TSG-6 treatment group and the dexamethasone treatment group were accepted separately10ug of recombinant TSG-6 (R&D, Cat. No. 2326-TS) or 100ug dexamethasone (Sigma-Aldrich, Cat. D4902), the drugs were dissolved in 100μL phosphate-buffered saline and given once a week for three consecutive weeks by tail vein injections. At 24 weeks, all mice were sacrificed and blood, thyroid and orbital tissues were collected. Serum samples were stored at −80°C until assayed for total serum thyroxine (T4) and TSHR antibodies (TSHRAbs). Thyroid tissue and orbital tissue are fixed with 4% paraformaldehyde.
Measurements of Thyroid Function and Serum TSHRAb
Serum thyroxine and TSHRAb levels were both determined by enzyme-linked immunosorbent assay (ELISA). Serum thyroxine was tested using mouse Thyroxine (T4) Competitive ELISA Kit (Invitrogen, Cat. EIAT4C) according to the instructions. TSHRAb levels in sera were measured using mouse TSHRAb ELISA kit (AssayGenie, Cat. MOF101166) similarly.
Histopathology and Immunohistochemistry of Thyroid Glands
The thyroid glands were about 2 mm long and attached to both sides of the thyroid cartilage, after fixation and paraffin embedding, thyroid sections (5µm) were hematoxylin and eosin (H&E) stained for histological analysis. Meanwhile, the sections were immune histologically stained for CD3. Images were generated using the Leica microscope (Scientific Research and Experimental Center of Anhui Medical University).
Histopathology and Immunohistochemistry of Orbits
The entire orbital tissues (including the orbital bones with the eyeball, extraocular muscles, and the optic nerve) were collected and fixed in 4% paraformaldehyde. Place the orbital tissue in 0.5 M EDTA decalcification solution for at least three weeks and change the fluid weekly. Orbital sections (5 µm) are stained by H&E and the area of optic nerve and fat tissue were measured with ImageJ. Sections were incubated with CD3 antibodies and F4/80 antibodies. Positive cells were counted in orbital fat and muscle tissue.
Measurement of Extracellular Matrix Accumulation
We evaluate extracellular matrix accumulation by examining the production of collagen fibers and mucopolysaccharides. Masson staining was performed on orbital sections for detection of collagen fibers following the protocols, the collagen fibers were dyed blue. Mucopolysaccharides were dyed red by periodic Acid-Schiff (PAS) staining. Positive area was measured with ImageJ.
Statistical Analysis
All data are presented as means and standard deviations (SD). Differences between groups were analyzed using ANOVA or the independent samples t-test. Statistical analysis was performed by GraphPad Prism 6.0 (GraphPad Software Inc.), and P-values <0.05 represent significant differences.
Result
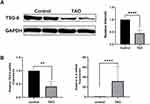
The Expression of TSG-6 is Decreased in TAO Patients Orbital Tissues
Compared to controls, TSG-6 protein expression had a significant decrease in TAO orbital tissues (Figure 1A), the Western Blot result was confirmed by Q-PCR analysis (Figure 1B), besides, the expression of IL-6 gene mRNA was considerably increased in TAO orbital tissues (P < 0.001).
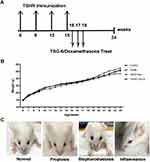
The Characterization of Body Weight and Orbital Symptoms in TSHR Model Mice
The experimental flowchart is shown in Figure 2A. All groups’ mice body weight changes were recorded (Figure 2B). During the first two immunization periods, the TSHR-immunized mice gained more body weight as compared to the control groups, and the opposite effect occurred during the last two immunization periods. At 24 weeks, Mice in the dexamethasone-treated group had a higher body weight compared with other groups. The body weight of TSHR group mice was the lowest. There was no significant difference in body weight between the TSG-6 group and the control group. Moreover, the untreated TSHR-immunized mice developed typical ocular symptoms similar to the TAO patients. Such as eyeball proptosis, eyelid oedema, inflammation and blepharodiastasis (Figure 2C).
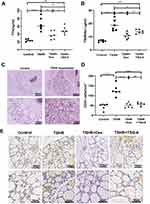
Effect of TSG-6 on Thyroid Function of TAO Model Mice
Serum TT4 concentrations are tested at week 24 in all groups’ mice (Figure 3A). TT4 levels were significantly higher in TSHR-immunized mice versus the control mice (P ≤ 0.0001). Dexamethasone applied to TSHR‐immunized mice reduced the concentration of TT4 (P ≤ 0.01), whereas TSG-6 did not have an obvious therapeutic effect compared to untreated TSHR‐immunized mice (P > 0.05). Besides, TSHR-stimulated antibody titers were significantly increased in the TSHR-immunization group mice (P ≤ 0.0001) (Figure 3B). Both TSG-6 (P ≤ 0.05) and Dexamethasone (P ≤ 0.001) could reduce the serum levels of TSHRAb to varying degrees, dexamethasone performed more effectively.
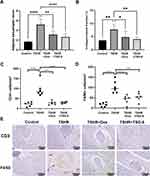
Almost all TSHR‐immunized mice (5/6) developed thyroid morphological changes (Figure 3C), the thyroid gland underwent glandular hyperplasia, the fluid in the follicular cavity decreased, and the follicular epithelial cells became high and columnar. In addition, immunohistochemistry for CD3 showed that thyroid follicles were surrounded by CD3 positive T cells in the TSHR-immunized group (Figure 3D and E). T cell infiltration was improved in TSG-6 and dexamethasone treatment groups. The severity of thyroid inflammation was assessed via counting CD3 positive cells under multiple fields of view (Figure 3D and E). The number of CD3 positive T cells in the TSHR group were significantly higher than control group (P ≤ 0.0001), while this change could be counteracted by TSG-6 (P ≤ 0.0001) and dexamethasone (P ≤ 0.0001) treatment. And there was no differ between TSG-6 and dexamethasone (P > 0.05).
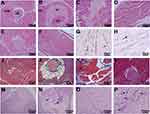
Histomorphological Changes in Mice Orbital
Histopathological analysis of mice orbital was helpful to assess the orbital lesions caused by TSHR plasmid immunization and the therapeutic effect of TSG-6 on TSHR-immunization mice. HE stain of orbital paraffin sections showed the area of fat in mice orbital. Compared to control mice (Figure 4A), the production of fat was increased in TSHR group (Figure 4B). The orbital volume was relatively insufficient, the fat tissue expanded outward and compressed the optic nerve, even separated the orbital muscle fiber bundles (Figure 4C), caused proptosis and blepharodiastasis. Besides, the extraocular muscles in the TSHR group were thickened and the muscle fibers were disordered (Figure 4D). Interestingly, TSG-6 improved lipogenesis and extraocular muscle lesions (Figure 4E and F). Moshkelgosha et al proposed that TAO mice orbital pathology has two subtypes: the lipohyperplasia type and the inflammation infiltrated type.7 Earlier we described the first type, the latter were showed by immunohistochemical staining of CD3. The muscle interbundle of the TSHR group mice were filled with CD3-positive cells (Figure 4G), and which was significantly improved in the TSG-6 treatment group (Figure 4H).
TSG-6 Suppresses the Progress of Retrobulbar Fibrosis
The degree of fibrosis in mice orbit depended on the concentration of retrobulbar collagen fibers, acidic mucin and glycosaminoglycan (GAG). Normal mice had very little collagen fiber in orbital tissue (Figure 4I). The increase of collagen fiber was observed around optic nerve and extraocular muscle bundles in TSHR group mice (Figure 4J and K), which could be improved by TSG-6 treatment (Figure 4L). TSG-6 and dexamethasone could reduce the production of collagen, and TSG-6 (P ≤ 0.01) performed better than dexamethasone (P ≤ 0.05). In addition, the area of acidic mucin and glycosaminoglycan (GAG) deposition were dyed purplish-red via PAS staining. Compared to the control mice (Figure 4M), TSHR-immunized mice orbital tissue developed acidic mucin and GAG deposition (Figure 4N and P), which was the main reason of orbital edema. After the use of TSG-6, acidic mucin and GAG deposition were reduced in extraocular muscles (Figure 4O). TSG-6 was able to inhibit orbital fibrosis in TAO model mice, and in this regard, TSG was superior to dexamethasone.
Orbital Inflammation is Improved by TSG-6 Treatment
By calculating the area ratio of adipose tissue to optic nerve (Figure 5A), we found that TSG-6 significantly reduced lipogenesis (P ≤ 0.0001), which was inseparable from the anti-inflammatory effect of TSG-6. The results of Masson staining were analyzed by ImageJ (Figure 5B), there was a significant difference between the control group and TSHR group (P ≤ 0.01). We investigated inflammatory infiltration in the orbit via counting CD3+ cells and F4/80+ cells (Figure 5C and D). There was a significant increase of T lymphocyte in TSHR-immunization mice versus the control group (P ≤ 0.0001). After the treatment of TSG-6 (P ≤ 0.001) or dexamethasone (P ≤ 0.0001), the lymphocyte infiltration was effectively mitigated. In addition, macrophages were massively increased in the orbit of TSHR group mice (P ≤ 0.0001), and a considerable part located in adipose tissue. Importantly, TSG-6 downregulated macrophages more strongly than dexamethasone (P ≤ 0.05), which might have association with the inhibition of fat production by TSG-6. To evaluate the therapeutic effect of TSG-6 on orbital inflammation, orbital sections of all mice were immunohistochemically stained for CD3 and F4/80. There were 5/6 of the mice in the TSHR group with obvious T cell and macrophage infiltration in the orbit, after dexamethasone and TSG-6 treatment, 1/6 and 2/6 of the mice achieved complete cured respectively, and the rest of the mice also had different degrees of remission. Immunohistochemistry images of each group mice were shown and macrophage were visible where the arrow pointed (Figure 5E).
Discussion
As far as we know, this is the first time TSG-6 had been found to be low expressed in thyroid-associated ophthalmopathy. In our study, we investigated the role of TSG-6 in TAO in reducing inflammatory infiltration, inhibiting lipogenesis and fibrosis. The TSHR-immunized mice developed typical TAO symptoms such as proptosis and weight loss, which could be improved by TSG-6. We demonstrated that TSG-6 reduced inflammatory cells in thyroid and orbital tissue like dexamethasone. Although TSG-6 does not have a significant effect on improving thyroid-related hormones and antibodies that could be alleviated by dexamethasone, the administration of TSG-6 led to a significant improvement of retrobulbar fibrosis and acidic mucin and glycosaminoglycan deposition. Furthermore, TSG-6 was superior to dexamethasone in inhibiting lipogenesis in the orbit of mice, hence demonstrating the therapeutic effect of TSG-6.
TSG-6 is a wide-ranging anti-inflammatory protein, which could be stimulated by TNF-α, IL-1, IL-6, LPS, etc.16,17 Most of the time, TSG-6 is significantly increased in inflammation and injury,18,19 however, there is considerable downregulation of TSG-6 expression in the fat-connective tissue of orbital in patients with thyroid-associated eye disease. Orbital fibroblasts obtained from patients with thyroid-associated eye disease have a similar phenotype of mesenchymal stem cells (MSCs),11 but do not have the powerful immunotherapeutic role that MSCs play in inflammatory and fibrotic diseases.20–22 The mesenchymal stem cell differentiation potential of TAO-OFs may underlie the diversity of disease symptoms, but more evidence is necessarily needed to prove it. The low expression of TSG-6 in TAO orbital tissues may be mediated by the impaired MSCs function of OFs, as TSG-6 is the critical mediator of mesenchymal stem cells to exert therapeutic roles.23–25 Based on this point, endogenous TSG-6 is hardly stimulated by the high expression of IL-6 in TAO. It is not clear whether TSG-6 downregulation directly promotes the progression of TAO, and further work is needed to understand the role of endogenous TSG-6 low-expression in TAO. In this study, we focused on how exogenous TSG-6 played therapeutic effects on TSHR-plasmid immunized mice.
Massive research have suggested TSHR is the leading autoantigen of thyroid and orbit immunoreaction, which causes the local infiltration of T lymphocytes and the secretion of multiple cytokines, leading to hyaluronic acid secretion, lipogenesis and the persistence of orbital inflammation.26–29 Besides, macrophage infiltration also play a significant role in the pathogenesis, which prompted the production of hypoxia marker HIF-1α and inflammatory cytokines.30,31 In consequence, T lymphocytes/Macrophage/OF interaction results in constant inflammation and tissue remodeling. Indeed, in our plasmid-immunized mice model, the orbital tissue and thyroid gland showed different degrees of T lymphocyte and macrophage infiltration, although only 4/6 mice developed ocular symptoms, all of them had varying degrees of elevated thyroid hormone and antibody levels. In addition, a representative TAO mice would show weight loss,29 which is also present in our model. Of note, the mice treated by dexamethasone had abnormal weight gain, which may be associated with the side effect of glucocorticoid.
Previous study found that TSG-6 exerts a powerful therapeutic effect in a variety of diseases such as arthritis,32 keratitis,33 and ulcerative colitis.34 Exogenous TSG-6 could inhibit neutrophil infiltration and reduce the production of cytokines and chemokines,35 thereby limiting the tissue damage caused by excessive inflammatory response. In our study, TSG-6 significantly reduced CD3+ T lymphocyte and F4/80+ inflammatory cell infiltration in the orbital and thyroid tissues of TAO model mice by intravenous administration and the effect was comparable to that of dexamethasone. This is the foundation of the fact that TSG-6 inhibits the expression of cytokines related with fibrosis and adipogenesis. Moreover, the mechanism by which TSG-6 exerts its anti-fibrosis and anti-adipogenesis effects will be further explored in vitro. The treatment of TAO is a longtime challenge, corticosteroids are effective in only 80% of TAO patients and have some unavoidable side effects as first-line drugs, besides, there are many new biological agents applied in clinical, like Rituximab,36 Tocilizumab37 and Teprotumumab,38 which have proven effective for TAO but have a less good safety profile. Therefore, new approaches to improve the symptoms of TAO were trialed in animal models constantly. Diana et al have identified that TSHR-derived cyclic peptide 19 (P19) was effective in TAO mice model that received long-term immunizations of adenovirus expressing thyrotropin receptor A-subunits (TSHR), P19 had a significant effect on improving thyroid function, but have less research on ocular symptoms,39 Plöhn et al find fingolimod could reduce even inhibit hyperthyroidism and the formation of TSHR‐stimulating autoantibodies, and also alleviate fat and hyaluronan deposition.40
Based on our results, we can easily find that TSG-6 does not have much effect on thyroid function, but has a very positive effect on orbital remodeling caused by TSHR overexpression. It is conceivable that combination of TSG-6 and other thyroid medication may lead to better efficacy. However, most studies of TSG-6 are based on animal models. As a new treatment, further studies are required on its safety and long-term complications. Exploration of specific mechanisms will help to accelerate the clinical application of TSG-6 and provide new therapeutic targets for a variety of inflammation diseases.
Conclusion
In summary, our study showed TSG-6 played a powerful role in improving inflammation and fibrosis in TAO model mice, which indicates that TSG-6 could be a potential candidate for the early treatment of TAO and extend the understanding of pathogenesis.
Ethics Approval and Informed Consent
This study was conducted with the approval of the Ethics Committee of the Second Affiliated Hospital of Anhui Medical University (approval number: YX2022-125), and written informed consent was obtained from all participants. All animal testing was in accordance with the guidelines set by the USA national institutes of health, and approved by the Anhui medical university animal ethics committee (approval number: LLSC20221257).
Funding
This project was supported by the Clinical research training program of the Second Affiliated hospital of Anhui Medical University (2021LCZD11), Research Fund of Anhui Institute of Translational Medicine (2022zhyx-C73) and the Postgraduate Innovation Research and Practice Program of Anhui Medical University(approval number:YJS20230087).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bahn RS. Graves ophthalmopathy. N Engl J Med. 2010;362(8):726–738. doi:10.1056/NEJMra0905750
2. Ing EB, Madjedi K, Hurwitz JJ, Nijhawan N, Oestreicher J, Torun N. Nomenclature: thyroid-associated orbitopathy, graves ophthalmopathy, or thyroid eye disease? Can J Ophthalmol. 2021;56(1):e22–e24. doi:10.1016/j.jcjo.2020.06.004
3. Smith TJ, Janssen JAMJ. Insulin-like growth factor-i receptor and thyroid-associated ophthalmopathy. Endocr Rev. 2019;40:953–958. doi:10.1210/er.2018-00066
4. Rotondo Dottore G, Torregrossa L, Caturegli P, et al. Association of T and B cells infiltrating orbital tissues with clinical features of graves orbitopathy. Jama Ophthalmol. 2018;136:613. doi:10.1001/jamaophthalmol.2018.0806
5. Taylor PN, Zhang L, Lee R, et al. New insights into the pathogenesis and nonsurgical management of graves orbitopathy. Nat Rev Endocrinol. 2020;16(2):104–116. doi:10.1038/s41574-019-0305-4
6. Smith TJ. TSH-receptor-expressing fibrocytes and thyroid-associated ophthalmopathy. Nat Rev Endocrinol. 2015;11:171–181.
7. Moshkelgosha S, So P, Deasy N, Diaz-Cano S, Banga JP. Cutting edge: retrobulbar inflammation, adipogenesis, and acute orbital congestion in a preclinical female mouse model of graves’ orbitopathy induced by thyrotropin receptor plasmid-in vivo electroporation. Endocrinology. 2013;154:3008–3015. doi:10.1210/en.2013-1576
8. Zhang M, Ding X, Wu L, et al. A promising mouse model of graves’ orbitopathy induced by adenovirus expressing thyrotropin receptor a subunit. Thyroid. 2021;31:638–648. doi:10.1089/thy.2020.0088
9. Bartalena L, Kahaly GJ, Baldeschi L, et al. The 2021 European group on graves’ orbitopathy (eugogo) clinical practice guidelines for the medical management of graves’ orbitopathy. Pract Guideline. 2021;185(4):G43–G67.
10. Day AJ. CMM. TSG-6: a multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. 2019;78–79:60–83. doi:10.1016/j.matbio.2018.01.011
11. Brandau S, Bruderek K, Hestermann K, et al. Orbital fibroblasts from graves’ orbitopathy patients share functional and immunophenotypic properties with mesenchymal stem/stromal cells. Invest Ophthalmol Vis Sci. 2015;56(11):6549–6557. doi:10.1167/iovs.15-16610
12. Kozdon K, Fitchett C, Rose GE, Ezra DG, Bailly M. Mesenchymal stem cell-like properties of orbital fibroblasts in graves’ orbitopathy. Invest Ophth Vis Sci. 2015;56:5743–5750. doi:10.1167/iovs.15-16580
13. Park M, Banga JP, Kim GJ, Kim M, Lew H. Human placenta-derived mesenchymal stem cells ameliorate orbital adipogenesis in female mice models of graves’ ophthalmopathy. Stem Cell Res Ther. 2019;10(1):246. doi:10.1186/s13287-019-1348-0
14. Kim JY, Park S, Lee H, Lew H, Kim GJ. Functionally enhanced placenta-derived mesenchymal stem cells inhibit adipogenesis in orbital fibroblasts with graves’ ophthalmopathy. Stem Cell Res Ther. 2020;11(1):469. doi:10.1186/s13287-020-01982-3
15. Wang M, Zhang M, Fu L, et al. Liver-targeted delivery of TSG-6 by calcium phosphate nanoparticles for the management of liver fibrosis. Theranostics. 2020;10(1):36–49. doi:10.7150/thno.37301
16. Milner CM, Day AJ. TSG-6: a multifunctional protein associated with inflammation. J Cell Sci. 2003;116(10):1863–1873. doi:10.1242/jcs.00407
17. Li C, Li X, Shi Z, et al. Exosomes from LPS-preconditioned bone marrow MSCs accelerated peripheral nerve regeneration via M2 macrophage polarization: involvement of TSG-6/NF-κB/NLRP3 signaling pathway. Exp Neurol. 2022;356:114139. doi:10.1016/j.expneurol.2022.114139
18. Bayliss MT, Howat SLT, Dudhia J, et al. Up-regulation and differential expression of the hyaluronan-binding protein TSG-6 in cartilage and synovium in rheumatoid arthritis and osteoarthritis. Osteoarthritis Cartilage. 2001;9(1):42–48. doi:10.1053/joca.2000.0348
19. Guo P, Zhang S, He H, Zhu Y, Tseng SCG. TSG-6 controls transcription and activation of matrix metalloproteinase 1 in conjunctivochalasis. Invest Ophthalmol Vis Sci. 2012;53:1372–1380. doi:10.1167/iovs.11-8738
20. Lanzoni G, Linetsky E, Correa D, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, Phase 1/2a, randomized controlled trial. Stem Cell Transl Med. 2021;10(5):660–673. doi:10.1002/sctm.20-0472
21. Liu H, Li R, Liu T, Yang L, Yin G, Xie Q. Immunomodulatory effects of mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles in rheumatoid arthritis. Front Immunol. 2020;11:1912.
22. Petrou P, Kassis I, Levin N, et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain. 2020;143(12):3574–3588. doi:10.1093/brain/awaa333
23. Prockop DJ, Youn OJ. Mesenchymal Stem/Stromal Cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20(1):14–20. doi:10.1038/mt.2011.211
24. Zhang S, Fang J, Liu Z, et al. Inflammatory cytokines-stimulated human muscle stem cells ameliorate ulcerative colitis via the IDO-TSG6 axis. Stem Cell Res Ther. 2021:12. doi:10.1186/s13287-020-02065-z
25. Song HB, Park SY, Ko JH, et al. Mesenchymal stromal cells inhibit inflammatory lymphangiogenesis in the cornea by suppressing macrophage in a TSG-6-dependent manner. Mol Ther. 2018;26(1):162–172. doi:10.1016/j.ymthe.2017.09.026
26. Gianoukakis AG, Khadavi N, Smith TJ. Cytokines, graves’ disease, and thyroid-associated ophthalmopathy. Thyroid. 2008;18(9):953–958. doi:10.1089/thy.2007.0405
27. Łacheta D, Miśkiewicz P, Głuszko A, et al. Immunological aspects of graves’ ophthalmopathy. Biomed Res Int. 2019;2019:7453212–7453260. doi:10.1155/2019/7453260
28. Zheng J, Duan H, You S, Liang B, Chen Y, Huang H. Research progress on the pathogenesis of graves’ ophthalmopathy: based on immunity, noncoding RNA and exosomes. Front Immunol. 2022;13:1.
29. Zhang M, Jiang W, Lu G, Wang R, Lv Z, Li D. Insight into mouse models of hyperthyroidism. Front Endocrinol. 2022;13:929750.
30. Görtz GE, Philipp S, Bruderek K, et al. Macrophage-orbital fibroblast interaction and hypoxia promote inflammation and adipogenesis in graves’ orbitopathy. Endocrinology. 2022;164(2):203. doi:10.1210/endocr/bqac203
31. Chen MH, Chen MH, Liao SL, et al. Role of macrophage infiltration in the orbital fat of patients with graves’ ophthalmopathy. Clin Endocrinol. 2008;69(2):332–337. doi:10.1111/j.1365-2265.2008.03219.x
32. Bardos T, Kamath RV, Mikecz K, Glant TT. Anti-inflammatory and chondroprotective effect of TSG-6 (tumor necrosis factor-alpha-stimulated gene-6) in murine models of experimental arthritis. Am J Pathol. 2001;159:1711–1721. doi:10.1016/S0002-9440(10)63018-0
33. Oh JY, Roddy GW, Choi H, et al. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci. 2010;107(39):16875–16880. doi:10.1073/pnas.1012451107
34. Song W, Li Q, Ryu M, et al. TSG-6 released from intraperitoneally injected canine adipose tissue-derived mesenchymal stem cells ameliorate inflammatory bowel disease by inducing M2 macrophage switch in mice. Stem Cell Res Ther. 2018;9(1). doi:10.1186/s13287-018-0841-1
35. Ding Y, Gong P, Jiang J, et al. Mesenchymal stem/stromal cells primed by inflammatory cytokines alleviate psoriasis-like inflammation via the TSG-6-neutrophil axis. Cell Death Dis. 2022;13(11):996. doi:10.1038/s41419-022-05445-w
36. Salvi M, Vannucchi G, Currò N, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015;100(2):422–431. doi:10.1210/jc.2014-3014
37. Sánchez-Bilbao L, Martínez-López D, Revenga M, et al. Anti-IL-6 receptor tocilizumab in refractory graves’ orbitopathy: national multicenter observational study of 48 patients. J Clin Med. 2020;9(9):2816. doi:10.3390/jcm9092816
38. Douglas RS, Kahaly GJ, Ugradar S, et al. Teprotumumab efficacy, safety, and durability in longer-duration thyroid eye disease and re-treatment. Ophthalmology. 2022;129(4):438–449. doi:10.1016/j.ophtha.2021.10.017
39. Diana T, Ungerer M, Wüster C, et al. A cyclic peptide significantly improves thyroid function, thyrotropin-receptor antibodies and orbital mucine /collagen content in a long-term graves’ disease mouse model. J Autoimmun. 2021;122:102666. doi:10.1016/j.jaut.2021.102666
40. Plöhn S, Hose M, Schlüter A, et al. Fingolimod improves the outcome of experimental graves’ disease and associated orbitopathy by modulating the autoimmune response to the thyroid-stimulating hormone receptor. Thyroid. 2019;29:1286–1301. doi:10.1089/thy.2018.0754
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.