Back to Journals » Journal of Pain Research » Volume 15
TRPV1 and GABAB1 in the Cerebrospinal Fluid-Contacting Nucleus are Jointly Involved in Chronic Inflammatory Pain in Rats
Authors Xu LL, Yan Y, Yuan YM, Li Y, Jiang J, Zhang LC
Received 12 August 2022
Accepted for publication 6 December 2022
Published 14 December 2022 Volume 2022:15 Pages 3931—3939
DOI https://doi.org/10.2147/JPR.S385810
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Ling-Ling Xu,1– 4,* Yao Yan,1– 3,* Yu-Min Yuan,1– 3 Ying Li,1– 3 Jun Jiang,1– 3 Li-Cai Zhang1– 3
1Jiangsu Province Key Laboratory of Anesthesiology, Xuzhou Medical University, Xuzhou, People’s Republic of China; 2Jiangsu Province Key Laboratory of Anesthesia and Analgesia Application Technology, Xuzhou Medical University, Xuzhou, People’s Republic of China; 3NMPA Key Laboratory for Research and Evaluation of Narcotic and Psychotropic Drugs, Xuzhou, People’s Republic of China; 4Department of Anesthesiology, Nanjing Gaochun People’s Hospital, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Li-Cai Zhang, Email [email protected]
Objective: To assess the receptors of TRPV1 and GABAB1 receptors that were colocalized in cerebrospinal fluid contacting nucleus (CSF-contact nucleus) of chronic inflammatory pain (CIP) rats bringing inspiration for reducing chronic pain.
Methods: A rat model of CIP was constructed by plantar injection of complete Freund’s adjuvant (CFA), and the paw withdrawal mechanical threshold (PWMT) and paw withdrawal thermal latency (PWTL) were measured 1, 3, 5, 7, 10, and 14 days after plantar injection. In the first part of the experiment, rats with CIP were divided into the immunofluorescence group and the coimmunoprecipitation (Co-IP) group (n = 6). Rats in the immunofluorescence group were injected with the retrograde tracer CB conjugated with Alexa Fluor 594 into the lateral ventricle two days before the injection of CFA into the plantar surface of the left paw. Three days later, rats that exhibited hyperalgesia were perfused, and their brains were extracted and used for double immunofluorescence staining of the CSF-contacting nucleus. Rats in the Co-IP group were anesthetized and dissected 3 days after CFA injection, and fresh brain segments containing the CSF-contacting nucleus were collected for Co-IP to assess the colocalization of TRPV1 and GABAB1 in the CSF-contacting nucleus (n = 6). In the second part of the experiment, SD rats were divided into the normal saline group (control group) and the CFA group. Fresh CSF-contacting nucleus-containing tissues were collected for Western blot analysis 3 days after plantar injection to observe the changes in TRPV1 and GABAB1 expression in the CSF-contacting nucleus.
Results: TRPV1 and GABAB1 were co-expressed in the CSF-contacting nucleus in rats with CIP, and their expression was upregulated.
Conclusion: TRPV1 and GABAB1 in the CSF-contacting nucleus are jointly involved in CIP in rats, and there is a direct or indirect link between TRPV1 and GABAB1.
Keywords: cerebrospinal fluid-contacting nucleus, TRPV1 receptor, GABAB1 receptor, chronic inflammatory pain
Introduction
Chronic inflammatory pain (CIP) is pain caused by persistent or unresolved inflammation. Multiple inflammatory stimuli can sensitize nociceptive neurons, thereby promoting pain hypersensitivity. However, animal experiments and clinical practice have shown that inhibition of individual inflammatory pathways is a problematic approach for relieving pain, as parallel signaling cascades can drive pathologic pain and thus promote pain sensitization.1 It is known that ion channels and receptors in the dorsal root ganglia (DRG) are responsible for the detection of noxious stimuli, and their plasticity contributes to the increased severity of pain. TRP (transient receptor potential) channels are emerging targets for understanding this process and developing novel treatments. Their ability to form multimeric complexes broadens the variety and complexity of channel regulation and the potential implications for pain modulation.2 Among TRP channels, the capsaicin receptor TRPV1, a non-selective cation channel, is a key molecular component of pain detection and modulation. Sensitization of TRPV1 is central to the initiation of pathological forms of pain, and multiple signaling cascades are known to enhance TRPV1 activity under inflammatory conditions.3 Hyperalgesia resulting from tissue injury or inflammation is often associated with sensitization of TRPV1 channel activity which can occur through several mechanisms such as phosphorylation, interaction with phospholipid PIP2, trafficking, and association with accessory proteins.4 The therapeutic glory of TRPV1 is well recognized in clinics which gives a promising insight into the treatment of pain.5 But the adverse effects associated with some of the antagonists directed the scientists towards other methods. The GABAB receptor is a G protein-coupled receptor (GPCR) composed of the GABAB1 (GB1) and GABAB2 (GB2) subunits. There are at least 14 subtypes of GB1 (GB1a-n), of which GB1a and GB1b are the most abundant subtypes and are mainly expressed in the central nervous system.6 Studies have confirmed that GABAB1 receptor subunits act as inhibitors of TRPV1 sensitization in different inflammatory settings, and the effect of GABAB on TRPV1 depends on the close juxtaposition of GABAB1 receptor subunits and TRPV1. Activation of GABAB1 receptor subunits does not attenuate the normal function of the TRPV1 receptor and only restores its sensitized state.7 Therefore, it is undoubtedly a meaningful scientific problem to find the neural inhibitory pain structure coexisting with TRPV1 and GABAB1.
The cerebrospinal fluid-contacting nucleus (CSF-contacting nucleus) was first discovered and named by our research group in the world. It locates in the ventral gray matter of the lower part of the midbrain aqueduct (Aq) and the upper part of the base of the fourth ventricle (4V). The distinguishing feature of this nucleus is that the somas are located in the brain parenchyma, and their processes extend into the CSF.8 This connection is not found in any known nerve or nucleus in the nervous system. The basic biological properties of the CSF-contacting nucleus, such as methods that can be used to specifically label it,9 its location and stereotactic coordinates,8 the distribution of receptors, neurotransmitters, and ion channels10–12 and its relationship with morphine dependence and withdrawal, stress, sodium appetite, ion channels10,13–15 have been revealed. A model animal with the CSF-contacting nucleus eliminated has been successfully established.16
Based on the expression of TRPV1 and GABA in CSF-contacting nuclei in both neuropathic and inflammatory pain conditions,17,18 we hypothesized that TRPV1 and GABAB1 receptors may coexist in the neurons of the CSF-contacting nucleus and play important roles in neuropathic and inflammatory pain regulation. In the present study, our data demonstrated that GABAB1 expressed and formed a complex with TRPV1 in the CSF-contacting nucleus and investigated the changes in TRPV1 and GABAB1 expression in CIP.
Methods
Reagents
CB conjugated with Alexa Fluor 594 (Invitrogen, Thermo Fisher), a rabbit anti-TRPV1 antibody (Novusbio, USA), a mouse anti-GABAB1 antibody (Santa Cruz, USA), mouse anti-GABAB1 (Abcam, UK), Alexa Fluor 405-conjugated donkey anti-mouse IgG H&L (Abcam, UK), Alexa Fluor 488-conjugated donkey anti-rabbit secondary antibody (Absin, China), Rabbit (Mouse) Control IgG (ABclonal, Wu Han, China), HRP-labeled goat anti-rabbit IgG (H+L), and HRP-labeled goat anti-mouse IgG (H+L) (Beyotime, China) were used.
Experimental Grouping
Adult Specific Pathogen Free (SPF)-grade male SD rats weighing 250±25 g were provided by the Experimental Animal Center of Xuzhou Medical University. All animals were adaptively housed in a housing room on a 12–12 h light/dark cycle for one week before the start of the experiment and were given free access to food and water. In the first part of the experiment, rats with CIP were divided into immunofluorescence and coimmunoprecipitation (Co-IP) groups (n=6); in the second part of the experiment, SD rats were divided into the control group (control group) and CIP group (complete Freund’s adjuvant (CFA) group) (n=6). All protocols were approved by the Committee for Ethical Use of Laboratory Animals of Xuzhou Medical University (L20211001001) and were carried out according to the Guidelines for the Care and Use of Laboratory Animals.
Animal Model
To induce chronic inflammatory pain, rats were administered 100 µL CFA intra-plantar injection after being anesthetized with 2.5% sevoflurane.19 The control group was injected with an equal amount of normal saline according to the same procedure.
Behavioral Assessment
(1) Paw withdrawal mechanical threshold (PWMT)
The rats were placed in a transparent enclosure (35 cm*30 cm*25 cm) with a wire mesh bottom and allowed to acclimate in a quiet environment for 15 min before the experiment. We measured the paw withdrawal threshold to von Frey filaments (Stoelting, USA). Each filament (1.4, 2, 4, 6, 8, 10, 15, 26 g) was applied for 6 to 8 s to test the mid-plantar left hind paw, avoiding the footpads according to the up-down method as described previously.19,20 Abrupt withdrawal, licking, and shaking of the hind paw in response to von Frey filament was considered a positive response. If a positive response occurred, the next smaller von Frey filament was used whereas the filament of higher force was used. Since the threshold is unknown, strings of similar responses may be generated when the threshold is approached from either direction. Thus, although all responses were recorded, the critical six data points were not counted until the response threshold was first crossed.21 So, we started with a lower force of 4 g filament. The stimuli were always presented continuously, either ascending or descending. The test was continued till five responses were assessed after the first crossing of the withdrawal threshold, or the upper/lower end of the von Frey filament set was reached before a positive/negative response had been obtained. The pattern of positive and negative responses was converted to a 50% threshold value using the formula according to the up-down method of Dixon.22 Pre-CFA baseline and 1 d, 3 d, 5 d,7d,10 d, and 14 d post-CFA-injected thresholds were measured.
(2) Paw withdrawal thermal latency (PWTL)
A 15 cm*15 cm*15 cm transparent Plexiglas box was placed on a 3 mm-thick glass plate, and the rats were acclimated to the box for at least 30 min. The experiment was performed after the rats had calmed down. A light beam was applied to the middle plantar surface of the left hind paw three times at 3 to 5 min intervals with a thermal pain stimulator (BME2410A, Institute of Biological Engineering, Chinese Academy of Medical Sciences) according to the Hargreaves method.23,24 The duration from the start of irradiation to a leg lifting or avoidance response was measured as the PWTL, and irradiation was stopped when the rat raised its hind paw. A cutoff time of 20s was set to prevent tissue damage caused by excessive irradiation. The intensity of the thermal stimulus was kept the same throughout the experiment. The average paw withdrawal latency of the three trials was used for data analysis.
Injection of Alexa Fluor 594-Conjugated CB into the Lateral Ventricle
Two days before plantar injection of CFA, the rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.), and their heads were fixed on a stereotaxic apparatus. CB was dissolved in PBS (0.01 M, pH 7.4) and microinjected into the lateral ventricle (400 ng/2 µL) at the following stereotaxic coordinates determined from the Paxinos and Watson brain atlas: 1.4±0.2 mm to the right of the midline, 1.2±0.4 mm posterior from bregma, and 3.2 ±0.4 mm deep. Successful targeting of the lateral ventricle was confirmed by aspiration of CSF. The rats were allowed to recover for two days before subsequent experimental manipulations.
Immunofluorescent Staining
The rats were anesthetized with pentobarbital sodium (40 mg/kg, i.p.). The lower edge of the xiphoid process was cut, the thoracic cavity was opened to expose the heart, the apex of the heart was gently clamped with vascular forceps, and a puncture needle was inserted into the ascending aorta from the left ventricle of the apex of the heart. Vascular forceps were used to fix the puncture needle, 200~300 mL of normal saline was quickly perfused, and the right atrial appendage was simultaneously cut so that the blood quickly flushed out and became clear. Then, the blood was replaced with 200~300 mL of chilled 4% paraformaldehyde. After perfusion, brain tissue was collected, placed in 4% paraformaldehyde for more than 6 h in a refrigerator at 4°C, and then transferred to 30% sucrose solution until it sank to the bottom of the tube. The brain containing the CSF-contacting nucleus was isolated and sectioned coronally on a cryostat (Leica CM1900, Germany) at 40 μm. The sections were collected in PBS and rinsed for 5 min × 3 times, permeabilized with 0.1% TritonX-100 in PBS for 15 min. The brain slices were transferred to a 24-well incubation tank containing 10% donkey serum for 2 h at room temperature. After blocking, they were incubated with rabbit anti-TRPV1 (1:800, Novus) and mouse anti-GABAB1 (1:800, Abcam) antibodies diluted in PBST (0.3%Tween-20 in PBS) overnight at 4°C on a shaker. After washing with PBST, the brain slices were incubated with Alexa Fluor 488-labeled donkey anti-rabbit (1:500) and Alexa Fluor 405-labeled donkey anti-mouse (1:500, Abcam) secondary antibodies in the dark at room temperature for 2 h. After washing three times as described above, sections were mounted in sequence on slides and coverslipped. Immunofluorescence images were acquired on an Olympus FV1000 laser confocal microscope and were processed for quantitative analysis using ImageJ software.
Co-Immunoprecipitation
Three days after plantar injection of CFA, brain tissues containing the CSF-contacting nucleus were rapidly isolated, and 10 times the tissue weight of RIPA Lysis Buffer (Strong)(GK10023, Glpbio) and 1/100 of the lysate volume of a phosphatase enzyme inhibitor (PMSF) (GK10023, Glpbio) were added. The supernatant was collected after ultrasonic homogenization and centrifugation (4°C,12000 rpm,15 min), and the total protein concentration of the sample was determined using a BCA kit (Beyotime, China). The samples were divided into three parts: (1) one part, which was mixed with 1X SDS-PAGE loading buffer and boiled for 10 min, was used as a positive control (input) for Western blot analysis; (2) another part was incubated with negative control IgG (ABclonal, Wu Han, China); and (3) the last part was incubated with rabbit anti-TRPV1 or mouse anti-GABAB1 primary antibody overnight at 4°C with shaking. Prepared rProtein A/G Plus MagPoly beads (ABclonal, Wu Han, China) were added to 1 mL of 3% BSA and incubated at 4°C with shaking for 1 h to eliminate nonspecific binding. The antibody-antigen binding complexes were mixed with the prepared magnetic beads and incubated at 4°C overnight. The magnetic bead-antibody-antigen complexes were rinsed three times, and the supernatant was discarded. Then, 30 µL of 2X SDS-PAGE loading buffer was added, and the samples were mixed well and heated at 95°C for 15 min for denaturing elution. The immunoprecipitated protein complexes were separated from the supernatant by SDS-PAGE. Western blot analysis with antibodies against TRPV1 and GABAB1 was performed.
Western Blot Analysis
SD rats were divided into the control and CFA groups, plantar injection three days later, and fresh brain tissues containing the CSF-contacting nucleus were collected. Ten times the tissue weight of RIPA lysis buffer (Beyotime, China) and 1/100 of the lysate volume of PMSF (Beyotime, China) was added. After the tissues were homogenized and centrifuged at 4°C for 15 min at 12,000 rpm, the supernatant was collected, and the total protein concentration of each sample was determined by a BCA protein assay kit (Beyotime, China). Then, the proteins were separated by 8% SDS-PAGE. After electrophoresis, the proteins were transferred to PVDF membranes, which were blocked with 5% skim milk powder for 2 h and incubated with rabbit anti-TRPV1 (1:2000, Novus Biologicals), mouse anti-GABAB1 (1:500, Abcam) and mouse anti-GAPDH (1:2000, Proteintech) antibodies at 4°C overnight. After half an hour at room temperature, the PVDF membranes were rinsed three times with TBST (TBS containing 1% Tween-20) and incubated with corresponding HRP-conjugated secondary antibodies (1:2000, Beyotime, China) for 2 h. Then, the membranes were rinsed six times with TBST. The protein bands were detected by a chemiluminescent reagent (Beyotime, China), and the relative grayscale values were analyzed using ImageJ software.
Statistical Analysis
GraphPad 8.0 software was used to statistically analyze the data. Statistical comparisons between two groups were conducted by two-tailed, unpaired Student’s t-test. Repeated-measures data were compared using a two-way ANOVA analysis accompanied by Bonferroni’s multiple comparison post hoc tests. P<0.05 was considered statistically significant. Normally distributed data are expressed as the mean ± SEM. MATLAB software was used to quantify the mechanical paw-withdrawal threshold using the registries of the up-down method test. ImageJ software was used to quantify the density of protein bands.
Results
Establishment of a CFA-Induced CIP Model
Rats in the CFA group, ie, CIP model rats, showed redness and swelling of the plantar surface of the left hind paw after CFA injection, and lifting and licking of the hind paw were observed. No such symptoms were observed in the control group before or after injection, and no significant changes in the PWMT and PWTL were observed before or after injection (P>0.05). The CFA group showed a lower pain threshold on the second day, and the PWMT and PWTL of rats in the CFA group were lower than the respective baseline values (P <0.01). The decrease in the PWMT and PWTL lasted for approximately two weeks (Figure 1).
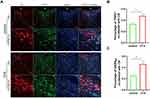
Immunofluorescence Analysis of TRPV1 and GABAB1 Coexpression in the CSF-Contacting Nucleus
Laser confocal microscopy was used to visualize the CSF-contacting nucleus. The CSF-contacting nucleus was indicated by red fluorescence, and the localization of the red fluorescence was consistent with the expected position of the CSF-contacting nucleus. TRPV1-positive cells were labeled with green fluorescence, and GABAB1-positive cells were labeled with blue fluorescence. Immunofluorescence staining revealed that TRPV1 and GABAB1 were co-expressed in the CSF-contacting nucleus. Quantitative analysis showed that the co-labeling of TRPV1 and GABAB1 receptors with the CSF-contacting nucleus respectively increased compared with the control group (Figure 2).
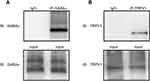
Co-Immunoprecipitation
TRPV1 and GABAB1 receptors in the CSF-contacting nucleus form complex. Co-IP was used to investigate the possible interaction between TRPV1 and GABAB1 at the protein level. Bidirectional Co-IP was performed, and TRPV1 and GABAB1 were detected in the CSF-contacting nucleus lysates, which were used as positive controls (“input” samples), but not in negative control samples incubated with normal IgG (“IgG” samples), indicating the specificity of the antibody. The results indicate that TRPV1 and GABAB1 bind to each other in neurons in the CSF-contacting nucleus (Figure 3).
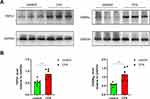
Changes in the Expression of TRPV1 and GABAB1 Receptors in the CSF-Contacting Nucleus in Chronic Inflammatory Pain Rat
To explore the changes in TRPV1 and GABAB1 expression in the CSF-contacting nucleus in CIP model rats, we first constructed a rat model of chronic inflammatory pain by injecting CFA into the plantar surface and then performed Western blot analysis. The results revealed that the expression of TRPV1 and GABAB1 in the CSF-contacting nucleus was significantly upregulated in the CIP model group compared with the control group (P<0.01) (Figure 4).
Discussion
In 2000, the World Health Organization stated that chronic pain is a disease that does harm health. The causes of chronic pain, especially neuropathic pain, can be very complex, but it is generally accepted that a variety of factors lead first to inflammation and finally to chronic pain and even neuropathic pain. Therefore, preventing chronic inflammatory pain from further transforming into neuropathic pain is one of the main strategies in the field of research and treatment. Although it has become the main goal of basic research and the basic measure of clinical treatment to find specific pain-causing factors and reduce pain by intervening in this factor, both basic research and clinical practice show that the analgesic effect of simply intervening in a specific target is not ideal. As parallel signaling cascades can still drive pathologic pain and thus promote pain sensitization.1
TRPV1 is a well-established ion channel activated by pain and heat. Gaurav G-N et al recently showed that natural products and some semi-synthetic analogs as potential TRPV1 ligands for attenuating neuropathic pain.25 Akhilesh et al reviewed Unlocking the potential of TRPV1 based siRNA therapeutics for the treatment of chemotherapy-induced neuropathic pain.5 Pathogenic sensitization of TRPV1 is characterized by a decrease in the activation threshold and an increase in responsiveness.26 Various mechanisms, such as alterations in channel dynamics, changes in TRPV1 levels in the neuronal plasma membrane, and changes in the levels of proteins that bind and regulate TRPV1, may play a role in this process.27–31 Therefore, targeting substances that interact with TRPV1, especially those that specifically regulate pathological states such as inflammatory pain, may be an interesting alternative to blocking TRPV1.32,33 American scientists David Julius and Ardem Patapoutian have won the 2021 Nobel Prize in Physiology/Medicine for their outstanding contributions to “finding TRPV1 in capsaicin and discovering it as a receptor for sensing temperature and touch”.
GABA is a major transmitter in the central nervous system. Its receptors include three types: GABAA and GABAC receptors can form ligand-gated chloride channels, while GABAB receptors belong to the G protein-coupled receptor family and are accompanied by K+ and Ca2+ channels. Stephen G. Brickley and Istvan Mody (2012) introduced the function of GABAA Receptors in the CNS and its Implications for Disease. GABAARs are known to be key targets of anesthetics, sleep-inducing drugs, neurosteroids, and alcohol. The network dynamics associated with epilepsy and Parkinson’s disease are likely to involve tonic GABAAR-mediated changes in conductance. Therefore, GABAARs may be a therapeutic target for the treatment of these disorders, with the potential to enhance cognition and aid functional recovery after stroke.34 GABABR is a G protein-coupled receptor. Recently, Marzia M (2018) and Dietmar B (2022) respectively systematically reviewed the biological and pharmacological research progress of GABAB receptors in pain. This receptor has been considered a valuable target for the treatment of chronic pain due to extensive evidence that they are involved in the regulation of pain signaling.35,36
The CSF-contacting nucleus is distinct from other brain nuclei, our previous studies have shown that neurons in this nucleus contain not only TRPV1 but also GABA and are involved in the regulation of various pain.12,37 In this study, we demonstrated for the first time that TRPV1 and GABAB1 receptors not only coexist but also form a complex in the CSF-contacting nucleus. This result will provide a new insight for reducing inflammatory pain by intervening GABAB1 or TRPV1 in the CSF-contacting nucleus through the cerebrospinal fluid pathway. Of course, further research is needed.
Conclusions
This study demonstrated that TRPV1 receptors and GABAB1 receptors are both expressed in the CSF-contacting nucleus and form complexes, which may be involved in the development of chronic inflammatory pain. This will provide a new idea for the treatment of chronic inflammatory pain.
Ethics Approval
Ethics approval was obtained from the Experimental Animal Ethics Committee of Xuzhou Medical University (L20211001001).
Author Contributions
All authors made a significant contribution to this work in the conception, study design, execution, acquisition of data, analysis, and interpretation; took part in composing the article or revising it critically; agreed to submit to this journal; reviewed and agreed on all versions of the article before submission; agree to take responsibility and be accountable for the contents of the article.
Funding
This research is supported by the National Natural Science Foundation of China, 2022 Original Exploration Program recommended by experts. Grant No. 82150007.
Disclosure
The authors declare no potential conflicts of interest in this research.
References
1. Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16(11):1248–1257. doi:10.1038/nm.2235
2. Weng HJ, Patel KN, Jeske NA, et al. Tmem100 is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain. Neuron. 2015;85(4):833–846. doi:10.1016/j.neuron.2014.12.065
3. Caterina M, Schumacher M, Tominaga M, Rosen T, Levine J, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi:10.1038/39807
4. Kim YS, Chu Y, Han L, et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron. 2014;81(4):873–887. doi:10.1016/j.neuron.2013.12.011
5. Akhilesh UA, Gadepalli A, Gadepalli A, et al. Unlocking the potential of TRPV1 based siRNA therapeutics for the treatment of chemotherapy-induced neuropathic pain. Life Sci. 2022;288:120187. doi:10.1016/j.lfs.2021.120187
6. Jiang X, Su L, Zhang Q, et al. GABAB receptor complex as a potential target for tumor therapy. J Histochem. 2012;60(4):269–279.
7. Hanack C, Moroni M, Lima WC, et al. GABA blocks pathological but not acute TRPV1 pain signals. Cell. 2015;160(4):759–770. doi:10.1016/j.cell.2015.01.022
8. Song SY, Li YH, Bao CY, et al. Stereotaxic coordinates and morphological characterization of a unique nucleus (CSF-Contacting Nucleus) in rat. Front Neuroanat. 2019;13:47. doi:10.3389/fnana.2019.00047
9. Zhou F, Wang J, Zhang H, et al. Evaluation of three tracers for labeling distal cerebrospinal fluid-contacting neurons. Neurosci Bull. 2013;29(5):576–580. doi:10.1007/s12264-013-1332-0
10. Lu XF, Li YY, Wang CG, Wei JQ, Cao JL. Substance P in the cerebrospinal fluid-contacting nucleus contributes to morphine physical dependence in rats. Neurosci Lett. 2011;488(2):188–192. doi:10.1016/j.neulet.2010.11.026
11. Wang XY, Yan WW, Zhang XL, Liu H, Zhang LC. ASIC3 in the cerebrospinal fluid-contacting nucleus of brain parenchyma contributes to inflammatory pain in rats. Neurol Res. 2014;36(3):270–275. doi:10.1179/1743132813Y.0000000297
12. Liu PF, Fang HZ, Yang Y, et al. Activation of P2X3 receptors in the cerebrospinal fluid-contacting nucleus neurons reduces formalin-induced pain behavior via PAG in a rat model. Neuroscience. 2017;358:93–102. doi:10.1016/j.neuroscience.2017.06.036
13. Wu YH, Song SY, Liu H, et al. Role of adrenomedullin in the cerebrospinal fluid-contacting nucleus in the modulation of immobilization stress. Neuropeptides. 2015;51:43–54. doi:10.1016/j.npep.2015.03.007
14. Xing D, Wu Y, Li G, et al. Role of cerebrospinal fluid-contacting nucleus in sodium sensing and sodium appetite. Physiol Behav. 2015;147:291–299. doi:10.1016/j.physbeh.2015.04.034
15. Zhang C, Li Y, Wang X, Fei Y, Zhang L. Involvement of neurokinin 1 receptor within the cerebrospinal fluid-contacting nucleus in visceral pain. Mol Med Rep. 2017;15(6):4300–4304. doi:10.3892/mmr.2017.6499
16. Song SY, Zhang LC. The establishment of a CSF-contacting nucleus “knockout” model animal. Front Neuroanat. 2018;12(22):54–64. doi:10.3389/fnana.2018.00022
17. Xu C, Zhao ZJ, Wu TT, Zhang LC. Distribution of TRPV1 in CSF contacting nucleus of rat brain parenchyma and its expression in neuropathic pain. J Neurol Neurophysiol. 2011;02(02):1–6. doi:10.4172/2155-9562.1000114
18. Chao CL, Lu XF, Zhang LC. Formalin-induced pain stimulation induced expression of GABA in the distal cerebrospinal fluid contacting neurons. Chin J Appl Physiol. 2010;26(1):36–38.
19. Uniyal A, Gadepalli A, Modi A, Tiwari V. Modulation of KIF17/NR2B crosstalk by tozasertib attenuates inflammatory pain in rats. Inflammopharmacology. 2022;30(2):549–563. doi:10.1007/s10787-022-00948-6
20. Tiwari V, Anderson M, Yang F, et al. Peripherally acting mu-opioid receptor agonists attenuate ongoing pain-associated behavior and spontaneous neuronal activity after nerve injury in rats. Anesthesiology. 2018;128(6):1220–1236. doi:10.1097/ALN.0000000000002191
21. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi:10.1016/0165-0270(94)90144-9
22. Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi:10.1146/annurev.pa.20.040180.002301
23. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi:10.1016/0304-3959(88)90026-7
24. Tiwari V, He SQ, Huang Q, et al. Activation of micro-delta opioid receptor heteromers inhibits neuropathic pain behavior in rodents. Pain. 2020;161(4):842–855. doi:10.1097/j.pain.0000000000001768
25. Naik GG, Uniyal A, Chouhan D, Tiwari V, Sahu AN. Natural products and some semi-synthetic analogues as potential TRPV1 ligands for attenuating neuropathic pain. Curr Pharm Biotechnol. 2022;23(6):766–786. doi:10.2174/1389201022666210719155931
26. Planells-Cases R, Garcìa-Sanz N, Morenilla-Palao C, Ferrer-Montiel A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflügers Archiv. 2005;451(1):151–159. doi:10.1007/s00424-005-1423-5
27. Lainez S, Valente P, Ontoria-Oviedo I, et al. GABAA receptor associated protein (GABARAP) modulates TRPV1 expression and channel function and desensitization. FASEB J. 2010;24(6):1958–1970. doi:10.1096/fj.09-151472
28. Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534(3):813–825. doi:10.1111/j.1469-7793.2001.00813.x
29. Morenilla-Palao C. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J Biol Chem. 2004;279(24):25665–25672. doi:10.1074/jbc.M311515200
30. Camprubi-Robles M, Planells-Cases R, Ferrer-Montiel A. Differential contribution of SNARE-dependent exocytosis to inflammatory potentiation of TRPV1 in nociceptors. FASEB J. 2009;23(11):3722–3733. doi:10.1096/fj.09-134346
31. Meng J, Wang J, Steinhoff M, Dolly JO. TNFα induces co-trafficking of TRPV1/TRPA1 in VAMP1-containing vesicles to the plasmalemma via Munc18–1/syntaxin1/SNAP-25 mediated fusion. Sci Rep. 2016;6:21226. doi:10.1038/srep21226
32. Fernández-Carvajal A, Fernández-Ballester G, Devesa I, González-Ros JM, Ferrer-Montiel A. New strategies to develop novel pain therapies: addressing thermoreceptors from different points of view. Pharmaceuticals. 2012;5(1):16–48. doi:10.3390/ph5010016
33. Fischer MJ, Btesh J, Mcnaughton PA. Disrupting sensitization of transient receptor potential vanilloid subtype 1 inhibits inflammatory hyperalgesia. J Neurosci. 2013;33(17):7407–7414. doi:10.1523/JNEUROSCI.3721-12.2013
34. Brickley SG, Mody I. Extrasynaptic GABA (A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73(1):23–34. doi:10.1016/j.neuron.2011.12.012
35. Malcangio M. GABAB receptors and pain. Neuropharmacology. 2018;136:102–105. doi:10.1016/j.neuropharm.2017.05.012
36. Benke D. GABAB receptors and pain. Curr Top Behav Neurosci. 2022;52:213–239.
37. Ping C, Min PC, Qsl D, Xzl A, Zl D. CSF-CN contributes to cancer-induced bone pain via the MKP-1-mediated MAPK pathway. Biochem Biophys Res Commun. 2021;547:36–43. doi:10.1016/j.bbrc.2021.02.010
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.