Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Triple Cross-Linked Hyaluronic Acid Based on Tri-Hyal Technique Has More Durable Effect on Dermal Renewal
Authors Chen R, Yang W, Sun J, Liu Y, An Q, Zhang F, Bai Z, Luan Q
Received 17 February 2022
Accepted for publication 7 April 2022
Published 15 April 2022 Volume 2022:15 Pages 691—701
DOI https://doi.org/10.2147/CCID.S362785
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Rong Chen,1 Wenbin Yang,2 Jing Sun,3 Yuan Liu,4 Qing An,2 Feijuan Zhang,2 Zhuanli Bai,5 Qi Luan2
1College of Life Sciences, Northwest University, Xi’an, 710069, People’s Republic of China; 2Department of Dermatology, Xi’an No.3 Hospital, the Affiliated Hospital of Northwest University, Northwest University, Xi’an, 710069, People’s Republic of China; 3Department of Anesthesiology, Northwest Women’s and Children’s Hospital, Xi’an, 710061, People’s Republic of China; 4Center of Medical Genetics, Northwest Women’s and Children’s Hospital, Xi’an, 710061, People’s Republic of China; 5Department of Plastic and Aesthetic Maxillofacial Surgery, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China
Correspondence: Qi Luan, Department of Dermatology, Xi’an No.3 Hospital, the Affiliated Hospital of Northwest University, Northwest University, Xi’an, 710069, People’s Republic of China, Tel/Fax +86-29-61816090, Email [email protected] Zhuanli Bai, Department of Plastic and Aesthetic Maxillofacial Surgery, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, People’s Republic of China, Email [email protected]
Background: Hyaluronic acid (HA)-based fillers are applied to treat facial wrinkles and volume loss. Many efforts have been made to improve properties of HA to prolong the duration in aesthetic indications. A new cross-linking technique called “Tri-Hyal”, could make HAs to achieve desired rheological characteristics. HAs synthesized by Tri-Hyal are triple cross-linked and sustained-release, which could increase duration of promoting skin rejuvenation after injection.
Purpose: To evaluate the efficiency and persistence of HAs with Tri-Hyal on skin rejuvenation and further investigate underlying mechanisms, we compared the performances of cross-linked HA (AF) based on Tri-Hyal with another highly acceptable HA filler (Res) in vivo and in vitro.
Methods: Male BALB/c mice were divided into three groups, treated with AF, Res and vehicle, respectively. Skin biopsies were taken on day 0, 30, 90 and 180 after injection and hematoxylin and eosin (H&E), Masson’s trichrome (MT), immunohistochemical (IHC) stainings for CD31, TGF-β and MMP9 were performed. EdU incorporation, cell counting kit-8 (CCK-8), SA-β-Gal staining and activity were measured by biochemical analysis. RFP-GFP-LC3 adeno virus was used for autophagic flux detection. Protein levels of CD44, P62 and LC3I/II were detected by Western blot. Reactive oxygen species (ROS) level was detected by flow cytometry with DCFH-DA probe.
Results: The AF synthesized by Tri-Hyal showed persistent dermal structural correction without attenuation up to 6 months, which was illustrated by skin thickness, formation of elastic fibers and vascular density. Consistently, in fibroblasts the AF improved cell proliferation and slowed the senescent in vitro. Furthermore, it promoted cellular autophagy to reduce ROS level, which would account for its function in skin renewal.
Conclusion: The HA with Tri-Hyal could stimulate the production of extracellular matrix components more persistently than traditional HA fillers. In terms of mechanisms, it delayed senescence in dermal fibroblasts through reducing oxidative stress mediated by induction of autophagy.
Keywords: skin rejuvenation, fibroblasts, senescence, ROS, autophagy
Introduction
Over the decades, products based on hyaluronic acid (HA), which can be naturally synthesis by fibroblasts and other resident cells in vivo, are widely accepted as dermal fillers as their biocompatible, non-immunogenic, and non-inflammatory properties. As a kind of glycosaminoglycan and a natural of the extracellular matrix, HA filler restores lost volumes of soft tissue as expected.1–3 Furthermore, it was reported that cross-linked HA–based fillers may have additional effects, including stimulation of procollagen and various growth factors in aging skin in addition to their primary function as tissue-filling devices.4–6
However, HA-based substances are technically differed in terms of HA source, cross-linkage (agent and degree), HA concentration, and so on, which affect the effectiveness and safety of these products. Many efforts on the improvement of HA cross-linking technique have been made to optimize HA products, especially on their duration. It is well accepted that free HA could effectively promote skin rejuvenation through multiple mechanisms, though non–crosslinked HA had a half-life of only a few weeks following intradermal injection.7 Now, most available HA products in aesthetic indications are cross-linked, which would prolong the duration from 6 to 12 months depending on the degree of HA cross-linking.8
ART FILLER (AF) is a new kind of dermal filler which has successfully applied “Tri-Hyal” technology. This unique novel technique nicely combined the long chain, very-long chain and free HA together to achieve the desired rheological characteristics in the dermal filler. Based on this technique, AF obtained better properties for the treatment of facial rejuvenation. AF improved the facial volume loss by adding volume or shape restoration of the aging face. Triple cross-linked HAs including the very-long chain, long chain cross-linked HA and non-cross-linked HAs, could provide more suitable microenvironment for dermal fibroblasts to produce several extracellular matrix components relative to skin self-renewal continuously, while the cross-linked HA would persist for a long time in the tissue. Additionally, the mixture of three HAs with three different molecular weights has the characteristic of a natural entanglement in “Tri-Hyal” technique.9 The presence of natural entanglement could minimize the amount of cross-linking agent such as 1,4-butanediol diglycidyl ether (BDDE), used in the majority of the market-leading HA fillers. It is well known that amount of cross-linking agents are one of important causes of side effects in aesthetic injection of HA fillers.10 Consistently, clinical data showed that after more than 18 months, there was neither inflammatory nodule nor granuloma formation detected by cutaneous high-frequency ultrasound imaging.11
The mechanism for the stimulation of skin self-renewal by cross-linked HA products is not well understood, especially in AF with the “Tri-Hyal”, which is a triple cross-linking technique for sustained release of free HAs. Oxidative stress caused by environmental influences plays a major role in the skin aging process.12,13 UV-irradiation especially leads to the generation of reactive oxygen species (ROS) that mainly contributes to the aging of skin.14 Therefore many attempts were made to quench these ROS by topical treatment of the skin or supplementation with anti-oxidants, in the hope to improve or even rejuvenate aged skin. Autophagy can maintain cellular homeostasis when faced with different stress conditions and is one of the survival mechanisms of cell resistance to intrinsic and extrinsic stress. Aging skin has chronic inflammation, and its cell proliferation and waste disposal rate are slow, which are all related to autophagy.13 Activated autophagy can alleviate the oxidative damage of photoaging skin induced by UV radiation. Therefore, the main objective of this study was to investigate the mechanism for the promotion and persistence of skin rejuvenation by triple cross-linked AF with the “Tri-Hyal” technique.
In the present study, AF showed the persistent dermal structural correction in dermis without any attenuation up to 6 months. AF could upregulate TGF-β and downregulate of MMPs more durably after AF injection into mouse dorsal skin, which was consistent with higher amount of elastic fiber in the dermis. Additionally, AF promoted the formation and maintenance of microvasculature in dermal microenvironment. In vitro, AF improved cell proliferation and delayed senescence in human primary fibroblasts, caused by AF continuously scavenged ROS by inducing autophagy.
Materials and Methods
Materials
The two HA products were ART FILLER Universal (Fillmed, Paris, Franch) and Restylane II (Q-Med AB, Uppsala, Sweden). The primary antibodies used for IHC were anti-TGF-β (ab215715, Abcam), anti-MMP9 (bs-0397R, Bioss), anti-CD31 (ab28364, Abcam) and used for Western blot analysis were anti-CD44 (ab6124, Abcam), anti-p62 (ab109012, Abcam), anti-LC3 (GTX127375, GeneTex), and anti-Tubulin (BS1699, Bioworld). Senescence β-Galactosidase staining kit was from Beytime Biotechnology (C0602, China). Senescence β-Galactosidase activity kit was from BioLab (SK170-2, China). The Cell Counting Kit-8 (CCK-8) was purchased from TOPSCIENCE (C0005, China), and the Cell-Light EdU Apollo 643 in vitro Kit was form RiboBio (C10310, China). RFP-GFP-LC3 adeno virus was from Hanbio (Shanghai, China).
Animal Experiments
Male BALB/c mice (6 weeks old) were obtained from the Experimental Animal Research Center of the Fourth Military Medical University (Xi’an, China). All experiments were performed in strict accordance with the NIH guidelines for the care and use of laboratory animals (8th edition, NIH), and approved by Animal Ethics Committee of Northwest University (Xi’an, China). A total of 6 BALB/c mice were fed a standard diet. Each mouse was injected subcutaneously with 50 μL of AF, Res and PBS on its dorsal skin at different sites, respectively. There were twelve injection sites in total on the dorsal skin of each mouse, including AF, Res and vehicle groups with four injection sites in each group. The sample collection of skin biopsies for each time point was performed on the same mouse on day 0, 30, 90 and 180 after injection and hematoxylin and eosin (H&E) staining, Masson’s trichrome (MT) staining and immunohistochemical (IHC) staining were performed to compare the effects of HA treatment with different cross-linking technology. The study was conducted with the approval of the local agency’s Animal Care and Use Committee.
Cell Culture and Treatment
Human dermal fibroblast cells were obtained from explants of mammoplasty and abdominoplasty provided by three separate female donors, according to the methods previously described.15 Cell culture and treatment was conducted in accordance with the Declaration of Helsinki. The samples were used after previous inform consent by the volunteers and approval by the Ethics Committee of Dermatology Unit, Xijing hospital. The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Cat. No. 10566016, Gibco) supplemented with 10% fetal bovine serum (FBS) (Cat. No. 10100154, Gibco) and 1% antimycotic-antibiotic solution (Cat. No. 15140122, Gibco), and maintained at 37°C in humid atmosphere containing 5% CO2. For all experimental assays in vitro, human dermal fibroblasts were seeded on precoated culture plates with AF and Res in advance.
Histomorphometric Evaluation
Skin biopsy specimens obtained from each injection site were fixed in 4% paraformaldehyde, embedded in paraffin, after treatment at different time points. Then, the tissue sections were stained for H&E staining to measure skin thickness. MT staining for visualizing elastin fibers was performed as described.16 Representative images were digitalized using an Olympus BX-51 light microscope.
Immunohistochemical (IHC) Staining
Immunohistochemical (IHC) stains of CD31, TGF-β and MMP9 were done for evaluation of the dermal collagen synthesis and vascular density after HA injection at different points.17 The specimens were fixed with 10% formalin for 72 hours, and then were paraffin-embedded and cut into sections with a 4-µm thickness. The sections were incubated with a primary antibody at 4°C overnight and were stained with an appropriate secondary antibody conjugated with horseradish peroxidase. Next, the sections were stained with the chromogen diaminobenzidine, washed with PBS, counterstained with hematoxylin and dehydrated. All the biopsies were independently examined by two pathologists. Five observations of each sample were made to evaluate the specimens.
EdU Incorporation and CCK-8
The EdU incorporation assay was conducted to evaluate fibroblast proliferation. Primary fibroblasts were seeded in a 24-well plate at 4000 cells/well precoated with AF, Res and PBS. EdU incorporation assay was done as previously described.18 The EdU positive cells were detected by a fluorescence confocal microscope (LSM800, Carl Zeiss) and analyzed by Image J program. The results were calculated by the following equation: Percentage of cells (%) = the count of EdU cells/the count of total cells × 100%.
Cell viability was detected with Cell Counting Kit-8 (CCK-8) according to the manufacturer's protocol. The fibroblasts were incubated with 100 μL medium containing 10 μL CCK-8 for 4 h. The absorbance was measured at 450 nm.
β-Galactosidase (SA-β-Gal) Analysis
For Cytochemical staining for SA-β-Gal, fibroblasts were fixed in 4% paraformaldehyde. Then the slides were immersed in β-galactosidase solution. After incubation in the dark at 37°C for 16–18 h β-Gal staining become visible. The main procedures were performed according to the introduction of the kit. Images were collected with an Olympus BX-51 light microscope. The SA-β-Gal activities in the fibroblasts were determined with the β-Gal activity detection kit. The main procedures were done according to the introduction of the kit.
Autophagic Flux Quantification and Western Blot
Fibroblasts were infected with RFP-GFP-LC3 adeno virus for autophagic flux detection. 30–60 cells for each treatment were quantified. The red puncta only was considered as autolysosome, and yellow puncta was early autophagosome (Red and Green = yellow). In acidic pH, the GFP fluorescence was reduced while RFP still remains stable The conversion of yellow puncta to red puncta provided a readout for autophagic flux. The puncta in cells were analyzed with a confocal laser scanning microscope (LSM800, Carl Zeiss), using a 40 × oil immersion objective. The yellow puncta and red only puncta were quantified with the Image J program.
Fibroblasts were lysed in RIPA lysis buffer. Proteins (20–40 μg) were separated on 8% to 10% SDS-PAGE gel and then transferred onto PVDF membranes. After blocking the membranes with 5% fat-free milk in TBS with Tween 20 for 1 hour at room temperature, the membranes were incubated with primary antibodies overnight at 4°C. After washing, the blots were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour and developed in enhanced chemiluminescent reagent (Cat. No. WBKLS0500, Millipore Sigma), and visualized with an image analyzer Quantity One System (Bio-Rad).
Cellular ROS Level
The production of ROS was in fibroblasts was detected by DCFH-DA probe as previously described.18 10 μM probe in 1 mL PBS at 37 °C for 30 min in the dark, and then harvested and resuspended in PBS. Fluorescence in the cells were then measured by Beckman CytoFlex S flow cytometer at channels λex = 488 nm, λem= 525 nm. The percentage of ROS positive cells was analyzed by using Image J program.
Statistical Analysis
The statistical significance of the differences observed between groups was determined using ANOVA test and P < 0.05 was considered a statistically significant difference. Data are presented as mean ± SEM.
Results
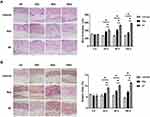
AF Based on Tri-Hyal Technique Increased Skin Thickness and Formation of Elastic Fibers in Mouse Dorsal Skin More Persistently.
To evaluate the effect of AF with sustained release Tri-Hyal technique on skin rejuvenation, the changes of skin thickness and the expression level of dermal elastic fibers at 0d, 30d, 90d and 180d after injection in mice were observed. BALB/c mice model is adopted and dermal injection was performed in the site on the dorsal skin. The skin biopsies were collected at planned time. Data from dermal skin biopsies with H&E and MT staining after the respective treatments showed that both cross-linked HA injection could stimulate dermal growth as early as 30 days and there was no difference between two groups. From 90 to 180 days, we observed a slight decrease of skin thickness and elastic fibers in skin injected with PBS. From 30 days on, HA injection promoted dermal thickness and elastic fibers formation. This elevating trend reached peak in 90 days in HA injection group, while the trend was kept until 180 days in AF group (P>0.05; Figure 1A and B). That judgement is proved by histological analysis. Totally, AF with sustained release Tri-Hyal technique consistently stimulates the dermal growth lasting for more than half a year without efficiency attenuation.
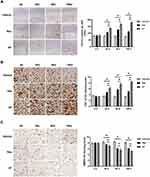
AF with Tri-Hyal Technique Increased the Expression Level of CD31 and TGF-β and Decreased the Expression of MMP9 More Durably.
Expression of CD31, an endothelial cell marker, in skin biopsies could provide further evidence for the role of angiogenesis in new tissue growth. Quantitative assessment of vascular density by IHC for CD31 showed a reduction of the cutaneous microvasculature in skin injected with vehicle and increased blood vessels in the mid-to-deep dermis in both filler-injected specimens after 30 days. In particularly, we noticed increased vessels staining by 50%, 85% and 57% in Res group and by 97%, 105% and 120% at 30, 90 and 180 days after filler injection, compared with vehicle group, respectively (P<0.05; Figure 2A right). It was worth mentioning that vascular density increased even 90 days after the AF injection.
Upregulation of TGF-β and downregulation of matrix metalloproteinase (MMP) are important markers of skin renewal. We then examined the protein expressions of TGF-β and MMP9 after dermal injection with these two HA products. Consistent with assessment of vascular density, the data showed that TGF-β expression was gradually decreased in the blank group and increased in the other two HA groups from 90 days after injection. From 90 days to 180 days, the expression of TGF-β in Res group began to decrease, while the TGF-β level in the AF group still showed a upward trend, which proved the effect on skin renewal from AF was more durable (Figure 2B). Conversely, MMP9 expression levels increased gradually over time in the blank control, while decreased in the other two HA-treating groups, although there was no significant difference between the two groups (P>0.05; Figure 2C). These IHC results suggested that HA with triple sustained-release cross-linking technique had a longer effect on skin rejuvenation.
AF with Tri-Hyal Technique Delayed the Fibroblasts Senescence in Vitro.
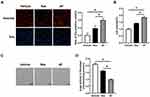
To further investigate the function of AF in delaying the cell senescence, we manipulated Edu incorporation and CCK-8 assays in fibroblast cultured on cell plates precoated with AF and Res. It was showed that compared with Res, AF treatment significantly improved the cell proliferation and viability (P<0.05; Figure 3A and B), although Res also mildly induced the cell proliferation and increased the cell viability compared with the vehicle.
As SA-β-gal was a widely accepted biomarker for the aging, SA-β-gal staining was then performed. Data showed that AF treatment robustly decreased the number of positive-stained cells compared with Res and vehicle (P<0.05; Figure 3C). Consistent with this, AF significantly inhibited the SA-β-gal activity compared with the Res and vehicle (P<0.05; Figure 3D). Collectively, all of these results suggested that AF more effectively delayed the senescence in human primary fibroblasts than other cross-linked HA filler.
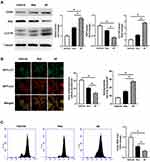
AF with Tri-Hyal Technique Reduced the ROS Level in Fibroblasts Through Inducing Autophagy.
To further investigate the mechanism of AF promoting skin rejuvenation and slowing skin aging, we monitored the expression of CD44 by Western blot in HA treated fibroblasts, which was known as the main HA receptor to mediate HA function in the cell. Meanwhile, we also detected the level of autophagy to clarify whether the cellular autophagy was induced by HA-CD44 binding in HA-treated fibroblasts. Data showed that AF and Res both increased the expression of CD44 and the ratio of LC3II to LC3I, decreased the expression of P62, which were molecular markers of autophagy (P<0.05; Figure 4A). Autophagy flux also showed that AF and Res promoted autophagy in fibroblasts compared with vehicle, while the effect of AF was more significant than Res (P<0.05; Figure 4B). It was reported that the augment of autophagy could reduce ROS level and attenuate the oxidative stress. We then detected the ROS level in fibroblast by flow cytometry with DCFH-DA, a ROS molecular detector. The result showed that AF could significantly reduce the ROS level in fibroblast compared with Res treatment, although Res also showed the inhibitory effect on ROS level (P<0.05; Figure 4C). All above suggested that although all kinds of HA could reduce the ROS level in fibroblasts through HA-CD44 binding mediated autophagy, non-cross linked HA would be more effective.
Discussion
The injection of cross-linked HA–based dermal fillers has provided an attractive nonsurgical alternative for the facial rejuvenation and volume compensation, because of its ability to function as a space-occupying device.19 The clinical efficacy, the effective duration, as well as the incidence of adverse effects for cross-linked HA fillers are related to their own characteristics, which included rheologic and physicochemical parameters, such as elasticity, viscosity, molecular weight, degree of cross-linking and cross-linker content.20
Many researches are focus on improving HA cross-linking technology to optimize these parameters as mentioned. The optimal cross-linking technique includes the fewest cross-linking agents, the longest duration of treatment, the most comfortable filling effect, and the most stimulating dermal rejuvenation.7 Many efforts have been put on it. AF, a novel cross-linked HA, was synthesized using a new technology called “Tri-Hyal”, which could make HAs to achieve the desired rheological characteristics. By means of “Tri-Hyal”, AF are formulated with a unique combination of three sizes of HA chains to give the desired ratio of very-long chain to long chain, cross-linking rate, and free HA concentration.21 Previous study has reported that AF showed the sustained efficiency in the nasolabial folds, crow’s feet as well as wrinkle volume.11 This suggested that as a kind of facial filler, AF rejuvenated the face by not only replenishing volume, but also being a rejuvenating agent through their regulatory mechanism on fibroblasts. Furthermore, it is possible that the slow release of the non-cross-linked HAs in AF to the dermis could provide suitable microenvironment and signal transduction regulation for dermal fibroblasts to produce several extracellular matrix components relative to skin self-renewal continuously. Additionally, the combination of three hyaluronic acids in “Tri-Hyal” has the characteristic of a natural entanglement,9 the presence of which could minimize the amount of BDDE, a popular cross-linking agent. The lifetime and adverse effects of HA-based fillers is dependent on the presence and amount of cross-linking agents in aesthetic injection.10,22 The triple sustained-release cross-linking technique could avoid unwanted artificial effects, as less cross-linking agent in AF. Clinical studies demonstrated the safety and lasting efficacy of AF detected by cutaneous high-frequency ultrasound imaging.11,21
In this study, it was further confirmed that AF with the triple sustained-release cross-linking technique had more lasting stimulating activity of dermal self-renewal than traditional HA through histological analysis in vivo, which included the measurement of skin thickness, vascular density and typical markers associated with dermal rejuvenation. The results in vitro suggested that AF synthesized by the new “Tri-Hyal” technique could stimulate the proliferation and activity of human primary fibroblasts by inducing autophagy, which laid a foundation for further research on the protective mechanism of fibroblasts by AF.
The free radical theory of aging in the skin has been widely accepted. Not only is the ROS load in this organ higher than in any other organ, but in many cases a clear correlation between the ROS originating from external and internal insults and a pro-aging effect can be found. Extrinsic aging is driven to a large extent by oxidative stress caused by UV irradiation.23 As this, lots of products with natural antioxidants has been demonstrated to effectively slow skin aging. For instance, vitamin E sequestered in hydrophobic lipids, can absorb the energy from UV light, and thus plays an important role in photo-protection from ROS.24 Although as a signal molecular, moderate ROS could stimulate cell proliferation, excessive ROS induce aging, especially in skin.25 Oxidative stress is always a result of a balance between the generation of ROS and the antioxidant defense system against oxidative stress ranging from enzymes like superoxide dismutase, catalase, peroxiredoxins, and GSH peroxidases, CoQ10, as well as glutathione.26 Reported features of aged human skin include fragmentation of collagen fibers by the action of the enzyme MMP-1 and increased mitochondrial ROS production and oxidative stress resulting in common damages to DNA, proteins, lipids.23,27
Autophagy can remove damaged proteins, lipids and other cytoplasmic substances, and act as a protective response in conditions of starvation or nutritional deficiency, and autophagy-lysosome pathway is the only way to clear entire organelles such as mitochondria.28 Activation of autophagy has been shown to significantly prolong the replication life of cells and inhibit stress-induced cellular senescence in many human and animal cell models, while inhibition of autophagy leads to premature aging.29 Treatment of primary bronchial epithelial cells with the cigarette smoke extract while adding the autophagy inhibitor 3-MA or knocking down LC-3 and ATG5 results in increased number of senescent cells and SASP accumulation.13 Activation of autophagy can delay aging, as it is an important mechanism for maintaining organelle stability. Damaged mitochondria produced more ROS which would be a burden for the skin. Autophagy reduced the ROS level by removing all of these impaired organelles.30
It has been reported that the signal from HA is transferred from the extracellular space to the intracellular space via several HA receptors, among which the principle one is CD44. CD44 can be expressed on the surface of many cell types, including fibroblasts, which express the short and best described standard variant of CD44.31 The interaction of HA and CD44 was shown to activate various signaling pathways to slow skin aging.
Conclusion
In this study in vivo, our data showed that cross-linked AF with triple sustained-release cross-linking Tri-Hyal technique consistently stimulated the dermal growth lasting for more than half year without efficiency attenuation, which effect lasted more persistently than that of traditional HA. In addition, AF with this unique technology could be associated with the proliferation, viability and activity of human primary fibroblasts in vitro, which would be mediated by cellular autophagy.
Acknowledgments
This research was funded by National Natural Science Foundation of China, grant number 81872217.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dai X, Li L, Peterson W, et al. Safety and effectiveness of hyaluronic acid dermal filler in correction of moderate-to-severe nasolabial folds in Chinese subjects. Clin Cosmet Investig Dermatol. 2019;12:57–62.
2. Czumbel LM, Farkasdi S, Gede N, et al. Hyaluronic Acid Is an Effective Dermal Filler for Lip Augmentation: a Meta-Analysis. Front Surgery. 2021;8:681028.
3. Berguiga M, Galatoire O. Tear trough rejuvenation: a safety evaluation of the treatment by a semi-cross-linked hyaluronic acid filler. Orbit. 2017;36(1):22–26.
4. Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143(2):155–163.
5. Paliwal S, Fagien S, Sun X, et al. Skin extracellular matrix stimulation following injection of a hyaluronic acid-based dermal filler in a rat model. Plast Reconstr Surg. 2014;134(6):1224–1233.
6. Landau M, Fagien S. Science of Hyaluronic Acid Beyond Filling: fibroblasts and Their Response to the Extracellular Matrix. Plast Reconstr Surg. 2015;136(5 Suppl):188S–195S.
7. Tezel A, Fredrickson GH. The science of hyaluronic acid dermal fillers. J Cosmetic Laser Therapy. 2008;10(1):35–42.
8. Ariyati N, Handono K, Nurdiana N, Wirohadidjojo YW. What is the Best Degree of Hyaluronic Acid Crosslinking in Increasing Growth Factors Level of Platelet-Rich Fibrin Lysate? J Stem Cells Regen Med. 2019;15(1):3–7.
9. Bian S, He M, Sui J, et al. The self-crosslinking smart hyaluronic acid hydrogels as injectable three-dimensional scaffolds for cells culture. Colloids Surf B Biointerfaces. 2016;140:392–402.
10. Keizers PHJ, Vanhee C, van den Elzen EMW, et al. A high crosslinking grade of hyaluronic acid found in a dermal filler causing adverse effects. J Pharm Biomed Anal. 2018;159:173–178.
11. Trevidic P, Andre P, Benadiba L, et al. Objective 18-month Comparison of the Tolerability of 2 Dermal Fillers Formulated with Tri-Hyal Technology. Plastic Reconstructive Surg Global Open. 2020;8(12):e3274.
12. Kozina LS, Borzova IV, Arutiunov VA, Ryzhak GA. The role of oxidative stress in skin aging. Adv Gerontol. 2012;25(2):217–222.
13. Gu Y, Han J, Jiang C, Zhang Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res Rev. 2020;59:101036.
14. Panich U, Sittithumcharee G, Rathviboon N, Jirawatnotai S. Ultraviolet Radiation-Induced Skin Aging: the Role of DNA Damage and Oxidative Stress in Epidermal Stem Cell Damage Mediated Skin Aging. Stem Cells Int. 2016;2016:7370642.
15. Arakawa M, Hatamochi A, Takeda K, Ueki H. Increased collagen synthesis accompanying elevated m-RNA levels in cultured Werner’s syndrome fibroblasts. J Invest Dermatol. 1990;94(2):187–190.
16. Chen Z, Han X, Ouyang X, Fang J, Huang X, Wei H. Transplantation of induced pluripotent stem cell-derived mesenchymal stem cells improved erectile dysfunction induced by cavernous nerve injury. Theranostics. 2019;9(22):6354–6368.
17. Wang Y, Dong L, Zhong H, et al. Extracellular Vesicles (EVs) from Lung Adenocarcinoma Cells Promote Human Umbilical Vein Endothelial Cell (HUVEC) Angiogenesis through Yes Kinase-associated Protein (YAP) Transport. Int J Biol Sci. 2019;15(10):2110–2118.
18. Yang L, Zhou X, Sun J, et al. Reactive oxygen species mediate anlotinib-induced apoptosis via activation of endoplasmic reticulum stress in pancreatic cancer. Cell Death Dis. 2020;11(9):766.
19. Highley CB, Prestwich GD, Burdick JA. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr Opin Biotechnol. 2016;40:35–40.
20. Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013;132(4Suppl 2):5S–21S.
21. Trevidic P, Andre P, Benadiba L, et al. Prospective, Split-Face, Randomized, Long-Term Blinded Objective Comparison of the Performance and Tolerability of Two New Hyaluronic Acid Fillers. Dermatologic Surgery. 2017;43(12):1448–1457.
22. Humphrey S, Jones DH, Carruthers JD, et al. Retrospective review of delayed adverse events secondary to treatment with a smooth, cohesive 20-mg/mL hyaluronic acid filler in 4500 patients. J Am Acad Dermatol. 2020;83(1):86–95.
23. Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5(2):545–589.
24. Solway J, McBride M, Haq F, Abdul W, Miller R. Diet and Dermatology: the Role of a Whole-food, Plant-based Diet in Preventing and Reversing Skin Aging-A Review. J Clin Aesthet Dermatol. 2020;13(5):38–43.
25. Murakami S, Motohashi H. Roles of Nrf2 in cell proliferation and differentiation. Free Radic Biol Med. 2015;88(Pt B):168–178.
26. Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem. 2015;97:55–74.
27. Merecz-Sadowska A, Sitarek P, Kucharska E, et al. Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells. Antioxidants. 2021;10(5):34.
28. Cao W, Li J, Yang K, Cao D. An overview of autophagy: mechanism, regulation and research progress. Bull Cancer. 2021;108(3):304–322.
29. Di Micco R, Krizhanovsky V, Baker D, d’Adda Di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22(2):75–95.
30. Ferrucci L, Gonzalez-Freire M, Fabbri E, et al. Measuring biological aging in humans: a quest. Aging Cell. 2020;19(2):e13080.
31. Rajarajan A, Bloor BK, Desai H, Stokes A, Odell EW. Variant CD44 expression by human fibroblasts. Biomarkers. 2008;13(3):307–318.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.