Back to Journals » International Medical Case Reports Journal » Volume 17
Trifluridine and Tipiracil Hydrochloride Combination-Induced Interstitial Pneumonia: A Case Report
Authors Nakazawa S , Kato M , Terayama Y, Sakamoto Matubara N, Sato Y, Murashima R, Hayakawa D, Okamoto S, Takahashi K
Received 12 October 2023
Accepted for publication 16 January 2024
Published 7 February 2024 Volume 2024:17 Pages 101—104
DOI https://doi.org/10.2147/IMCRJ.S444330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ronald Prineas
Shun Nakazawa, Motoyasu Kato, Yuriko Terayama, Naho Sakamoto Matubara, Yoshihiko Sato, Ryoko Murashima, Daisuke Hayakawa, Shouichi Okamoto, Kazuhisa Takahashi
Department of Respiratory Medicine, Juntendo University Graduate School of Medicine, Bunkyo-ku, Tokyo, Japan
Correspondence: Shun Nakazawa, Department of Respiratory Medicine, Juntendo University Graduate School of Medicine, 3-1-3 Hongo, Bunkyo-ku, Tokyo, 113-8431, Japan, Tel +81 3-3813-3111, Fax +81 3-3812-7560, Email [email protected]
Abstract: We report a case of a 62-year-old male who was diagnosed with advanced rectal cancer. The attending gastro-enterologist initiated chemotherapy using capecitabine plus oxaliplatin and bevacizumab; however, this treatment regimen was discontinued, as the patient developed a skin rash. Once the skin rash improved, chemotherapy was re-initiated using a combination of trifluridine and tipiracil hydrochloride (TAS-102). The patient developed high fever and dyspnea 2 months after initiation of TAS-102. Chest high-resolution computed tomography showed bilateral diffuse ground glass opacities in all lung lobes with traction bronchiectasis. At this time, the gastro-enterologist consulted our department. The patient was put on non-invasive positive pressure ventilation due to worsening respiratory symptoms. The patient was suspected to develop TAS-102-induced interstitial pneumonia based on positive TAS-102 drug-induced lymphocyte stimulation test. The patient’s respiratory symptoms and radiological findings improved after corticosteroid treatment. The corticosteroid dose was gradually decreased by 5 mg. Thereafter, chemotherapy was re-initiated using different anti-cancer agents.
Keywords: TAS-102, colorectal cancer, DAD, corticosteroid
Introduction
Trifluridine/tipiracil hydrochloride (TAS-102) is an orally administered combination drug. It improves the bioavailability of trifluridine by inhibiting the trifluridine-degrading enzyme thymidine phosphorylase. TAS-102 is effective when administered in 28-day cycles, each comprising 5 days of treatment followed by a 2-day rest period for 2 weeks and a 14-day rest period thereafter.1 Recently, the drug has been used as a late-line chemotherapeutic agent for colorectal cancer.2 The results of a placebo-controlled, double-blind, Phase 3 clinical trial indicated that treatment with TAS-102 achieved a significant improvement in the overall and progression-free survival in patients with colorectal cancer refractory to standard chemotherapies. Common adverse effects of TAS-102 include neutropenia, leukopenia, anemia, fatigue, and loss of appetite; interstitial pneumonia has rarely been reported as an adverse effect.1,3 In the Phase 2 trial, there were 457 cases of adverse events, but only one suspected case of interstitial pneumonia was reported.3 Conti et al performed a multicenter, retrospective, cohort study on TAS-102 for refractory metastatic colorectal cancer; however, they did not report on the incidence of interstitial pneumonia.4 We present a case of interstitial lung disease development with severe respiratory failure during TAS-102 treatment in a patient with advanced rectal cancer.
Case Presentation
A 62-year-old Japanese male was diagnosed with Stage IV advanced rectal cancer with liver metastasis. There was no personal medical history other than rectal cancer, and he has smoked 20 cigarettes per day for 40 years. He was initially treated with capecitabine plus oxaliplatin and bevacizumab as first-line chemotherapy. After 11 cycles of the regimen, the patient developed a skin rash, which was induced by infusion of these agents; thus, the treatment was changed to TAS-102 regimen. Approximately 2 months after initiation of TAS-102, the patient developed high fever and was emergently admitted to our hospital. The patient had received antibiotics from a few days before this visit, and vital signs on admission were as follows: blood pressure 100/68 mmHg, heart rate 82 beats/min, body temperature 37.4°C, and oxygen saturation was 90% on room air. High-resolution computed tomography (HRCT) of the chest revealed bilateral diffuse ground glass opacities in all lung lobes with traction bronchiectasis (Figure 1A and B). Laboratory findings were as follows: white blood cell count 3400/μL (31.1% of lymphocytes, 1.8% of eosinophils), C-reactive protein level 9.96 mg/dL, and lactate dehydrogenase level 275 IU/L. Serum Krebs von den Lungen-6 and surfactant protein D levels were 528 IU/mL (normal < 500 IU/L) and 235 ng/mL (normal < 110 ng/mL), respectively. There were no elevated levels of serum antibodies associated with autoimmune diseases, and no evidence of any infectious agent, including general bacteria and acid-fast bacillus in sputum, on urine and blood culture. Although we initiated broad-spectrum antibiotics after emergent admission, the patient’s oxygen levels worsened. The patient then received non-invasive positive pressure ventilation, followed by nasal high-flow oxygen therapy and steroid pulse therapy using methylprednisolone 1 g for 3 days as treatment for suspected TAS-102-induced interstitial pneumonia. The patient was treated with 1 mg/kg of corticosteroid after steroid pulse therapy. The corticosteroid dose was then gradually decreased. During these treatments, the drug lymphocyte stimulation test result for TAS-102 was positive (stimulation index: 251%); therefore, the patient was diagnosed with TAS-102-induced interstitial pneumonia based on the Japanese Respiratory Society (JRS) guideline for management of drug-induced lung disease. Oxygenation and chest radiological findings (Figure 2A and B) improved after corticosteroid treatment, and the patient was discharged 23 days after emergency admission.
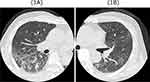 |
Figure 1 (A and B) Chest high resolution computed tomography findings on admission revealed bilateral diffuse ground glass opacities in all lung lobes with traction bronchiectasis. |
 |
Figure 2 (A and B) Chest high resolution computed tomography findings at 20 days after admission revealed bilateral diffuse ground glass opacities improved after corticosteroid treatment. |
Discussion
The incidence of interstitial pneumonia was reportedly 0.9% (only one case) during treatment with TAS-102 in Phase II trial.3 Although the patient in this reported case was diagnosed with interstitial pneumonia due to viral infection during TAS-102 treatment based on the clinical course and lung biopsy results, the association between the development of interstitial pneumonia and TAS-102 treatment was unclear. However, in the serious adverse events of treatment with TAS-102 reported between March 2014 and August 2019, 33 patients developed interstitial pneumonia, 9 of whom died of TAS-102-induced interstitial pneumonia.5 Furthermore, three cases of TAS-102-induced interstitial pneumonia were reported in Japan.6–8 The two main mechanisms involved in the pathogenesis of drug-induced lung injury are as follows: first, direct cytotoxic pulmonary injury to the alveolar epithelial or vascular endothelial cells and activation of immune system. The cytotoxic mechanism is known to be related to the dosage and duration of administration and is associated with the development of diffuse alveolar damage (DAD). This DAD pattern is seen as ground-glass opacity (GGO) with traction bronchiectasis on HRCT,9,10 which is consistent with the findings in our case. In contrast, the second pathogenesis involves immune cell-related pneumonia and is characterized by the activation of immune system cells that induce lung damage, regardless of the initial dose or low quantities of the drug, and is associated with hypersensitivity, pneumonitis, eosinophilic pneumonia, or organizing pneumonia.10–13 TAS-102 is a combination of two compounds: trifluridine and tipiracil. Trifluridine is a derivative of deoxyuridine, which contains a nucleoside and has a crucial role of anticancer efficacy.14 Gemcitabine, tegafur/gimeracil/oteracil combination, and fluorouracil contain a type of nucleoside or degradation product of nucleoside, similar to the ones found in TAS-102.15 The mechanism of gemcitabine-induced lung toxicity has been reported as cytokine-mediated inflammation of the alveolar capillary wall and development of increased membrane permeability, which causes DAD.16 The type of nucleoside in TAS-102 is a similar to that in gemcitabine; thus, the mechanism of the development of drug-induced interstitial pneumonia in TAS-102 may be similar to that in gemcitabine. In this case, although we could not perform a lung biopsy due to severe respiratory failure, chest HRCT demonstrated a DAD pattern at admission, which was also reported in the other two cases. Kamei et al reported that TAS-102-induced interstitial pneumonia occurred 4 days after receiving the treated with TAS-102, and CT scan showed diffuse areas of defined ground-glass opacities with fine reticulation and pleural effusions in both lung fields. Kono et al also reported that TAS-102-induced interstitial pneumonia developed 16 days after TAS-102 administration; a CT scan revealed a diffuse GGOs in both lungs. A histological biopsy was not performed in both these cases; therefore, TAS-102-induced interstitial pneumonia was suspected on the basis of clinical manifestations and high-dose intravenous corticosteroid therapy was initiated.6,7 Therefore, during TAS-102 treatment, if patients present with symptoms such as coughing and dyspnea or if shadows are observed on chest radiography, the possibility of TAS-102-induced interstitial pneumonia must be considered. The use of TAS-102 may increase cytokine release and affect membrane permeability, thereby leading to DAD. After considering TAS-102-induced interstitial pneumonia, high-dose intravenous corticosteroid therapy should be initiated. The exact mechanism of TAS-102-induced interstitial pneumonia remains unclear, and more cases should be accumulated in the future.
Conclusion
We experienced a case of severe TAS-102-induced interstitial pneumonia. While the frequency of TAS-102-induced interstitial is low, there is a potential for a rapid and severe course. It is crucial to suspect TAS-102-induced interstitial pneumonia when respiratory symptoms occur during TAS-102 use.
Abbreviations
TAS-102, trifluridine and tipiracil hydrochloride; HRCT, High-resolution computed tomography; JRS, Japanese Respiratory Society; DAD, diffuse alveolar damage; GGO, ground-glass opacity.
Consent
We received informed consent from the patient to have the case details and any accompanying images published. Ethical approval was also obtained from the Juntendo University Graduate School of Medicine Institutional Review Board (IRB approval number: JHS22-028).
Acknowledgments
We thank Editage for English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mayer RJ, Van Cutsem E, Falcone A, et al.; RECOURSE Study Group. Randomized trial of Tas-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. doi:10.1056/NEJMoa1414325
2. Salvatore L, Rossini D, Moretto R, et al. Tas-102 for the treatment of metastatic colorectal cancer. Expert Rev Anticancer Ther. 2015;15(11):1283–1292. doi:10.1586/14737140.2015.1105746
3. Yoshino T, Mizunuma N, Yamazaki K, et al. Tas-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012;13(10):993–1001. doi:10.1016/S1470-2045(12)70345-5
4. Conti M, Bolzacchini E, Luchena G, et al. Tas-102 for refractory metastatic colorectal cancer: a multicenter retrospective cohort study. Cancers. 2023;15(13):3465. doi:10.3390/cancers15133465
5. List of serious adverse effects of Tas-102. Reporting period: march 24, 2014 (approval date) to August 31, 2019 [home page on the Internet]. Japan: TAIHO Phamaceutical Co., Ltd.; 2019. Available from: https://www.taiho.co.jp/medical/safety/files/pdf/728ecfd9b3c01399747f27473b4b4c92.pdf.
6. Kamei H, Ishibashi N, Tanigawa M, et al. Interstitial pneumonia in a patient treated with Tas-102 for metastatic colorectal cancer: a case report. J Med Case Rep. 2016;10(1):310. doi:10.1186/s13256-016-1097-y
7. Kono E, Hiramatsu M, Kobayashi T, et al. A case of interstitial pneumonitis induced by Tas-102 for liver and lung metastasis of colorectal cancer. Gan To Kagaku Ryoho. 2018;45(9):1365–1368. The data that support the findings of this study are available on request from the corresponding author.
8. Hasegawa Y, Ota T, Tsukuda H, et al. Drug-induced pneumonitis following the administration of Tas-102. Intern Med. 2016;55(19):2855–2859. doi:10.2169/internalmedicine.55.6629
9. Lind JS, Smit EF, Grünberg K, et al. Fatal interstitial lung disease after erlotinib for non-small cell lung cancer. J Thorac Oncol. 2008;3(9):1050–1053. doi:10.1097/JTO.0b013e318183a9f5
10. Distefano G, Fanzone L, Palermo M, et al. HRCT patterns of drug-induced interstitial lung diseases: a review. Diagnostics. 2020;10(4):244. doi:10.3390/diagnostics10040244
11. Nemery B, Bast A, Behr J, et al. Interstitial lung disease induced by exogenous agents: factors governing susceptibility. Eur Respir J Suppl. 2001;32:30s–42s.
12. Bartal C, Sagy I, Barski L. Drug-induced eosinophilic pneumonia: a review of 196 case reports. Medicine. 2018;97(4):e9688. doi:10.1097/MD.0000000000009688
13. Ishiwata T, Ebata T, Iwasawa S, et al. Nivolumab-induced Acute Fibrinous and Organizing Pneumonia (AFOP). Intern Med. 2017;56(17):2311–2315. doi:10.2169/internalmedicine.8271-16
14. Temmink OH, Emura T, de Bruin M, et al. Therapeutic potential of the dual-targeted Tas-102 formulation in the treatment of gastrointestinal malignancies. Cancer Sci. 2007;98(6):779–789. doi:10.1111/j.1349-7006.2007.00477.x
15. Thalambedu N, El-Habr AH. Gemcitabine induced pneumonitis: a case report and review of literature. J Community Hosp Intern Med Perspect. 2020;10(6):579–582. doi:10.1080/20009666.2020.1811071.
16. Barlési F, Villani P, Doddoli C, et al. Gemcitabine-induced severe pulmonary toxicity. Fundam Clin Pharmacol. 2004;18:85–91. doi:10.1046/j.0767-3981.2003.00206.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
