Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Treatment-resistant prurigo nodularis: challenges and solutions
Authors Kowalski EH , Kneiber D , Valdebran M , Patel U , Amber KT
Received 7 November 2018
Accepted for publication 22 January 2019
Published 28 February 2019 Volume 2019:12 Pages 163—172
DOI https://doi.org/10.2147/CCID.S188070
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Eric H Kowalski,1 Diana Kneiber,1 Manuel Valdebran,2 Umangi Patel,1 Kyle T Amber1
1Department of Dermatology, University of Illinois at Chicago, Chicago, IL, USA; 2Department of Dermatology, University of California, Irvine, Irvine, CA, USA
Abstract: Prurigo nodualris (PN) is a chronic condition with highly pruritic, hyperkeratotic papules or nodules arising in the setting of chronic pruritus. While PN may serve as a phenotypic presentation of several underlying conditions such as atopic dermatitis, chronic kidney disease-related pruritus, and neurological diseases, it represents a distinct clinical entity that may persist despite the removal of the underlying cause, if one is identified. Neuronal proliferation, eosinophils, mast cells, and small-fiber neuropathy play a role in the production of pruritus in PN, although the exact mechanism has not yet been established. Identifying an underlying cause, if present, is essential to prevent recurrence of PN. Due to often present comorbidities, treatment is typically multimodal with utilization of topical and systemic therapies. We performed a PubMed/MEDLINE search for PN and present a review of recent developments in the treatment of PN. Treatment typically relies on the use of topical or intralesional steroids, though more severe or recalcitrant cases often necessitate the use of phototherapy or systemic immunosuppressives. Thalidomide and lenalidomide can both be used in severe cases; however, their toxicity profile makes them less favorable. Opioid receptor antagonists and neurokinin-1 receptor antagonists represent two novel families of therapeutic agents which may effectively treat PN with a lower toxicity profile than thalidomide or lenalidomide.
Keywords: pruritus, chronic prurigo, neurokinin 1, thalidomide, atopic dermatitis
A Letter to the Editor has been published for this article.
Introduction
PN is an intensely pruritic, chronic skin condition characterized by localized or generalized hyperkeratotic papules and nodules typically in a symmetrical distribution.1 PN is accompanied by long-standing pruritus and thought to develop as a reaction to repeated scratching in patients with CP from various etiologies including dermatological, systemic, infectious, and psychiatric.2–4 Although most patients present with several associated conditions that may explain the development of CP, there is a significant percentage (~13%) who do not have an identifiable illness or predisposing condition that would serve as an initial trigger.2 The initiation of an itch–scratch cycle perpetuates the development of PN and explains the propensity for symmetrical distribution of lesions and the characteristic absence on the upper mid back.5 Lesions can number from several to hundreds, and can vary greatly in size.4
Recent work on the pathogenesis of PN has pointed to a complex interplay of pro-inflammatory and pruritogenic substances in addition to increased local concentrations of neuropeptides in lesional skin that may be responsible for the alterations in nerve density and cutaneous inflammation found in PN.6–9 Despite these findings, our understanding of the pathophysiology remains unclear. In an effort to simplify terminology, it was recently proposed to utilize chronic prurigo as an all-encompassing clinical term for the various subtypes (nodular, papular, umbilicated) of prurigo based on the unifying core symptoms of CP (>6 weeks), signs of repeated scratching, and the development of pruriginous lesions.10 Still, cases such as pemphigoid nodularis, where the underlying disease requires a significantly different treatment regimen, complicate utilization of such terminology.11 As such, we will make distinctions between treating prurigo and addressing underlying causes of the prurigo.
Pruritus as a symptom is present in many diseases, and a certain subset of patients may be predisposed to a higher sensitivity or lower tolerance to pruritus, developing a clinical prurigo response under the influence of the itch–scratch cycle.12 Resolution of the underlying etiology with eventual neuronal sensitization can ensue leading to perpetuation and spread of this secondary response.13,14 Although the evolution of this prurigo response is dependent on the underlying systemic illness inducing CP, chronic scratching itself appears to alter the environment in the dermis and epidermis as evidenced by increased levels of neuropeptides and neurohyperplasia.8,9,15 This results in a chronic condition that may no longer be dependent on the underlying etiology that originally caused the CP.
In light of the difficulties in adequately categorizing PN, epidemiological and treatment studies are often limited to smaller, less-powered studies. We performed a PubMed/MEDLINE review of PN and herein review its etiology and various treatment options.
Epidemiology
Despite PN’s fairly frequent occurrence in the clinical setting, studies on the prevalence and incidence of PN have to date consisted of small case studies and case reports. To ascertain the incidence of PN, Pereira et al performed a survey study across 14 countries and demonstrated that 60% of respondents, on average, saw fewer than five PN patients per month presenting to clinic.16 Overall, epidemiological studies are lacking.
A majority of patients with PN present between the ages of 51 and 65, though several cases in other age groups have been described, including pediatric patients.2,17–19 Multiple groups have demonstrated that individuals with an atopic predisposition have an earlier age of onset.2,20,21 More recently, the largest study to investigate the demographics and comorbidities associated with PN determined that African Americans are 3.4 times more likely to have PN than white patients.19 In that same study, significant novel associations with a variety of systemic diseases including COPD, and heart failure were found.19
Clinical presentation
PN is a chronic condition defined by the presence of highly pruritic, hyperkeratotic papules or nodules arising in the setting of CP and the induction of an uncontrollable itch–scratch cycle. Repeated scratching can lead to excoriation, further lichenification, or crusting, often resulting in a hyperpigmented border secondary to the inflammatory stimulus (Figure 1). Lesions are distributed in areas accessible to chronic scratching and are often found in a symmetrical distribution over the extensor surfaces, trunk, and lower extremities.5,22 PN can also be localized in the setting of an underlying local dermatosis such as venous stasis, postherpetic neuralgia, or brachioradial pruritus.4,14,23 Regardless of the distribution, PN is thought to harbor the highest itch intensity among the most frequent causes of CP.2,12 Patients experience a combination of pruritic sensations including burning, stinging, and alterations in lesional temperature.2 These varying sensations and the underlying etiology do not, however, appear to affect the severity or course of PN.10
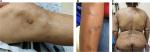  | Figure 1 Clinical images of patients with prurigo nodularis. |
PN has a significant effect on the quality of life which can lead to psychological distress, and sleep disturbances.24,25 There is a significant association between PN, depression, and increased anxiolytic and antidepressant use further underscoring the biopsychosocial nature of this condition.26,27
Pathology and dermoscopy
Histological features of PN have been characterized by Weigelt et al upon assessment of 136 histological samples. The most frequent epidermal features included compact orthohyperkeratosis (84%), irregular elongation of the rete ridges (58%), and focal or broad hypergranulosis (52.2%). Dermal changes consisted of fibrosis of the papillary dermis (71.3%) with vertical arrangement of collagen fibers (64%), and an increased number of capillaries (65.4%) (Figure 2). An inflammatory infiltrate was present in virtually all the patients; most of it was composed by lymphocytes and histiocytes. However, half of the specimens showed eosinophils and neutrophils as well.28 Dermatoscopic features described in small series consisted of white areas with peripheral striations, hyperkeratosis and scaling, crusts, erosions and follicular plugging, red dots, and red globules.29,30
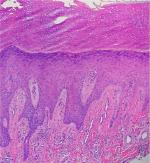  | Figure 2 A section showing orthohyperkeratosis, hypergranulosis, elongation of the rete ridges, vertical arrangement of collagen fibers, and increased number of capillaries. |
Underlying conditions
Dermatological diseases
Several dermatoses have established associations with PN, all of which have a documented high level of pruritic intensity which typically precedes the development of pruriginous lesions through the evolution of an itch–scratch cycle and neuronal sensitization.2,12 The most documented and well studied is the association between PN and an atopy.2 Atopic prurigo may be the dominant manifestation of atopic dermatitis and is more commonly seen in children.31
Additional underlying dermatoses that generate pruriginous lesions include venous stasis dermatitis, epidermolysis bullosa acquisita, and mycosis fungoides.4,32,33 Aside from underlying dermatological diseases, infectious etiologies limited to the skin may also predispose a patient to chronic itching and initiate a prurigo response. Patients with lesions typical of PN have in rare cases been found to harbor various species of mycobacteria, exhibiting favorable responses with initiation of antituberculosis therapy.34,35
Systemic and neurological diseases
Systemic diseases with an intense pruritic manifestation can lead to the development of PN. HIV may initially present with pruritus, and patients are known to develop an intractable itch with disease progression.36–38 PN lesions in HIV patients correlate with depleted CD4 counts.39 Recently, in a large study at a single academic institution, black patients with PN were 8.5–10 times more likely to have HIV than race-matched controls with atopic dermatitis or psoriasis.19 Because of PN’s association with an advanced state of immunosuppression, and improvement of PN symptoms with initiation of therapy, it is suggested for patients to undergo HIV testing.19,39
Between 25% and 42% of patients receiving hemodialysis experience CKD-ap.40,41 In another study investigating the dermatological findings associated with CKD-ap, 10% of patients presented with excoriations and scratched nodules consistent clinically with PN.42 Overwhelmingly, the most common skin finding in patients receiving hemodialysis was xerosis cutis.42 In a recent study, 60% of female patients with PN had concomitant xerosis cutis, 66% of which was from a nonatopic underlying condition.43 These patients were more likely to see PN development at a later age and to have multiple underlying conditions associated with PN.43 The most common diseases associated with this rare PN subtype were chronic kidney disease and diabetes mellitus.44
Lesions characteristic of an acquired reactive perforating dermatosis observed in patients with underlying metabolic diseases are histologically representative of PN.45,46 There are case reports showing associations between a variety of malignancies47–49 including lymphoproliferative disorders, such as Sèzary syndrome, Hodgkin’s lymphoma, and polycythemia vera.50–52
Neurological diseases causing damage to peripheral nerves through pathological compression, or through an SFN, have been associated with PN.53 The herpes zoster virus damages small cutaneous nerve fibers and has been posited to serve as a trigger for development of postherpetic neuralgia and PN.54 Pathological compression of nerves seen in brachioradial pruritus, and notalgia paresthetica with a resulting neuropathic itch may also produce PN lesions in a dermatomal distribution.14,23,53,55
Pathophysiology
Following the initial discovery of dermal neural hyperplasia in prurigo nodules by Pautrier in 1934, the role of cutaneous nerves has been repeatedly investigated.56 Although dermal neuronal hyperplasia has been disputed by recent studies which demonstrated that increased nerve fiber density is not seen in the majority of patients, neuronal plasticity still appears to play a significant part in the development of PN.4,28,57,58
Prurigo nodules have higher concentrations of PGP 9.5, p75 NGFr, and CGRP nerve fibers in the upper dermis when compared to healthy skin.6,8,59–61 NGF, which binds to NGFr, is a neurotrophin that is necessary for the development and survival of neurons.62–64 In the skin, NGF is secreted by keratinocytes, mast cells, eosinophils, and T lymphocytes.65–71 Using NGF and p75 NGFr double-staining, Johansson et al found an increase in high- and low-affinity NGFr in dermal nerve fibers, and an increased production of NGF in dermally infiltrating inflammatory cells.8 Additionally, NGF-producing eosinophils and mast cells were found in close proximity to NGFr-positive nerve fibers, suggesting a potential function for these inflammatory cells in promoting dermal neuronal hyperplasia in PN.6,7 Epidermal hyperplasia may also be explained by an increase in NGF given that NGF has been shown to induce keratinocyte proliferation in a dose-dependent manner.66,72,73 NGF additionally has various biologic effects on the activity of inflammatory cells, such as lymphocytes, mast cells, basophils, and eosinophils, which is consistent with the finding that NGF promotes the production of SP by sensory neurons and histamine expression and release by mast cells.67,69,70,74–76
SP, a tachykinin which binds to the NK1r, induces erythema, edema, vascular dilatation, plasma protein extravasation, and pruritus in cutaneous tissues.77–79 Increased density of SP-positive nerve fibers in PN lesions may promote inflammation and pruritus in PN.9 This is supported by the finding that aprepitant, an anti-neurokinin-1 antagonist, and topical capsaicin, which depletes SP, reduce pruritus and the size of PN lesions.80,81 Histamine, a potent pruritogen, appears to play a role in the pruritus of PN as increased numbers of histamine-containing mast cells are found to be near NGFr-positive nerve fibers in the dermis of PN.82,83 Increased quantities of eosinophils containing eosinophilic cationic protein and eosinophil-derived neurotoxin are similarly found to be in close proximity to PGP 9.5-positive nerve fibers and with an increased density of granular proteins.7 Furthermore, IL-31, a cytokine and pruritogen produced by eosinophils, has a 50-fold increased mRNA expression in PN biopsies when compared with healthy skin likely contributing to the pruritus in PN.84,85 Together, these pruritogens are believed to result in the intractable pruritus felt by patients with PN. Recently, PGP 9.5-positive IENFs were found to be reduced in PN and between lesions, with the restoration of the neuronal density in healed nodules, thus implicating the epidermis in pruritus.15,86
SFN has been proposed as a possible etiology for the itch associated with prurigo nodules.15 SFNs involving aδ fibers and c fibers in the epidermis are characterized by IENF hypoplasia and are commonly associated with sensations of pruritus, burning, stinging, and prickling, symptoms which are frequently reported by patients with PN.2,15,86–90 In addition, patients who are treated with medications which are commonly used for the treatment of neuropathy, such as gabapentin, pregabalin, and capsaicin, show significant improvement of pruritus and severity of nodules.81,91–96 However, a small study of 12 patients using quantitative sensory testing, a measure of SFN, did not find a significant difference between PN and control skin.97,98
IL-6 and serotonin have also been hypothesized to act as a link between psychiatric disease and PN. IL-6 levels and serum serotonin levels – directly and indirectly, respectively – correlate with increased severity of pruritus suggesting a possible common pathway for the development of PN and depression.99
Treatment of PN
The treatment of PN presents a challenge to the clinician as there are few RCTs delineating therapy options. Therapies should be tailored to the patient’s age, comorbidities, severity of PN, quality of life, and expected side effects.100 Discussions with the patient should include advantages and disadvantages of the therapy, side effects, and possible use of off-label medications. Patient education can thus promote treatment adherence. Potential length of therapy should also be discussed as PN is difficult to treat and patients may become frustrated with lack of improvement. Multimodal therapies which include both topical and systemic therapies may need to be implemented to achieve the goals of therapy – pruritic relief and healing of PN lesions. The addition of emollients is also recommended as a base therapy.
Identifying an underlying cause, if present, is essential to properly treating a patient with PN to prevent any recurrent pruritus that may lead to recurrence, as well as to avoid any treatments which may be contraindicated. We reported a patient who had received PUVA empirically for treatment of clinically diagnosed PN.11 After several treatments, the patient developed a bullous eruption with workup confirming a diagnosis of pemphigoid nodularis, a variant of bullous pemphigoid. Thus, treatment for PN is not necessarily universal, and depends on addressing underlying dermatoses, if present. Herein, we discuss PN treatments in the case of recalcitrant PN lesions or adequate treatment of any identifiable underlying causes.
Topical and intralesional therapy
Direct injection of triamcinolone acetonide into PN lesions has produced clinical improvement and is often required for thicker lesions into which topical therapy cannot adequately penetrate.101 These intralesional injections can be accompanied with cryotherapy.102,103 Likewise, high-potency topical corticosteroids can be used, due to their immunosuppressive effect on T-cells and cytokines implicated in the generation of pro-inflammatory and pruritogenic neuropeptides SP and CGRP.6,104,105
The efficacy of less potent steroids, pimecrolimus, and calcipotriol has been investigated in RCT. Betamethasone valerate 0.1%-impregnated occlusive tape significantly decreased itch compared with an antipruritic moisturizing cream containing feverfew, a traditional medicinal herb.105
Topical calcineurin inhibitors have strong antipruritic effects stemming from their immunomodulatory role in cytokine release and inhibition of the TRPV1 receptor which stimulates neurogenic inflammation.106,107 In a randomized, controlled, double-blind study, Siepmann et al compared the efficacy of 0.1% pimecrolimus cream to 0.1% hydrocortisone cream applied twice daily over 8 weeks in 30 nonatopic PN patients.107 There was a significant reduction in itch, assessed by the VAS, with 2.7 points for pimecrolimus and 2.8 for hydrocortisone.107 This effect could be observed 10 days after initiation of therapy. Comparatively, however, there was not a significant difference in reduction of scratch lesions, serum neuropeptide levels, or itch between the two treatment arms.107 Pimecrolimus was as effective as hydrocortisone and offers an alternative topical treatment that may be implemented in a long-term regimen.107
Calcipotriol ointment, a synthetic form of vitamin D, was compared to betamethasone valerate 0.1% ointment in a small RCT, with calcipotriol ointment showing greater efficacy and more rapid clearance of prurigo lesions.108
Antihistamines and leukotriene inhibitors
Antihistamines are utilized in PN therapy given an increased number of mast cells detected in PN lesions.83 High-dose nonsedating antihistamine for daytime followed by sedating antihistamine during night has exhibited beneficial effects in patients with CP in a case series.109 Combination therapy of fexofenadine and montelukast improved lesions and pruritus in 11 of 15 patients with both PN and pemphigoid nodularis. One patient achieved remission with this regimen.110 Currently, there are insufficient data from RCTs on the use of antihistamines for PN.
Phototherapy/excimer
Phototherapy can be utilized for the treatment of various inflammatory skin conditions and is a therapeutic alternative for patients with multiple comorbidities or generalized PN. UV-light exposure provides an anti-inflammatory effect and can decrease itch in inflammatory skin disorders including atopic dermatitis and PN.111 UVB radiation decreases the levels of NGF and CGRP, and has immunosuppressive effects which may decrease the levels of IL-31.112,113
Narrowband UVB results in a significant improvement in PN at an average dose of 23.88±26.00 J/cm2.114 The combination therapy of narrowband 308 nm UVB and PUVA accelerated healing of PN lesions when compared with PUVA alone.111 UVA monotherapy demonstrated improvement of PN nodules in a case series of 19 patients. Totally, 23 UVA phototherapy sessions were provided. Among the patients, 79% experienced at least slight improvement of their lesions and two patients achieved complete remission.115 Excimer laser proved more beneficial than topical Clobetasol.104
Modified Goeckerman therapy, which consists of daily multistep broadband UVB therapy followed by occlusive coal tar and topical steroid application, improved prurigo nodules in a case series.116 Logistical consideration and potential carcinogenicity of coal tar limits utilization of this protocol.117
Oral immunosuppressants
Oral immunosuppressive therapy should be considered for patients with severe, recalcitrant PN. A single-institution retrospective study demonstrated clinical improvement with fewer lesions and decreased pruritus using cyclosporine in an average time of 3 weeks at a mean dose of 3.1 mg/kg.118 In this small study consisting of eight patients, six showed complete remission with no recurrence following treatment cessation.118
There have been two retrospective studies investigating MTX use in difficult-to-treat PN. The initial small study showed remission or marked improvement with resolution of >50% of nodules in 76% of patients on a weekly 7.5–20 mg subcutaneous injection of MTX.119 Conclusions, however, were limited by the continuation of various adjuvant treatments during MTX use. More recently, a multicenter report of 39 patients receiving 5–25 mg MTX weekly demonstrated a mean objective efficacy, defined as achieving complete or partial remission, of 2.4 months.120 Cases were limited to PN refractory to conventional therapies and showed a mean duration of response of 19 months. With a median 16-month follow-up, 64% of patients were still well controlled but the majority required weekly maintenance MTX of 5–20 mg.120 A significant proportion, 38%, experienced adverse effects attributable to MTX including nausea, gastrointestinal symptoms, and transaminitis.120
Treatment with azathioprine and cyclophosphamide has also been reported to be successful.121,122 Oral tacrolimus therapy dramatically reduced pruritus in a patient who was previously treated with cyclosporine for PN.123 Lastly, a combination therapy of three cycles of intravenous immunoglobulin followed by MTX and topical steroids had antipruritic effect on PN related to atopic dermatitis in a case study.124
Novel treatments
Thalidomide and lenalidomide
Thalidomide is an immunomodulatory agent, which also acts as a central and peripheral depressant, and exhibits anti-inflammatory properties as a tumor necrosis factor-α inhibitor.125 It has been used in dermatological diseases refractory to traditional therapies.126 The therapeutic action against PN is thought to derive from its neurotoxic effects.126 To date, the largest study investigating thalidomide use in refractory PN included 42 patients treated with an average of 100 mg of thalidomide for 105 weeks.127 Each patient had prior treatments that proved insufficient, or began to suffer from adverse effects.127 Efficacious treatment was observed in 32 patients, with one experiencing complete clearing.127 Termination of therapy was most commonly due to adverse side effects with 59% of patients experiencing peripheral neuropathy.127
A meta-analysis of over 280 patients with refractory CP treated with thalidomide described improvement of itch secondary to a variety of etiologies, most commonly PN.125 Lenalidomide, a more potent molecular form of thalidomide, has a lower frequency of peripheral neuropathy and has demonstrated efficacy with decreased PN lesions and pruritus in a case series.128,129 However, despite the success of these therapies, significant side effects including teratogenicity, fatigue, neuropathy, and hypercoagulability relegate their use to severe and recalcitrant cases.128,130
Opioid receptor antagonists
Systemic µ-opioid receptor antagonists, such as naloxone and naltrexone, are utilized in the treatment of pruritus associated with several systemic and dermatological diseases, including PN.131,132 The antipruritic effect is exerted through inhibition of µ-opioid receptors on nociceptive neurons and interneurons resulting in suppression of itch.131 In this case series, 67.7% of patients with PN noted improvement of symptoms and 38% reported complete remission of PN.131 Although gastrointestinal and neurological side effects are commonly observed with naltrexone use, they are generally limited to the initial 2 weeks of use.132 Contraindications include use of active opiate, use of opioid containing medications, and significant liver disease.132,133 Currently, a clinical trial (NCT02174419) is conducting an ongoing investigation on the therapeutic benefits of nalbuphine for the treatment of PN. Nalbuphine is a dual µ-opioid receptor antagonist and κ-agonist (NCT02174419). Intranasal Butorphanol, also a dual µ-opioid receptor antagonist and κ-agonist, has demonstrated efficacy in the treatment of intractable pruritus in a case series.133
NK1r antagonists
SP, a tachykinin with strong affinity for NK1r found in the skin and central nervous system, has been implicated in stimulating pro-inflammatory and pruritogenic pathways involved in the induction and maintenance of itch.134,135 NK1r antagonists, aprepitant and serlopitant, could prevent SP-mediated signaling in the pathogenesis of PN.9 Significant relief of itch was achieved in PN patients on aprepitant monotherapy.80 The most significant response rate was among those with PN.80 Histologically, PN lesions display an increased density of SP-positive nerve fibers which may explain the high response rates seen with NK1r antagonist therapy. This was followed up by Ohanyan et al who investigated the efficacy of topical aprepitant 1% gel compared to a placebo vehicle in a study with 19 patients.136 Significantly elevated serum SP levels and increased NK1r expression were shown in PN patients when compared to controls.136 The difference in the reduction of mean VAS score between the placebo (66%) and the topical aprepitant group (58%) was not significant indicating poor efficacy of topical NK1r antagonists.136 Ständer et al compared the effects of daily 5 mg serlopitant to placebo for 8 weeks in 127 PN patients. Findings revealed significant reduction in VAS score in the serlopitant group (3.6 cm) compared to the placebo group (1.9 cm).137
IL-31 receptor antibody
IL-31 is considered to play a bridging role between itch induction and maintenance of inflammation in pruritic skin disorders.138,139 Immunotherapy targeting the IL-31 pathway and halting the release of inflammatory cytokines may prove fruitful. Nemolizumab, a humanized monoclonal antibody to the IL-31 receptor A, found on dorsal root ganglion neurons, provided significant improvement in pruritus scores among patients with moderate-to-severe atopic dermatitis.140 However, its role in PN remains unclear.
Conclusion
PN is a chronic condition, typically preceded by an underlying cause of pruritis. PN is a distinct entity from these underlying causes and can persist despite resolution of the predisposing condition. Current pathogenesis indicates that neuronal sensitization and local changes in factor concentrations in the cutaneous microenvironment lead to PN. While treating the underlying factors of PN has an important role in preventing recurrence, the treatment of PN is distinct. Treatment typically relies on the use of topical or intralesional steroids, though more severe or recalcitrant cases often necessitate the use of phototherapy or systemic immunosuppressives. Thalidomide and lenalidomide can both be used in severe cases; however, their toxicity profile makes them less favorable. Opioid receptor antagonists and NK1r antagonists represent two novel families of therapeutic agents which may effectively treat PN with a lower toxicity profile than thalidomide or lenalidomide.
Abbreviations
CKD-ap, chronic kidney disease-associated pruritus; CP, chronic pruritus; IENF, intraepidermal nerve fiber; MTX, methotrexate; NGF, nerve growth factor; NGFr, nerve growth factor receptor; NK1r, neurokinin 1 receptor; PGP, protein gene product; PN, prurigo nodularis; PUVA, psoralen ultraviolet A; RCT, randomized controlled trial; SFN, small-fiber neuropathy; SP, substance P; UVB, ultraviolet B.
Disclosure
The authors report no conflicts of interest in this work.
References
Zeidler C, Ständer S. The pathogenesis of Prurigo nodularis--’Super-Itch’ in exploration. Eur J Pain. 2016;20(1):37–40. | ||
Iking A, Grundmann S, Chatzigeorgakidis E, Phan NQ, Klein D, Ständer S. Prurigo as a symptom of atopic and non-atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol. 2013;27(5):550–557. | ||
Winhoven SM, Gawkrodger DJ. Nodular prurigo: metabolic diseases are a common association. Clin Exp Dermatol. 2007;32(2):224–225. | ||
Rowland Payne CM, Wilkinson JD, Mckee PH, Jurecka W, Black MM. Nodular prurigo – a clinicopathological study of 46 patients. Br J Dermatol. 1985;113(4):431–439. | ||
Vaidya DC, Schwartz RA. Prurigo nodularis: a benign dermatosis derived from a persistent pruritus. Acta Dermatovenerol Croat. 2008;16(1):38–44. | ||
Liang Y, Jacobi HH, Reimert CM, Haak-Frendscho M, Marcusson JA, Johansson O. CGRP-immunoreactive nerves in prurigo nodularis--an exploration of neurogenic inflammation. J Cutan Pathol. 2000;27(7):359–366. | ||
Johansson O, Liang Y, Marcusson JA, Reimert CM. Eosinophil cationic protein- and eosinophil-derived neurotoxin/eosinophil protein X-immunoreactive eosinophils in prurigo nodularis. Arch Dermatol Res. 2000;292(8):371–378. | ||
Johansson O, Liang Y, Emtestam L. Increased nerve growth factor- and tyrosine kinase A-like immunoreactivities in prurigo nodularis skin -- an exploration of the cause of neurohyperplasia. Arch Dermatol Res. 2002;293(12):614–619. | ||
Haas S, Capellino S, Phan NQ, et al. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. J Dermatol Sci. 2010;58(3):193–197. | ||
Pereira MP, Steinke S, Zeidler C, et al. European Academy of dermatology and venereology European prurigo project: expert consensus on the definition, classification and terminology of chronic prurigo. J Eur Acad Dermatol Venereol. 2018;32(7):1059–1065. | ||
Amber KT, Korta DZ, de Feraudy S, Grando SA. Vesiculobullous eruption in a patient receiving psoralen ultraviolet A (PUVA) treatment for prurigo nodules: a case of PUVA-aggravated pemphigoid nodularis. Clin Exp Dermatol. 2017;42(7):833–835. | ||
Mollanazar NK, Sethi M, Rodriguez RV, et al. Retrospective analysis of data from an itch center: integrating validated tools in the electronic health record. J Am Acad Dermatol. 2016;75(4):842–844. | ||
Bharati A, Wilson NJ. Peripheral neuropathy associated with nodular prurigo. Clin Exp Dermatol. 2007;32(1):67–70. | ||
Yosipovitch G, Samuel LS. Neuropathic and psychogenic itch. Dermatol Ther. 2008;21(1):32–41. | ||
Schuhknecht B, Marziniak M, Wissel A, et al. Reduced intraepidermal nerve fibre density in lesional and nonlesional prurigo nodularis skin as a potential sign of subclinical cutaneous neuropathy. Br J Dermatol. 2011;165(1):85–91. | ||
Pereira MP, Basta S, Moore J, Ständer S. Prurigo nodularis: a physician survey to evaluate current perceptions of its classification, clinical experience and unmet need. J Eur Acad Dermatol Venereol. 2018. | ||
Amer A, Fischer H. Prurigo nodularis in a 9-year-old girl. Clin Pediatr. 2009;48(1):93–95. | ||
Vachiramon V, Tey HL, Thompson AE, Yosipovitch G. Atopic dermatitis in African American children: addressing unmet needs of a common disease. Pediatr Dermatol. 2012;29(4):395–402. | ||
Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018;79(4):714–719.e713. | ||
Tanaka M, Aiba S, Matsumura N, Aoyama H, Tagami H. Prurigo nodularis consists of two distinct forms: early-onset atopic and late-onset non-atopic. Dermatology. 1995;190(4):269–276. | ||
Tan WS, Tey HL. Extensive prurigo nodularis: characterization and etiology. Dermatology. 2014;228(3):276–280. | ||
A D, Rivet J, Moulonguet I, et al. Atypical presentation of adult T-cell leukaemia/lymphoma due to HTLV-1: prurigo nodularis lasting twelve years followed by an acute micropapular eruption. Acta Derm Venereol. 2010;90(3):287–290. | ||
Pereira MP, Lüling H, Dieckhöfer A, Steinke S, Zeidler C, Ständer S. Brachioradial pruritus and Notalgia paraesthetica: a comparative observational study of clinical presentation and morphological pathologies. Acta Derm Venereol. 2018;98(1):82–88. | ||
Weisshaar E, Szepietowski JC, Darsow U, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563–581. | ||
Tessari G, Dalle Vedove C, Loschiavo C, et al. The impact of pruritus on the quality of life of patients undergoing dialysis: a single centre cohort study. J Nephrol. 2009;22(2):241–248. | ||
Jørgensen KM, Egeberg A, Gislason GH, Skov L, Thyssen JP. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31(2):e106–e107. | ||
Dazzi C, Erma D, Piccinno R, Veraldi S, Caccialanza M. Psychological factors involved in prurigo nodularis: a pilot study. J Dermatolog Treat. 2011;22(4):211–214. | ||
Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37(5):578–586. | ||
Bs A, Beergouder SL. Hypertrophic lichen planus versus prurigo nodularis: a dermoscopic perspective. Dermatol Pract Concept. 2016;6(2):9–15. | ||
Errichetti E, Piccirillo A, Stinco G. Dermoscopy of Prurigo nodularis. J Dermatol. 2015;42(6):632–634. | ||
Pugliarello S, Cozzi A, Gisondi P, Girolomoni G. Phenotypes of atopic dermatitis. J Dtsch Dermatol Ges. 2011;9(1):12–20. | ||
Furukita K, Ansai S, Hida Y, Kubo Y, Arase S, Hashimoto T. A case of epidermolysis bullosa acquisita with unusual clinical features. Clin Exp Dermatol. 2009;34(8):e702–e704. | ||
Jerković Gulin S, Čeović R, Lončarić D, Ilić I, Radman I. Nodular Prurigo Associated with Mycosis Fungoides Ethnic differences and comorbidities of 909 prurigo nodularis patients Case Report. Acta Dermatovenerol Croat. 2015;23(3):203–207. | ||
Mattila JO, Vornanen M, Vaara J, Katila ML. Mycobacteria in prurigo nodularis: the cause or a consequence? J Am Acad Dermatol. 1996;34(2 Pt 1):224–228. | ||
Saporito L, Florena AM, Colomba C, Pampinella D, di Carlo P. Prurigo nodularis due to Mycobacterium tuberculosis. J Med Microbiol. 2009;58(Pt 12):1649–1651. | ||
Singh F, Rudikoff D. HIV-associated pruritus: etiology and management. Am J Clin Dermatol. 2003;4(3):177–188. | ||
Tarikci N, Kocatürk E, Güngör Şule, Oğuz Topal I, Ülkümen Can P, Singer R. Pruritus in systemic diseases: a review of etiological factors and new treatment modalities. ScientificWorldJournal. 2015;2015(2):1–8. | ||
Milazzo F, Piconi S, Trabattoni D, et al. Intractable pruritus in HIV infection: immunologic characterization. Allergy. 1999;54(3):266–272. | ||
Magand F, Nacher M, Cazorla C, Cambazard F, Marie DS, Couppié P. Predictive values of Prurigo nodularis and herpes zoster for HIV infection and immunosuppression requiring HAART in French Guiana. Trans R Soc Trop Med Hyg. 2011;105(7):401–404. | ||
Pisoni RL, Wikström B, Elder SJ, et al. Pruritus in haemodialysis patients: international results from the dialysis outcomes and practice patterns study (DOPPS). Nephrol Dial Transplant. 2006;21(12):3495–3505. | ||
Weiss M, Mettang T, Tschulena U, Passlick-Deetjen J, Weisshaar E. Prevalence of chronic itch and associated factors in haemodialysis patients: a representative cross-sectional study. Acta Derm Venereol. 2015;95(7):816–821. | ||
Hayani K, Weiss M, Weisshaar E. Clinical findings and provision of care in haemodialysis patients with chronic itch: new results from the German epidemiological haemodialysis itch study. Acta Derm Venereol. 2016;96(3):361–366. | ||
Akarsu S, Ozbagcivan O, Ilknur T, Semiz F, Inci BB, Fetil E. Xerosis cutis and associated co-factors in women with prurigo nodularis. An Bras Dermatol. 2018;93(5):671–679. | ||
Kestner RI, Ständer S, Osada N, Ziegler D, Metze D. Acquired reactive perforating dermatosis is a variant of Prurigo nodularis. Acta Derm Venereol. 2017;97(2):249–254. | ||
White CR, Heskel NS, Pokorny DJ. Perforating folliculitis of hemodialysis. Am J Dermatopathol. 1982;4(2):109–116. | ||
Hurwitz RM, Melton ME, Creech FT, Weiss J, Handt A. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4(2):101–108. | ||
Khaled A, Sfia M, Fazaa B, et al. Prurigo chronique rélévant un lymphome T angioimmunoblastique [Chronic prurigo revealing an angioimmunoblastic T cell lymphoma]. Tunis Med. 2009;87(8):534–537. French. | ||
Funaki M, Ohno T, Dekio S, et al. Prurigo nodularis associated with advanced gastric cancer: report of a case. J Dermatol. 1996;23(10):703–707. | ||
Lin JT, Wang WH, Yen CC, Yu IT, Chen PM. Prurigo nodularis as initial presentation of metastatic transitional cell carcinoma of the bladder. J Urol. 2002;168(2):631–632. | ||
Dumont S, Péchère M, Toutous Trellu L. Chronic prurigo: an unusual presentation of Hodgkin lymphoma. Case Rep Dermatol. 2018;10(2):122–126. | ||
Shelnitz LS, Paller AS. Hodgkin’s disease manifesting as prurigo nodularis. Pediatr Dermatol. 1990;7(2):136–139. | ||
Seshadri P, Rajan SJ, George IA, George R. A sinister itch: prurigo nodularis in Hodgkin lymphoma. J Assoc Physicians India. 2009;57:715–716. | ||
A S, Ständer S. Neuropathic itch: diagnosis and management. Dermatol Ther. 2013;26(2):104–109. | ||
De D, Dogra S, Kanwar AJ. Prurigo nodularis in healed herpes zoster scar: an isotopic response. J Eur Acad Dermatol Venereol. 2007;21(5):711–712. | ||
Mirzoyev SA, Davis MD. Brachioradial pruritus: mayo Clinic experience over the past decade. Br J Dermatol. 2013;169(5):1007–1015. | ||
Pautrier LM. A propos de la maladie de besnier-boeck-schaumann a forme de prurigo [Besnier-Boeck-Schaumann’s disease of prurigo type]. Ann Dermatol Syphiligr. 19541954;81(5):481–490. French | ||
Harris B, Harris K, Penneys NS. Demonstration by S-100 protein staining of increased numbers of nerves in the papillary dermis of patients with prurigo nodularis. J Am Acad Dermatol. 1992;26(1):56–58. | ||
Lindley RP, Payne CM. Neural hyperplasia is not a diagnostic prerequisite in nodular prurigo. A controlled morphometric microscopic study of 26 biopsy specimens. J Cutan Pathol. 1989;16(1):14–18. | ||
Liang Y, Heilborn J, Marcusson J, Johansson O. Increased NGFR immunoreactive, dermal nerve fibers in prurigo nodularis. Eur J Dermatol. 1996;6:563. | ||
Liang Y, Marcusson JA, Johansson O. Light and electron microscopic immunohistochemical observations of p75 nerve growth factor receptor-immunoreactive dermal nerves in prurigo nodularis. Arch Dermatol Res. 1999;291(1):14–21. | ||
Abadía Molina F, Burrows NP, Jones RR, Terenghi G, Polak JM. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127(4):344–351. | ||
Johnson EM, Gorin PD, Brandeis LD, Pearson J. Dorsal root ganglion neurons are destroyed by exposure in utero to maternal antibody to nerve growth factor. Science. 1980;210(4472):916–918. | ||
Goedert M, Otten U, Hunt SP, et al. Biochemical and anatomical effects of antibodies against nerve growth factor on developing rat sensory ganglia. Proc Natl Acad Sci USA. 1984;81(5):1580–1584. | ||
Mobley WC, Woo JE, Edwards RH, et al. Developmental regulation of nerve growth factor and its receptor in the rat caudate-putamen. Neuron. 1989;3(5):655–664. | ||
di Marco E, Marchisio PC, Bondanza S, Franzi AT, Cancedda R, de Luca M. Growth-regulated synthesis and secretion of biologically active nerve growth factor by human keratinocytes. J Biol Chem. 1991;266(32):21718–21722. | ||
Pincelli C, Sevignani C, Manfredini R, et al. Expression and function of nerve growth factor and nerve growth factor receptor on cultured keratinocytes. J Invest Dermatol. 1994;103(1):13–18. | ||
Groneberg DA, Serowka F, Peckenschneider N, et al. Gene expression and regulation of nerve growth factor in atopic dermatitis mast cells and the human mast cell LINE-1. J Neuroimmunol. 2005;161(1–2):87–92. | ||
Kobayashi H, Gleich GJ, Butterfield JH, Kita H. Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli. Blood. 2002;99(6):2214–2220. | ||
Solomon A, Aloe L, Pe’er J, et al. Nerve growth factor is preformed in and activates human peripheral blood eosinophils. J Allergy Clin Immunol. 1998;102(3):454–460. | ||
Nilsson G, Forsberg-Nilsson K, Xiang Z, Hallböök F, Nilsson K, Metcalfe DD. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur J Immunol. 1997;27(9):2295–2301. | ||
Lambiase A, Bracci-Laudiero L, Bonini S, et al. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J Allergy Clin Immunol. 1997;100(3):408–414. | ||
Matsumura S, Terao M, Murota H, Katayama I. Th2 cytokines enhance TRKA expression, upregulate proliferation, and downregulate differentiation of keratinocytes. J Dermatol Sci. 2015;78(3):215–223. | ||
Wilkinson DI, Theeuwes MJ, Farber EM. Nerve growth factor increases the mitogenicity of certain growth factors for cultured human keratinocytes: a comparison with epidermal growth factor. Exp Dermatol. 1994;3(5):239–245. | ||
Otten U, Ehrhard P, Peck R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc Natl Acad Sci USA. 1989;86(24):10059–10063. | ||
Bischoff SC, Ca D, Dahinden CA. Effect of nerve growth factor on the release of inflammatory mediators by mature human basophils. Blood. 1992;79(10):2662–2669. | ||
Kessler JA, Black IB. Nerve growth factor stimulates the development of substance P in sensory ganglia. Proc Natl Acad Sci USA. 1980;77(1):649–652. | ||
Hägermark O, Hökfelt T, Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978;71(4):233–235. | ||
Lembeck F, Donnerer J, Tsuchiya M, Nagahisa A. The non-peptide tachykinin antagonist, CP-96,345, is a potent inhibitor of neurogenic inflammation. Br J Pharmacol. 1992;105(3):527–530. | ||
Almeida TA, Rojo J, Nieto PM, et al. Tachykinins and tachykinin receptors: structure and activity relationships. Curr Med Chem. 2004;11(15):2045–2081. | ||
Ständer S, Siepmann D, Herrgott I, Sunderkötter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS One. 2010;5(6):e10968. | ||
Ständer S, Luger T, Metze D. Treatment of Prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44(3):471–478. | ||
Schmelz M, Schmidt R, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89(5):2441–2448. | ||
Liang Y, Marcusson JA, Jacobi HH, Haak-Frendscho M, Johansson O. Histamine-containing mast cells and their relationship to NGFr-immunoreactive nerves in prurigo nodularis: a reappraisal. J Cutan Pathol. 1998;25(4):189–198. | ||
Sonkoly E, Muller A, Lauerma AI, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117(2):411–417. | ||
Arai I, Tsuji M, Takeda H, Akiyama N, Saito S. A single dose of interleukin-31 (IL-31) causes continuous itch-associated scratching behaviour in mice. Exp Dermatol. 2013;22(10):669–671. | ||
Bobko S, Zeidler C, Osada N, et al. Intraepidermal nerve fibre density is decreased in lesional and Inter-lesional prurigo nodularis and reconstitutes on healing of lesions. Acta Derm Venereol. 2016;96(3):404–406. | ||
Hoeijmakers JG, Faber CG, Lauria G, Merkies IS, Waxman SG. Small-fibre neuropathies – advances in diagnosis, pathophysiology and management. Nat Rev Neurol. 2012;8(7):369–379. | ||
Lauria G, Hsieh ST, Johansson O, et al. European Federation of neurological Societies/Peripheral nerve Society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint Task Force of the European Federation of Neurological societies and the peripheral nerve Society. Eur J Neurol. 2010;17(7):903–e49e944-909. | ||
Holland NR, Crawford TO, Hauer P, Cornblath DR, Griffin JW, Mcarthur JC. Small-fiber sensory neuropathies: clinical course and neuropathology of idiopathic cases. Ann Neurol. 1998;44(1):47–59. | ||
Gorson KC, Herrmann DN, Thiagarajan R, et al. Non-length dependent small fibre neuropathy/ganglionopathy. J Neurol Neurosurg Psychiatry. 2008;79(2):163–169. | ||
Rosenberg JM, Harrell C, Ristic H, Werner RA, de Rosayro AM. The effect of gabapentin on neuropathic pain. Clin J Pain. 1997;13(3):251–255. | ||
Ho TW, Backonja M, Ma J, Leibensperger H, Froman S, Polydefkis M. Efficient assessment of neuropathic pain drugs in patients with small fiber sensory neuropathies. Pain. 2009;141(1-2):19–24. | ||
Rosenstock J, Tuchman M, Lamoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110(3):628–638. | ||
Forst T, Pohlmann T, Kunt T, et al. The influence of local capsaicin treatment on small nerve fibre function and neurovascular control in symptomatic diabetic neuropathy. Acta Diabetol. 2002;39(1):1–6. | ||
Gencoglan G, Inanir I, Gunduz K. Therapeutic Hotline: treatment of Prurigo nodularis and lichen simplex chronicus with gabapentin. Dermatol Ther. 2010;23(2):194–198. | ||
Mazza M, Guerriero G, Marano G, Janiri L, Bria P, Mazza S. Treatment of Prurigo nodularis with pregabalin. J Clin Pharm Ther. 2013;38(1):16–18. | ||
Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008;131(Pt 7):1912–1925. | ||
Pereira MP, Pogatzki-Zahn E, Snels C, et al. There is no functional small-fibre neuropathy in prurigo nodularis despite neuroanatomical alterations. Exp Dermatol. 2017;26(10):969–971. | ||
Konda D, Chandrashekar L, Rajappa M, Kattimani S, Thappa DM, Ananthanarayanan PH. Serotonin and interleukin-6: association with pruritus severity, sleep quality and depression severity in prurigo nodularis. Asian J Psychiatr. 2015;17:24–28. | ||
Ständer S, Zeidler C, Augustin M, et al. S2k Guidelines for the diagnosis and treatment of chronic pruritus – update – short version. J Dtsch Dermatol Ges. 2017;15(8):860–872. | ||
Richards RN. Update on intralesional steroid: focus on dermatoses. J Cutan Med Surg. 2010;14(1):19–23. | ||
Waldinger TP, Wong RC, Taylor WB, Voorhees JJ. Cryotherapy improves prurigo nodularis. Arch Dermatol. 1984;120(12):1598–1600. | ||
Stoll DM, Fields JP, King LE. Treatment of Prurigo nodularis: use of cryosurgery and intralesional steroids plus lidocaine. J Dermatol Surg Oncol. 1983;9(11):922–924. | ||
Brenninkmeijer EE, Spuls PI, Lindeboom R, van der Wal AC, Bos JD, Wolkerstorfer A. Excimer laser vs. clobetasol propionate 0·05% ointment in prurigo form of atopic dermatitis: a randomized controlled trial, a pilot. Br J Dermatol. 2010;163(4):823–831. | ||
Saraceno R, Chiricozzi A, Nisticò SP, Tiberti S, Chimenti S. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21(6):363–366. | ||
El-Batawy MM, Bosseila MA, Mashaly HM, Hafez VS. Topical calcineurin inhibitors in atopic dermatitis: a systematic review and meta-analysis. J Dermatol Sci. 2009;54(2):76–87. | ||
Siepmann D, Lotts T, Blome C, et al. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: results of a randomized, hydrocortisone-controlled, double-blind phase II trial. Dermatology. 2013;227(4):353–360. | ||
Wong SS, Goh CL. Double-blind, right/left comparison of calcipotriol ointment and betamethasone ointment in the treatment of Prurigo nodularis. Arch Dermatol. 2000;136(6):807–808. | ||
Schulz S, Metz M, Siepmann D, Luger TA, Maurer M, Ständer S. Antipruritische Wirksamkeit einer hoch dosierten Antihistaminikatherapie [Antipruritic efficacy of a high-dosage antihistamine therapy. Results of a retrospectively analysed case series]. Hautarzt. 2009;60(7):564–568. | ||
Shintani T, Ohata C, Koga H, et al. Combination therapy of fexofenadine and montelukast is effective in prurigo nodularis and pemphigoid nodularis. Dermatol Ther. 2014;27(3):135–139. | ||
Hammes S, Hermann J, Roos S, Ockenfels HM. UVB 308-nm excimer light and Bath PUVA: combination therapy is very effective in the treatment of Prurigo nodularis. J Eur Acad Dermatol Venereol. 2011;25(7):799–803. | ||
Wallengren J, Sundler F. Phototherapy reduces the number of epidermal and CGRP-positive dermal nerve fibres. Acta Derm Venereol. 2004;84(2):111–115. | ||
Tartar D, Bhutani T, Huynh M, Berger T, Koo J. Update on the immunological mechanism of action behind phototherapy. J Drugs Dermatol. 2014;13(5):564–568. | ||
Tamagawa-Mineoka R, Katoh N, Ueda E, Kishimoto S. Narrow-band ultraviolet B phototherapy in patients with recalcitrant nodular prurigo. J Dermatol. 2007;34(10):691–695. | ||
Bruni E, Caccialanza M, Piccinno R. Phototherapy of generalized prurigo nodularis. Clin Exp Dermatol. 2010;35(5):549–550. | ||
Sorenson E, Levin E, Koo J, Berger TG. Successful use of a modified Goeckerman regimen in the treatment of generalized prurigo nodularis. J Am Acad Dermatol. 2015;72(1):e40–e42. | ||
Paghdal KV, Schwartz RA. Topical TAR: back to the future. J Am Acad Dermatol. 2009;61(2):294–302. | ||
Wiznia LE, Callahan SW, Cohen DE, Orlow SJ. Rapid improvement of Prurigo nodularis with cyclosporine treatment. J Am Acad Dermatol. 2018;78(6):1209–1211. | ||
Spring P, Gschwind I, Gilliet M. Prurigo nodularis: retrospective study of 13 cases managed with methotrexate. Clin Exp Dermatol. 2014;39(4):468–473. | ||
Klejtman T, Beylot-Barry M, Joly P, et al. Treatment of prurigo with methotrexate: a multicentre retrospective study of 39 cases. J Eur Acad Dermatol Venereol. 2018;32(3):437–440. | ||
Lear JT, English JS, Smith AG. Nodular prurigo responsive to azathioprine. Br J Dermatol. 1996;134(6):1151. | ||
Gupta R. Treatment of Prurigo nodularis with dexamethasone-cyclophosphamide pulse therapy. Indian J Dermatol Venereol Leprol. 2016;82(2):239. | ||
Halvorsen JA, Aasebø W. Oral tacrolimus treatment of pruritus in prurigo nodularis. Acta Derm Venereol. 2015;95(7):866–867. | ||
Feldmeyer L, Werner S, Kamarashev J, French LE, Hofbauer GF. Atopic prurigo nodularis responds to intravenous immunoglobulins. Br J Dermatol. 2012;166(2):461–462. | ||
Sharma D, Kwatra SG. Thalidomide for the treatment of chronic refractory pruritus. J Am Acad Dermatol. 2016;74(2):363–369. | ||
Chen M, Doherty SD, Hsu S. Innovative uses of thalidomide. Dermatol Clin. 2010;28(3):577–586. | ||
Andersen TP, Fogh K. Thalidomide in 42 patients with prurigo nodularis Hyde. Dermatology. 2011;223(2):107–112. | ||
Kanavy H, Bahner J, Korman NJ. Treatment of refractory prurigo nodularis with lenalidomide. Arch Dermatol. 2012;148(7):794–796. | ||
Lim VM, Maranda EL, Patel V, Simmons BJ, Jimenez JJ. A review of the efficacy of thalidomide and lenalidomide in the treatment of refractory prurigo nodularis. Dermatol Ther. 2016;6(3):397–411. | ||
Liu H, Gaspari AA, Schleichert R. Use of lenalidomide in treating refractory prurigo nodularis. J Drugs Dermatol. 2013;12(3):360–361. | ||
Phan NQ, Lotts T, Antal A, Bernhard JD, Ständer S. Systemic kappa opioid receptor agonists in the treatment of chronic pruritus: a literature review. Acta Derm Venereol. 2012;92(5):555–560. | ||
Lee J, Shin JU, Noh S, Park CO, Lee KH. Clinical efficacy and safety of naltrexone combination therapy in older patients with severe pruritus. Ann Dermatol. 2016;28(2):159–163. | ||
Dawn AG, Yosipovitch G. Butorphanol for treatment of intractable pruritus. J Am Acad Dermatol. 2006;54(3):527–531. | ||
Devane CL. Substance P: a new era, a new role. Pharmacotherapy. 2001;21(9):1061–1069. | ||
Metz M, Krull C, Hawro T, et al. Substance P is upregulated in the serum of patients with chronic spontaneous urticaria. J Invest Dermatol. 2014;134(11):2833–2836. | ||
Ohanyan T, Schoepke N, Eirefelt S, et al. Role of substance P and its receptor neurokinin 1 in chronic prurigo: a randomized, proof-of-concept, controlled trial with topical aprepitant. Acta Derm Venereol. 2018;98(1):26–31. | ||
Ständer S, Kwon P, Luger TA. Randomized, double-blind, placebo-controlled phase 2 clinical trial of serlopitant effects on multiple measures of pruritus in patients with prurigo nodularis. Paper presented at: 9th World Congress on Itch; October 15–17, 2017; Wroclaw. | ||
Cornelissen C, Lüscher-Firzlaff J, Baron JM, Lüscher B. Signaling by IL-31 and functional consequences. Eur J Cell Biol. 2012;91(6–7):552–566. | ||
Bağci IS, Ruzicka T. IL-31: A new key player in dermatology and beyond. J Allergy Clin Immunol. 2018;141(3):858–866. | ||
Ruzicka T, Hanifin JM, Furue M, et al. Anti-Interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med. 2017;376(9):826–835. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
