Back to Journals » Patient Related Outcome Measures » Volume 11
Treatment Outcomes and Associated Factors among Children Hospitalized with Acute Bacterial Meningitis in Eastern Ethiopia: A Cross-Sectional Study
Authors Adem F, Tasew A, Siraj A , Mohammed M
Received 19 August 2020
Accepted for publication 25 November 2020
Published 23 December 2020 Volume 2020:11 Pages 241—248
DOI https://doi.org/10.2147/PROM.S277586
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Liana Bruce
Fuad Adem,1 Amanuel Tasew,2 Ammas Siraj,1 Mesud Mohammed3
1Department of Clinical Pharmacy, School of Pharmacy, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 2School of Pharmacy, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 3Department of Pharmacy, School of Medicine, Madda-Walabu University, Goba, Ethiopia
Correspondence: Fuad Adem Tel +251-96-753-8374
Email [email protected]
Background: Bacterial meningitis is a common central nervous system infection that is associated with high morbidity and mortality in pediatrics. In Ethiopia, little is known about treatment outcomes of acute bacterial meningitis and associated factors among hospitalized children.
Objective: To assess treatment outcomes of acute bacterial meningitis and associated factors among hospitalized children with acute bacterial meningitis in the Hiwot Fana Specialized University Hospital pediatric ward.
Methods: A retrospective cross-sectional study was conducted at the pediatric ward of Hiwot Fana Specialized University Hospital, eastern Ethiopia. Relevant data were collected using a structured data-collection tool from patients’ medical charts. Bivariate and multivariate logistic regression analyses were done to identify predictors of treatment outcomes. OR with 95% CI and P≤ 0.05 was used for statistical significance.
Results: A total of 200 children with acute bacterial meningitis were included in the study, of which 92% were aged ≥ 2 months and the majority (128, 64%) had delayed (≥ 72 hours) presentation to the hospital. At admission, 181 (90.5%) were febrile, 92 (46%) had depressed level of consciousness, and 40 (20%) had had seizures. Most (126, 63%) had documented medical comorbidities. The antibiotic combination of ampicillin and gentamycin had been frequently administered in children aged < 2 months while ceftriaxone was commonly prescribed for those aged > 2 months. Of the total study participants, 154 (77%) showed successful treatment outcomes, while 46 (23%) experienced poor treatment outcomes (died or “self”-discharged). Level of consciousness (AOR 3.25, 95% CI 1.21– 8.75), duration of illness before admission (AOR 3.74, 95% CI 1.76– 7.98), and antibiotic-regimen change (AOR 4.7, 95% CI 2.4– 10) were predictors of treatment outcomes.
Conclusion: The majority of study participants experienced good treatment outcomes. Unconsciousness, antibiotic-regimen change, and duration of illness before hospitalization were significantly associated with treatment outcomes. Early treatment, linkage of primary-health facilities to tertiary health–care centers, and availability of diagnostics should be promoted to improve patient outcomes.
Keywords: acute bacterial meningitis, treatment outcomes, pediatrics, eastern Ethiopia
Background
Meningitis is an inflammation of the meninges that involves the subarachnoid space or spinal fluid.1 It can arise from an infectious etiology, such as bacteria, mycobacteria, viruses, fungi, or parasites, or be associated with autoimmunity, cancer, or reactions to medications.2 Meningitis is a common infectious cause of morbidity and mortality in pediatric age-groups. Each year, it affects about 2.81 million children, of which a third are <5 years of age.2 If acute bacterial meningitis is not properly treated, its mortality rate can be as high as 50%, and thus it has been labeled one of the top-ten bacterial causes of mortality worldwide.3
The mortality rate from acute bacterial meningitis ranges from 5% to 30%, and 30% of survivors experience neurological sequelae, such as hearing impairment, seizure disorders, and learning and behavioral problems.4 Globally, it is responsible for about 288,000 annual cases of all-age mortality, of which more than half (53%) are children aged <5 years.5 In Africa, the highest burden of bacterial meningitis occurs in an area of sub-Saharan Africa commonly known as the “meningitis belt”, stretching from Senegal through South Sudan and Ethiopia.2
The causative microorganism for acute bacterial meningitis varies with age, immunofunction, immunization status, and geographic region.6 However, Haemophilus influenzae type B, Streptococcus pneumoniae, and Neisseria meningitides are the three commonest etiologic agents.6 Once an infection is contracted, clinical features vary with the stage of the disease. Aspecific symptoms, such as fever, headache, and malaise, manifest in the early stage of the disease, whereas neck stiffness, photophobia, and signs of meningeal irritation (Kernig’s and Brudziński’s signs) manifest later in the course of the disease and are commoner in older children.7 Suspected bacterial meningitis is a medical emergency, and appropriate empirical antimicrobial therapy should be initiated as soon as possible, then modified to definitive therapy based on established laboratory-investigation results.8 However, in developing countries case management relies mainly on empirical therapy, which contributes to the emergence of antimicrobial resistance to commonly used drugs, affecting treatment outcomes.9
Ethiopia has a high burden of bacterial meningitis. Accordingly, bacterial meningitis underlies 6%–8% of pediatric hospitalizations in the country.10,11 The case-fatality rate is estimated as high as 22%–28%, putting Ethiopia among the ten countries with the highest mortality rate from acute bacterial meningitis in sub-Saharan Africa.2,10 Despite the high morbidity and mortality from bacterial meningitis in the country, studies reporting treatment outcomes of acute bacterial meningitis and contributing factors are limited. Further, to the best of our knowledge, no study has been conducted to determine treatment outcomes of acute bacterial meningitis and associated factors among pediatrics in the study area. Taking this into account, we aimed to assess treatment outcomes of acute bacterial meningitis and associated factors among pediatrics at Hiwot Fana Specialized University Hospital (HFSUH).
Methods
Study Design and Setting
A retrospective cross-sectional study was conducted from February 1 to March 30, 2019, at HFSUH in eastern Ethiopia. HFSUH is a teaching hospital in Harar town, 526 km east of Addis Ababa, the capital of Ethiopia. It serves as a referral hospital for the entire eastern part of the country, ie, Eastern Oromia region, Dire Dawa City Administration, Somali Regional State, and Harari Regional State. The hospital provides inpatient, outpatient, and emergency services. It has an internal medicine ward, surgery ward, pediatric ward, and gynecology and obstetrics ward, and antenatal care clinics, dental clinics, tuberculosis clinics, antiretroviral therapy clinics, dermatology clinics, and ophthalmological clinics.
Data Collection
Patient charts of pediatric patients admitted to the pediatric ward due to acute bacterial meningitis were used to collect the data. The data-collection tool was prepared after a thorough literature review of published studies on acute bacterial meningitis in pediatrics. The content of the tool was reviewed by one pediatrician and one clinical pharmacist, and modifications made. Two pharmacy interns and one supervisor with a master’s degree were assigned to the data-collection process. Before actual data collection started, 1day’s training was given to data collectors and the supervisor on how to collect and record data appropriately. The questionnaire was pretested on the 5% of the sample size and slight modifications made, and these were not included in the actual sample of study participants. Patients’ medical records were reviewed retrospectively, and relevant patient data — sociodemographic and clinical characteristics, time of presentation to the health-care facility from symptom onset, management characteristics, and final treatment outcomes — were extracted.
Statistical Analysis
Data collected were checked for completeness and entered and analyzed using SPSS 21.0 for Windows. Descriptive statistics were derived and bivariate and multivariate logistic regression analyses performed. Significant variables on bivariate logistic regression analyses (P≤0.25) were included in multivariate logistic regression analyses to identify independent predictors of treatment outcomes. P≤0.05 with 95% CI was considered significant in all cases.
Ethics
The study was approved by the Institutional Research and Ethical Review Committee (IRERC) of the College of Health and Medical Sciences, Haramaya University. Administrative permission was obtained from HFSUH. Confidentiality was maintained by using a patient card number instead of a patient name, and other patient information kept confidential. The study was performed in accordance with the Declaration of Helsinki.
Operational Definitions
Young infants were defined in this study as <2 months of age, and older infants and children as 2 months to 14 years of age, based on the treatment-protocol difference. Therefore, pediatrics according to the current study comprised infants and children aged from 1 day to 14 years.
Bacterial meningitis was defined according to a physician’s clinical diagnosis, or probable cases and if no changes in treatment considered until discharge rather than antibiotic regimen changes for meningitis treatment protocols.
Antibiotic-regimen changes was defined as a change in empirical antibiotics within 2–3 days in cases where the patient was not improving with initial empiric antibiotics.
Treatment outcomes were defined as outcomes of bacterial meningitis detected until discharge only. These included good and poor outcomes.
Good outcome meant the patient improved and was discharged without acute complications.
Poor outcome was defined as death within the ward and “self”-discharge against medical advice according to this study. Here, “self”-discharge was categorized as poor outcome, because such patients were “self”-discharged against medical advice before the completion of the prescribed antibiotic regimen. Progression, final status, and outcome of the patient could not be assessed, which most likely would have resulted in complications and/or death, as effective antibiotic treatment was the outcome determinant.
Results
Demographic and Baseline Characteristics of Study Participants
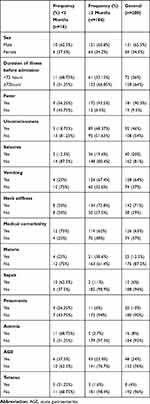
Of the total 200 study participants, 65.5% were male and the majority (92%) aged ≥2 months. Most participants (128, 64%) had delayed presentation to the hospital (≥72 hours). On admission, 181 (90.5%) were febrile, 92 (46%) had depressed levels of consciousness, and 40 (20%) had had seizures. Most (63%) had documented medical comorbidities: 48 (24%) acute gastroenteritis, 25 (12.5%) malaria, 20 (10%) pneumonia, 16 (8%) anemia, and 12 (6%) sepsis (Table 1).
Empirical Treatments and Treatment Outcomes
As microbiological evaluations were not readily available in the setting of this study, our findings of were based on clinical diagnosis and empirical treatments. Accordingly, ampicillin plus gentamycin was the commonest (75%) used initial empirical antibiotic regimen in children aged <2 months, whereas ceftriaxone was the most (81.5%) frequently prescribed initial antibiotic in those aged ≥2 months. Adjuvant dexamethasone was prescribed for 92% of children. Of all children treated for acute bacterial meningitis, 154 (77%) experienced good treatment outcomes and the remaining 46 (23%) poor treatment outcomes, of which 22 (11%) died and 24 (12%) “self”-discharged against medical advice. On the other hand, median time to improvement for children who improved was 5 (4–6) days for those aged <2 months and 4 (3–5) days for those aged ≥2 months (Table 2).
 |
Table 2 Treatment Outcomes of Acute Bacterial Meningitis Among Pediatrics Hospitalized at Hiwot Fana Specialized University Hospital, Eastern Ethiopia from November 1, 2018 to May 1, 2019 (n=200) |
Factors Associated with Treatment Outcomes
Multivariate logistic regression analyses revealed that level of consciousness, antibiotic-regimen change, and duration of illness before admission were significantly associated with treatment outcomes. Accordingly, children who were unconscious at admission were 3.25 times more likely to encounter a poor treatment outcome (died or “self”-discharged: AOR 3.25, 95% CI 1.21–8.75). Likewise, children for whom the initial antibiotic regimen changed were 4.6 times more likely to experience a good treatment outcome (AOR 4.66, 95% CI 2.173–10). On the other hand, patients who presented to the hospital early (within 72 hours of symptom onset) were 3.7 times more likely to benefit from treatment than those who presented late (AOR 3.74, 95% CI 1.76–7.98; Table 3).
Discussion
This study assessed treatment outcomes of acute bacterial meningitis and associated factors among children hospitalized at HFSUH, and is the first of its kind to be conducted in the study area. A total of 200 children with acute bacterial meningitis were included in the study, of which 65.5% were male and most (92%) aged ≥2 months. The majority (64%) presented late to the hospital, 90.5% had fever, 46% had depressed levels of consciousness, and 20% had had seizures at admission. Most (63%) had documented medical comorbidities. Regarding treatment, ampicillin plus gentamycin was frequently administered in children aged <2 months, while ceftriaxone was commonly prescribed for those >2 months of age. As for treatment outcomes, of all participants, 154 (77%) showed successful treatment outcomes, while 46 (23%) experienced poor treatment outcomes (died and “self”-discharged). Despite the inclusion of variables related to sociodemographic and clinical characteristics in bivariate analysis, level of consciousness, duration of illness before admission, and antibiotic-regimen change remained predictors of treatment outcomes on multivariate logistic regression analysis.
The majority of study participants were aged ≥2 months and slightly predominated by male sex. In contrast to our study, a majority (86.8%) of study participants were young infants and children in a study conducted in Jimma.12 This inconsistency could be attributed to the differences study area and sample size. On the other hand, our study is consistent with one done in Karachi in which male predominance was reported (74.5%).13
Duration of illness before hospital presentation is an important outcome determinant. The findings of this study showed that most children had had delayed presentation to the hospital (>72 hours from the onset of the symptoms). Reasons for hospital-presentation delay may be complex and related to multiple factors, including poor health-seeking behavior, limited access to health-care facilities, poor infrastructure, and health care–related factors, such as misdiagnosis of acute bacterial meningitis for simple febrile illness in primary-care settings, and lack of a proper referral system. This delays proper case management and treatment, which may consequently be associated with poor outcomes. In contrast to this study finding, the majority of participants presented to the healthcare facility early (within 1 hour of symptom onset) in a study conducted in Italy.14 A possible explanation for this disagreement could be the fact that the health-care system of Ethiopia is relatively fragile, where referral linkage between primary-care and tertiary centers is poor. Moreover, differences in health-seeking behavior and infrastructure (roads and transport) might be other explanations. However, in line with our study findings, most (70%) subjects had presented late to a health-care facility in another study done in Ethiopia.15
From assessment of clinical presentations, fever was identified to be the commonest sign at hospitalization, followed by nuchal rigidity. In agreement with our results, fever (97.5%) and nuchal rigidity (84%) were identified in a majority of study participants in a Turkish study.16 This may be explained by the fact that fever and nuchal rigidity are among the commonest clinical features of acute bacterial meningitis, regardless of setting variations.
The combination of ampicillin and gentamycin was frequently prescribed as initial antibiotics in children aged <2 months, whereas ceftriaxone was the commonest prescribed initial empirical antibiotic in those aged ≥2 months. However, the initial antibiotic regimen was changed in 16% of cases, based on poor clinical response. This is supported by the trends of clinical practice of the treating physicians and recommendations by the treatment guidelines of the country. Current WHO and Ethiopian standard-treatment guidelines recommend narrowing of the empirical regimen as soon as the causative agent is identified or a change of empirical antibiotics within 2–3 days if it is not possible to identify the causative agent and the patient is not improving on initial empirical antibiotics. Similarly to our finding, ampicillin plus gentamycin was the most frequently (86.8%) prescribed initial empirical antibiotic regimen in young infants and children in the study conducted in Jimma. However, in contrast to our study, ceftriaxone was less frequently (5.6%) prescribed for older infants and children.12 This inconsistency might be due to differences in clinical characteristics, clinical practice guidelines, antimicrobial-resistance patterns, and presence of comorbidity among the study participants.
In this study, less than a third of study participants encountered poor treatment outcomes. In contrast to our findings, more than half the study subjects experienced poor treatment outcomes in a study done in Angola.17 This inconsistency could have resulted from differences in time of presentation to the health-care facility from symptom onset, clinical characteristics, health-seeking behavior and awareness of the community, geographic variations, and experience and practice differences of the health-care settings.
Children for whom the initial antibiotic regimen changed were more likely to experience good treatment outcomes in our study. Patients are usually started on treatment at an initial level empirically, then antibiotics are changed based on response to the initial regimen or microbiologic evaluation of cerebrospinal fluid analysis. As such, in the setting of our study, antibiotic-regimen changes most commonly occurred because of poor responses of patients to the initial antibiotics. These regimen changes were consequently accompanied by good response and positive outcomes. In contrast to our findings, children for whom initial antibiotics were changed were more likely to experience poor treatment outcomes in the study conducted in Jimma.12 This might be due to differences in adherence to treatment protocols, etiological agents, medical comorbidities, and complications among study participants.
The findings of this study showed that children who presented early (within 72 hours of symptom onset) to the health-care facility were more likely to experience good treatment outcomes. In line with our findings, early (within 72 hours) presentation was identified to be linked to good treatment outcomes (recovery without complication) in a study conducted in Pristina, Kosovo.18 Early health-facility visits facilitate early case management and intervention, which improve prognosis before complications from acute bacterial meningitis can result.
Pediatrics with impaired levels of consciousness (unconscious) at admission were more likely to experience poor treatment outcomes. This could be explained by the fact that prolonged altered level of consciousness increases the risk of severe neurological sequelae, which adversely affect patient prognosis. The presence of depressed levels of consciousness at hospital admission is implication complications from untreated prolonged bacterial meningitis, which might result from delayed presentation to hospital. It would have been better if patients had been followed for some time after discharge to assess the full impact of the disease so that complete outcomes were able to be measured. Since long-term severe neurologic sequelae could not be detected within this short study period, the data were limited to short-term acute complications. In line with this study, altered levels of consciousness at hospitalisation were significantly associated with poor treatment outcomes in the study conducted in Angola.17
Conclusion
Most of the study participants experienced good treatment outcomes. It was found that changes in initial antibiotic regimen, level of unconsciousness at hospitalization and time of presentation to the health-care facility from symptom onset significantly influenced treatment outcomes of acute bacterial meningitis in children. Early treatment-seeking practices should be encouraged and linkage of primary-health facilities to tertiary health–care centers promoted. Locally applicable diagnostics and guidelines that enhance early and accurate diagnosis of patients with suspected meningitis are essential to improve patient outcomes.
Limitations
This study was the first of its kind to be conducted in the study area, thus providing baseline data about the outcomes of children hospitalized with acute bacterial meningitis and its predictors. Apart from such important findings, this study is not without limitations. This was a single-center study, and outcomes reported may not be representative of the nation. Moreover, as is common secondary data–based studies, some information was incomplete from the records, other than baseline measures. The lack of laboratory-based diagnostics, instead of clinical diagnoses that may have been less accurate than laboratory-assisted ones, might have also resulted in misdiagnosis of acute bacterial meningitis, and this might have affected the reported outcomes. Lastly, the retrospective nature of the study could be a limitation. Therefore, nationwide multicenter studies with better design are recommended to generaterepresentative data on outcomes in pediatric patients with bacterial meningitis.
Acknowledgments
The authors acknowledge the health-care professional practicing in the pediatric ward at Hiwot Fana Specialized University Hospital.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval to the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Kliegman RM, Stanton BF, St Geme JW
2. Kassebaum DNJ. Global, regional, and national burden of meningitis, 1990 – 2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:1061–1082. doi:10.1016/S1474-4422(18)30387-9
3. Ahmed A Etiology of Bacterial Meningitis in Ethiopia, 2007–2011: A Retrospective Study (Master’s thesis); 2012. Available from: https://www.duo.uio.no/handle/10852/34243.
4. Namani S, Milenković Z, Koci B. A prospective study of risk factors for neurological complications in childhood bacterial meningitis. J Pediatr (Rio J). 2013;89(3):256–262. doi:10.1016/j.jped.2012.10.001
5. Roth DG. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980 – 2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1980–2017.
6. Agrawal S, Nadel S. Acute bacterial meningitis in infants and children. Pediatr Drugs. 2014;13:385–400. doi:10.2165/11593340-000000000-00000
7. Fayyaz J, Hamid A. Age related clinical manifestation of acute bacterial meningitis in children. J Pak Med Assoc. 2014;64(3):296–299.
8. Le Saux N; Society CP, Diseases I. Guidelines for the management of suspected and confirmed bacterial meningitis in Canadian children older than one month of age. Paediatr Child Health. 2014;19(3):141–146. doi:10.1093/pch/19.3.141
9. WHO. World Health Organization. Antimicrobial and support therapy for bacterial meningitis in children.
10. Gudina EK, Tesfaye M, Wieser A, Pfister H, Klein M. Outcome of patients with acute bacterial meningitis in a teaching hospital in Ethiopia: a prospective study. PLoS One. 2018;13(7):e0200067. doi:10.1371/journal.pone.0200067
11. Amare AT, Kebede ZT, Welch HD. Epidemiology of bacterial meningitis in children admitted to Gondar University Hospital in the post pneumococcal vaccine era. Pan Afr Med J. 2018;31(193):1–9. doi:10.11604/pamj.2018.31.193.10254
12. Addo HA, Hussen S, Chelkeba L. Childhood bacterial meningitis: antimicrobial use pattern and treatment outcomes: a prospective observational study. Clin Pract. 2018;15(SI):587–602.
13. Rabbani MA, Khan AA, Ali SS, et al. Spectrum of complications and mortality of bacterial meningitis: an experience from a developing country. Clin Infect Dis. 2001;53(580).
14. Ciofi M, Esposito S, Parola L, Ravà L, Gargantini G, Longhi R. In-hospital management of children with bacterial meningitis in Italy. Ital J Pediatr. 2014;40(87):1–7.
15. Gudina EK, Tesfaye M, Adane A, Lemma K, Shibiru T, Wieser A. Adjunctive dexamethasone therapy in unconfirmed bacterial meningitis in resource limited settings: is it a risk worth taking? BMC Neurol. 2016;16(153):1–8. doi:10.1186/s12883-016-0678-0
16. Turel O, Hospital A, Odyoloji A, Bakir M. Clinical characteristics and prognostic factors in childhood bacterial meningitis: a multicenter study. Balk Med J. 2013;30:80–84. doi:10.5152/balkanmedj.2012.092
17. Pelkonen T, Roine I, Monteiro L, et al. Risk factors for death and severe neurological sequelae in childhood bacterial meningitis in sub-Saharan Africa. Clin Infect Dis. 2009;48(8):1107–1110. doi:10.1086/597463
18. Namani S, Kuchar E. Mortality from bacterial meningitis in children in Kosovo. J Child Neurol. 2011;20(1):46–50.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.