Back to Journals » Clinical Ophthalmology » Volume 15
Treatment Outcome of Tubercular Uveitis in a High TB and HIV Setting: A Prospective Cohort Study
Authors Alli HD , Ally N , Mayet I , Joseph L, Omar S, Madhi S
Received 8 October 2021
Accepted for publication 10 December 2021
Published 30 December 2021 Volume 2021:15 Pages 4839—4846
DOI https://doi.org/10.2147/OPTH.S342268
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Hassan Dawood Alli,1 Naseer Ally,1 Ismail Mayet,1 Lavania Joseph,2 Shaheed Omar,2,3 Shabir Madhi4
1Department of Neurosciences, Division of Ophthalmology, St John Eye Hospital, Faculty of Health Sciences, University of the Witwatersrand, Soweto, Gauteng, South Africa; 2Centre for Tuberculosis, National TB Reference Laboratory, WHO TB Supranational Laboratory Network, National Institute for Communicable Diseases, National Health Laboratory Service, Johannesburg, South Africa; 3Department of Molecular Medicine & Haematology, School of Pathology, Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa; 4Medical Research Council Vaccines and Infectious Diseases Analytics Research Unit (VIDA), Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
Correspondence: Hassan Dawood Alli
Department of Neurosciences, Division of Ophthalmology, St John Eye Hospital, Faculty of Health Sciences, University of the Witwatersrand, 26 Chris Hani Road, Diepkloof, Soweto, 1862, Gauteng, South Africa
Tel +27833078152
Email [email protected]
Purpose: To determine the time to resolution of inflammation in tubercular uveitis (TBU) cases on standard anti-tubercular treatment. Sub-analysis of time to resolution according to HIV status was also performed.
Patients and Methods: A prospective cohort study of presumed idiopathic uveitis cases > 18 years underwent the tuberculin skin test, QuantiFERON-TB Gold test, and ocular tuberculosis (TB) polymerase chain reaction test. Adult TBU cases were treated with standard anti-tubercular therapy (and corticosteroids) for 9 months. Cases were followed-up for resolution of inflammation at 1.5, 3, 6, 9, 12 and 15 months post-diagnosis. Outcome measure was resolution of inflammation on ≤ 10 mg oral prednisone per day.
Results: Seventy-nine presumed idiopathic uveitis cases were enrolled in the study, 49 (62%) were diagnosed with TBU. The mean (SD) age of TBU cases at diagnosis was 41.8 (13.4) years. Using a multilevel mixed effects model, resolution was achieved at 6 months in the TBU cases (OR = 1.21; 95% CI, 1.03– 1.41; P = 0.017). Using generalized estimating equations, resolution was also achieved at 6 months in the TBU cases (OR = 1.21; 95% CI, 1.05– 1.39; P = 0.008). The HIV-positive cases (OR = 1.62; 95% CI, 1.13– 2.31; P = 0.008) and the HIV-negative cases (OR = 1.25; 95% CI, 1.06– 1.48; P = 0.009) achieved resolution at 9 months.
Conclusion: Resolution of inflammation in TBU cases on anti-tubercular treatment with corticosteroids was achieved at 6 months and maintained throughout the study. Our study suggests a minimum of 6 months treatment is required for significant resolution. Resolution of inflammation in HIV-positive and HIV-negative TBU cases needs to be further explored.
Keywords: Mycobacterium tuberculosis, anti-tubercular treatment, inflammation, HIV, resolution
Introduction
Tubercular uveitis (TBU) is defined as intraocular inflammation secondary to Mycobacterium tuberculosis infection.1 There is no gold standard for its diagnosis, and therefore the diagnosis of TBU is challenging.2 Tubercular uveitis is defined as definite if the microbiological/molecular tests of intraocular fluid are positive. However, the poor positivity rate (37.7 – 58.8%) of these tests has resulted in the diagnosis of TBU in most cases being mainly presumptive.3–5 A diagnosis of presumed TBU, following exclusion of other causes of uveitis, is often based on a combination of clinical signs of uveitis, tuberculin skin test (TST) or interferon-gamma release assay (IGRA) reactivity, chest radiography and/or non-ocular microbiological/molecular tests, and/or a positive response to anti-tubercular treatment.2,6
Treatment outcomes of TBU vary; this is partly due to the misdiagnosis of TBU, resistance to anti-tubercular treatment, variation in anti-tubercular treatment regimen (including treatment duration), and the variation in the concomitant corticosteroid-use to control inflammation.7 Good recovery rates on anti-tubercular treatment with corticosteroids have been reported in individuals with presumed (93–100%)8–10 and definite (90–92%)3,11 TBU. However, lower recovery rates (24% to 67%) in presumed TBU cases have been reported.12–14 The Collaborative Ocular Tuberculosis Study (COTS) reported a recovery rate of 87.0% in presumed TBU cases 6 months after completing anti-tubercular treatment.15 A follow-up of the same cohort of cases yielded a long-term recovery rate of 77.0% at 2 years.16 A meta-analysis of treatment outcomes reported an overall global recovery rate of 82% in TBU cases after completing anti-tubercular treatment.7
The optimal duration of anti-tubercular treatment yielding a good treatment response with minimal risk of adverse events has been debated. Alvarez et al and Vos et al mentioned that treatment for presumed TBU should be stopped in cases responding poorly after 2–4 months of anti-tubercular treatment.17,18 However, this may be too early to consider terminating treatment as other studies have reported poor treatment outcomes in cases treated for a shorter duration.12,19 Cases receiving anti-tubercular treatment and concomitant corticosteroids for 3 months had a lower recovery rate (50%) than cases treated for 9 months or longer (77%).19 A longitudinal study assessing recurrence rates in TBU cases treated with concomitant anti-tubercular treatment and corticosteroids for at least 12 months reported a low recurrence rate (16%).20 Another longitudinal study reported a recurrence rate of 30% in TBU cases treated with a similar regimen for 6 months.21
Although studies seem to suggest that a shorter anti-tubercular treatment duration for TBU is inadequate for a good outcome, longitudinal studies assessing the minimum treatment duration needed to achieve significant resolution are sparse. We, therefore, performed a prospective cohort study to determine the timeframe when significant resolution of inflammation in TBU cases on standard anti-tubercular treatment for 9 months occurs, and the duration of time resolution is maintained. Additionally, we sub-analyzed the timeframe for resolution according to HIV status.
Materials and Methods
We undertook a prospective, descriptive cohort study of individuals referred to the uveitis clinic at St John Eye Hospital from 2014 until 2018. St John Eye Hospital is a tertiary hospital in Johannesburg, South Africa, a country which is endemic for TB and which has the highest prevalence of human immunodeficiency virus (HIV) infection in the world.22,23 Individuals were included in the study if they, i. had active uveitis, ii. were ≥18 years of age, iii. had no prior or concurrent pulmonary or other extrapulmonary TB, and iv. had no previous anti-tubercular treatment. Excluded from the study were individuals that had: i. traumatic uveitis or post-surgical uveitis; ii. clinically diagnosed uveitis such as acute retinal necrosis (ARN), progressive outer retinal necrosis (PORN), cytomegalovirus (CMV) retinitis, Behcet’s disease, Vogt-Koyanagi-Harada (VKH) disease, Fuchs heterochromic iridocyclitis (FHI), sympathetic ophthalmia, HLA-B27-associated acute anterior uveitis (AAU), birdshot chorioretinopathy, multiple evanescent white dot syndrome (MEWDS), punctate inner choroidopathy (PIC) and acute posterior multifocal placoid pigment epitheliopathy (APMPPE); and iii. uveitis caused by toxoplasmosis, syphilis, systemic lupus erythematosus (SLE) and sarcoid on blood workup and chest radiography. Uveitis was defined as “presumed idiopathic” in participants included in the study, if no cause was found on clinical examination, blood workup and chest radiography.
The investigative work-up to exclude other causes of uveitis before study entry were 1. chest radiograph; and 2. laboratory evaluation, such as full blood count (FBC) and differential, erythrocyte sedimentation rate (ESR), human immunodeficiency virus (HIV) ELISA, CD4+ lymphocyte count if HIV-positive, rapid plasma reagin (RPR) and Treponema pallidum hemagglutination assay (TPHA), serum angiotensin converting enzyme (sACE) levels, Toxoplasma antibodies, antinuclear antibodies (ANA) and antineutrophil cytoplasmic antibodies (ANCA). We performed an ophthalmological assessment and investigative evaluation on all included participants which included 1. anterior and posterior segment examination; 2. Tuberculin skin test (Mantoux method [Statens Serum Institute, Copenhagen, Denmark])), QuantiFERON-TB Gold test (QFT-G [Cellestis Limited, Carnegie, Victoria, Australia]), and anterior chamber or vitreous tap which was sent for PCR to identify MTB (Xpert MTB/RIF [Cepheid, Sunnyvale, CA], in-house MPB 64 PCR and in-house IS6110 PCR). Participants presenting with bilateral uveitis had ocular fluid from one eye (the eye with the worse visual acuity and inflammatory activity) sampled for PCR testing. Based on the results of the investigative evaluation, TBU cases were identified from the cohort of presumed idiopathic uveitis cases.
We diagnosed TBU as follows: i. Confirmed or definite TBU if TB PCR was positive and possible or presumed TBU if TST and/or QFT-G were positive in the presence of uveitis; and ii. All other causes of uveitis were excluded. A TST ≥ 10 mm induration 48 hours after intradermal injection in HIV-negative patients was considered positive for TBU, and in HIV-positive patients ≥5mm.24 The TB antigen value minus the negative control value ≥0.35 IU/mL in the QFT-G test was considered positive for TBU. The TST was performed after the QFT test. The IS6110 and MPB64 gene sequence of Mycobacterium tuberculosis were the targets used for PCR.
All cases diagnosed with TBU were treated with fixed dose combination anti-tubercular treatment. Rifafour e-275 (Rifampicin [R] 150 mg, Isoniazid [H] 75 mg, Pyrazinamide [Z] 400 mg, and ethambutol hydrochloride [E] 275 mg) was prescribed for the first 2 months, and RIFINAH-150 (Rifampicin 150 mg and Isoniazid 100 mg) or RIFINAH-300 (Rifampicin 300 mg and Isoniazid 150 mg) for the remaining 7 months. The total duration of anti-tubercular treatment was 9 months, and the dose was weight dependent. To control the inflammatory activity, TBU cases were additionally treated with corticosteroids during and after completion of anti-tubercular treatment. Topical corticosteroids were prescribed for TBU cases with anterior uveitis; oral and/or periocular corticosteroids for intermediate and posterior uveitis; and oral and/or topical and/or periocular corticosteroids for panuveitis. Periocular steroids were mainly advocated for cystoid macular oedema. Tubercular uveitis cases were followed up for a further 6 months after completion of anti-tubercular treatment, totaling 15 months follow-up.
We assessed all TBU cases for intraocular inflammation at 1.5, 3, 6, 9, 12 and 15 months post-diagnosis. At each follow-up visit, the grading and outcome of intraocular inflammation was according to the standardization of uveitis (SUN) criteria.25 Resolution, which was the outcome measured during the study, was defined as no intraocular inflammation on ≤10 mg oral prednisone.25 Remission was defined as no inflammatory activity and being on ≤10 mg oral prednisone for 6 months duration after completion of 9 months anti-tubercular treatment.25 Participants who had bilateral uveitis were regarded as having achieved resolution or remission when they had no intraocular inflammation in both eyes.
The study was approved by the Human Research Ethics Committee (HREC) of the University of the Witwatersrand (M130942) and followed the tenets of the Declaration of Helsinki. Informed consent was obtained from all included participants prior to study entry.
Statistical Analysis
All data were collected and managed using the REDCap (Research Electronic Data Capture) tools hosted at the University of the Witwatersrand.26,27 Data was analysed in Stata 16.1 (StataCorp, College Station, Texas). Continuous variables were summarized as means (standard deviations) if they were normally distributed and medians (interquartile range) if they were skewed. Missing data for the longitudinal analysis of resolution was addressed using multiple imputation with chained equations as the pattern of missingness was non-monotone. For the longitudinal analysis of resolution as the outcome across all visits we used a two-level multilevel mixed effects model as well as generalized estimating equations, the former to evaluate the individual-level response and the latter to evaluate the population-level response. An alpha-level of 0.05 was taken to be statistically significant.
Results
Seventy-nine presumed idiopathic uveitis cases were enrolled in the study; 49 (62%) were diagnosed with TBU of whom 41 (52%) cases were presumed TBU and 8 (10%) confirmed TBU (Table 1). The mean (SD) age of the TBU cases at diagnosis was 41.8 (13.4) years. Cases with TBU were more likely to be female (82%) and HIV-negative (76%), and to have chronic uveitis (73%) (Table 1). Ninety-six percent of the TBU anatomical classification type was panuveitis and 69% of cases had multifocal choroiditis (Table 1). Of the 49 TBU cases treated with anti-tubercular medication, concomitant oral corticosteroids were initiated in 46 (94%) cases, of which 43 cases were additionally treated with topical corticosteroids and six cases with periocular corticosteroids; one TBU case was treated with topical corticosteroids only (Table 1). Two TBU cases had no concomitant corticosteroid treatment. Thirty-five TBU cases (71%) completed study follow-up through to 15-months post-diagnosis, of whom 15 (43%) were in remission (Table 1).
 |  |
Table 1 Baseline and Clinical Characteristics, and Treatment Outcomes of Tubercular Uveitis Cases |
Using a multilevel mixed effects model for the analysis of repeated outcomes at the individual level, the TBU cases achieved significant resolution at 6 months post-diagnosis (OR = 1.21; 95% CI, 1.03–1.41; P=0.017) (Table 2). Resolution was maintained at subsequent visits (Table 2). This relationship was significant in both the univariate and multivariate models.
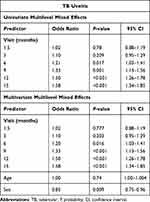 |
Table 2 Individual-Level Response Using Two-Level Multilevel Mixed Effects Model |
When using generalized estimating equations to assess the overall TBU population response (Table 3), the TBU population achieved significant resolution at 6 months post-diagnosis (OR = 1.21; 95% CI, 1.05–1.39; P=0.008). Again, using this method of analysis resolution was maintained at subsequent visits (Table 3). This association was maintained in both the univariate and multivariate analyses (Table 3).
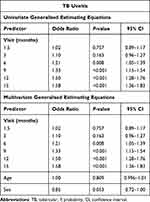 |
Table 3 Population-Level Response Using Generalized Estimating Equation |
A time-series plot, after multiple imputation, showed increasing number of TBU cases achieving resolution from 1.5 months through to 3-, 6-, 9-, 12-, and 15-months (Figure 1).
 |
Figure 1 Proportion of tubercular uveitis (TBU) cases achieving resolution at follow-up visits. |
Using a multilevel mixed effects model for the analysis of repeated outcomes, the HIV-positive cases (OR = 1.62; 95% CI, 1.13–2.31; P=0.008) and the HIV-negative cases (OR=1.25; 95% CI, 1.06–1.48; P=0.009) achieved significant resolution at 9 months post-diagnosis (Table 4).
 |
Table 4 Multilevel Mixed Effects Model Comparing the HIV-Positive and HIV-Negative Tubercular Uveitis Cases |
Discussion
Our study provides a timeframe for when a significant proportion of TBU cases will achieve resolution, and suggests a minimum duration required for anti-tubercular treatment with corticosteroid medication to be effective.
Our study measured outcomes in terms of time to resolution of inflammation in TBU cases on 9 months anti-tubercular treatment and corticosteroids. In both the models used for analysis, the odds of resolution of inflammation increased in the follow-up visits and reached statistical significance at 6 months post-diagnosis of TBU. Also, resolution was subsequently significantly maintained throughout the study.
The resolution of inflammation at 6 months in our study suggests that the minimum duration of anti-tubercular treatment should be 6 months. However, since all the TBU cases in our study were treated for 9 months, we do not know if the same level of significance would have been maintained throughout the study if all participants had been treated with 6 months of anti-tubercular treatment. Although studies in which TBU cases treated for 6 months with anti-tubercular treatment reported good treatment outcomes,7–9,11 Ang et al reported an eleven-fold decrease in the likelihood of recurrence of inflammation in TBU cases treated with anti-tubercular treatment for ≥ 9 months compared to cases treated < 9 months.12 Anti-tubercular treatment is associated with significant adverse effects, including optic neuropathy, which can be minimized with a shorter duration of exposure to anti-tubercular treatment.2 Therefore, it is important to determine if 6 months of anti-tubercular treatment will have the same effect as 9 months of anti-tubercular treatment. Large prospective cohort studies with longer follow-up comparing 6 months versus 9 months anti-tubercular treatment are needed to compare the length of time significant resolution can be maintained.
Our study also highlights the issue regarding the concomitant use of corticosteroids to control inflammation in TBU. Corticosteroids were prescribed in 47 of the 49 TBU cases in our study. Concomitant corticosteroids are advocated to limit ocular tissue damage caused by the immune-mediated reaction to Mtb bacilli, Mtb antigens or retinal antigens.2,28 Most studies report the use of corticosteroids, together with anti-tubercular medication, in the treatment of TBU; however, there is no standardization in the corticosteroid regimen (route, dose and duration).8,9,12,29 Although the corticosteroid regimen in our study varied, the outcome measured on corticosteroid treatment was standardized; resolution in our study was defined as minimal or no oral corticosteroids (≤10mg) according to the SUN classification.25
The resolution of inflammation in the HIV-positive group and the HIV-negative group was achieved at 9 months post-diagnosis. However, there were a small number of cases in the two HIV groups (especially in the HIV-positive group); therefore, these results, although significant, should be treated with caution. To my knowledge, there are no studies comparing resolution or recovery rates between these two groups. This needs to be explored in large prospective multicenter TBU studies. The small proportion of HIV-positive individuals diagnosed with TBU in our study highlights the issue of decreased sensitivity of the TST and QFT-G in immunosuppressed individuals.30,31 Although a lower (≥5mm) TST measurement corrected for this, it is possible that TBU may have been underdiagnosed in HIV-positive individuals.
There was a higher proportion of TBU cases with chronic uveitis in our study. Chronicity highlights the reluctance of the physicians at our hospital to diagnose TBU and initiate anti-tubercular treatment. Chronicity of uveitis, before anti-tubercular treatment is started, is associated with poor visual outcomes due to complications.32–34 Thus, a lower threshold for the diagnosis of TBU, and initiation of anti-tubercular treatment to control inflammation and prevent visual-impairing complications at our institution is needed.
Different anatomical classification types of TBU are associated with different treatment outcomes. Depending on the study, higher recurrence of inflammation has been associated with either anterior uveitis, intermediate uveitis or posterior uveitis.12,20,21 There was a high proportion of TBU cases that had panuveitis in our study, and this may have been due to referral bias from the general clinic to the Uveitis clinic at our hospital; cases with panuveitis and poor visual function may have preferably been referred for specialist assessment. Because of the very small number of cases with the other anatomical classification types, it was not possible to do subgroup analysis comparing resolution between the different anatomical types.
Limitations of the study are: (1) the limited number of cases; (2) the limited follow-up; (3) the missing data in the follow-up visits; and (4) the concomitant corticosteroid-use. (1) The limited number of cases meant that subgroup analyses, such as comparing the different choroiditis types and anatomical phenotypes, was not possible. Although we compared the HIV groups, a meaningful conclusion could not be drawn because of the small number of cases in each group. (2) A longer follow-up would have enabled us to see for how long significant resolution would have been maintained. (3) Although there were missing data in the follow-up visits of the cases in the study, these were addressed by using multiple imputation in the statistical analyses. (4) Although corticosteroids were prescribed in most of the TBU cases, the outcome measure in terms of the resolution of inflammation (on ≤10 mg corticosteroids) was standardized according to the SUN criteria.25 Strengths of the study are that it is a prospective cohort study where the evaluation of all cases and collection of all the data were done by one Ophthalmologist (HA), and the anti-tubercular treatment regimen and outcome measure were standardized.
Conclusion
Resolution of inflammation in TBU achieved at 6 months suggests that treating these cases with anti-tubercular treatment for at least 6 months is advisable. Future large prospective cohort studies are needed to compare 6 months to 9 months of anti-tubercular treatment to determine whether stopping treatment at 6 months will maintain resolution. Additionally, large prospective studies are warranted comparing resolution of inflammation between HIV-positive and HIV-negative individuals.
Acknowledgments
The authors would like to acknowledge the National TB Reference Laboratory staff.
Disclosure
Professor Shabir Madhi reports grants, personal fees from BMGF, grants from Pfizer, grants from GSK, grants from Minervax, grants from South Africa Medical Research Council, grants from National Research Foundation, outside the submitted work. The authors declare no other conflicts of interest in this work.
References
1. Agrawal R, Agarwal A, Jabs DA, et al. Standardization of nomenclature for ocular tuberculosis - results of Collaborative Ocular Tuberculosis Study (COTS) workshop. Ocul Immunol Inflamm. 2019;12:1–11. doi:10.1080/09273948.2019.1653933.
2. Gupta V, Gupta A, Rao NA. Intraocular tuberculosis - an update. Surv Ophthalmol. 2007;52(6):561–587. doi:10.1016/j.survophthal.2007.08.015
3. Gupta V, Arora S, Gupta A, Ram J, Bambery P, Sehgal S. Management of presumed intraocular tuberculosis: possible role of the polymerase chain reaction. Acta Ophthalmol Scand. 1998;76(6):679–682. doi:10.1034/j.1600-0420.1998.760609.x
4. Sudheer B, Lalitha P, Kumar AL, Rathinam S. Polymerase chain reaction and its correlation with clinical features and treatment response in tubercular uveitis. Ocul Immunol Inflamm. 2018;26(6):845–852. doi:10.1080/09273948.2017.1287925
5. Arora SK, Gupta V, Gupta A, Bambery P, Kapoor GS, Sehgal S. Diagnostic efficacy of polymerase chain reaction in granulomatous uveitis. Tuber Lung Dis. 1999;79(4):229–233. doi:10.1054/tuld.1999.0210
6. Gupta B, Agrawal R, Swampillai AJ, et al. Ocular manifestations of tuberculosis: an update. Expert Rev Ophthalmol. 2016;11(2):145–154. doi:10.1586/17469899.2016.1152887
7. Alli HD, Ally N, Mayet I, Dangor Z, Madhi SA. Global prevalence and clinical outcomes of tubercular uveitis: a systematic review and meta-analysis. Surv Ophthalmol. 2021. doi:10.1016/j.survophthal.2021.10.001
8. Manousaridis K, Ong E, Stenton C, Gupta R, Browning AC, Pandit R. Clinical presentation, treatment, and outcomes in presumed intraocular tuberculosis: experience from Newcastle upon Tyne, UK. Eye. 2013;27(4):480–486. doi:10.1038/eye.2013.11
9. Shakarchi FA. Mode of presentations and management of presumed tuberculous uveitis at a referral center. Iraqi Postgrad Med J. 2015;14(1):91–95.
10. Chung CY, Li KKW. The efficacy of latent tuberculosis treatment for immunocompetent uveitis patients with a positive T-SPOT.TB test: 6-year experience in a tuberculosis endemic region. Int Ophthalmol. 2018;38(6):2273–2282. doi:10.1007/s10792-017-0716-y
11. Balne PK, Modi RR, Choudhury N, et al. Factors influencing polymerase chain reaction outcomes in patients with clinically suspected ocular tuberculosis. J Ophthalmic Inflamm Infect. 2014;4(1):10. doi:10.1186/1869-5760-4-10
12. Ang M, Hedayatfar A, Wong W, Chee SP. Duration of anti-tubercular therapy in uveitis associated with latent tuberculosis: a case-control study. Br J Ophthalmol. 2012;96(3):332–336. doi:10.1136/bjophthalmol-2011-300209
13. Ng KK, Nisbet M, Damato EM, Sims JL. Presumed tuberculous uveitis in non-endemic country for tuberculosis: case series from a New Zealand tertiary uveitis clinic. Clin Exp Ophthalmol. 2017;45(4):357–365. doi:10.1111/ceo.12881
14. Krassas N, Wells J, Bell C, Woodhead M, Jones N. Presumed tuberculosis-associated uveitis: rising incidence and widening criteria for diagnosis in a non-endemic area. Eye. 2018;32(1):87–92. doi:10.1038/eye.2017.152
15. Agrawal R, Gunasekeran DV, Grant R, et al. Clinical features and outcomes of patients with tubercular uveitis treated with antitubercular therapy in the Collaborative Ocular Tuberculosis Study (COTS)-1. JAMA Ophthalmol. 2017;135(12):1318–1327. doi:10.1001/jamaophthalmol.2017.4485
16. Agarwal A, Agrawal R, Raje D, et al. Twenty-four month outcomes in the Collaborative Ocular Tuberculosis Study (COTS)-1: defining the “Cure” in ocular tuberculosis. Ocul Immunol Inflamm. 2020:1–9. doi:10.1080/09273948.2020.1761401.
17. Alvarez GG, Roth VR, Hodge W. Ocular tuberculosis: diagnostic and treatment challenges. Int J Infect Dis. 2009;13(4):432–435. doi:10.1016/j.ijid.2008.09.018
18. Vos AG, Wassenberg MWM, de Hoog J, Oosterheert JJ. Diagnosis and treatment of tuberculous uveitis in a low endemic setting. Int J Infect Dis. 2013;17(11):e993–e999. doi:10.1016/j.ijid.2013.03.019
19. Agrawal R, Gupta B, Gonzalez-Lopez JJ, et al. The role of anti-tubercular therapy in patients with presumed ocular tuberculosis. Ocul Immunol Inflamm. 2015;23(1):40–46. doi:10.3109/09273948.2014.986584
20. Bansal R, Gupta A, Gupta V, Dogra MR, Bambery P, Arora SK. Role of anti-tubercular therapy in uveitis with latent/manifest tuberculosis. Am J Ophthalmol. 2008;146(5):772–779. doi:10.1016/j.ajo.2008.06.011
21. Tomkins-Netzer O, Leong BCS, Zhang X, Lightman S, McCluskey PJ; Sydney-London Latent Ocular TB Study Group. Effect of antituberculous therapy on uveitis associated with latent tuberculosis. Am J Ophthalmol. 2018;190:164–170. doi:10.1016/j.ajo.2018.03.032
22. UNAIDS programme coordinating board sees South Africa’s AIDS response first-hand; 2018. Available from: https://www.unaids.org/en/resources/presscentre/featurestories/2018/november/pcb-field-visit-south-africa.
23. World Health Organization. Global tuberculosis report 2019. Available from: http://www.who.int/tb/publications/global_report/en/.
24. Centers for disease control and prevention. Fact sheets | Testing & Diagnosis | Fact sheet - Tuberculin skin testing | TB. CDC; 2020. Available from: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.htm.
25. Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. 2005;140(3):509–516. doi:10.1016/j.ajo.2005.03.057
26. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi:10.1016/j.jbi.2008.08.010
27. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
28. Basu S, Wakefield D, Biswas J, Rao NA. Pathogenesis and pathology of intraocular tuberculosis. Ocul Immunol Inflamm. 2015;23(4):353–357. doi:10.3109/09273948.2015.1056536
29. Kee AR, Gonzalez-Lopez JJ, Al-Hity A, et al. Anti-tubercular therapy for intraocular tuberculosis: a systematic review and meta-analysis. Surv Ophthalmol. 2016;61(5):628–653. doi:10.1016/j.survophthal.2016.03.001
30. Huebner RE, Schein MF, Bass JB. The tuberculin skin test. Clin Infect Dis. 1993;17(6):968–975. doi:10.1093/clinids/17.6.968
31. Leidl L, Mayanja-Kizza H, Sotgiu G, et al. Relationship of immunodiagnostic assays for tuberculosis and numbers of circulating CD4+ T-cells in HIV infection. Eur Respir J. 2010;35(3):619–626. doi:10.1183/09031936.00045509
32. Patel SS, Saraiya NV, Tessler HH, Goldstein DA. Mycobacterial ocular inflammation: delay in diagnosis and other factors impacting morbidity. JAMA Ophthalmol. 2013;131(6):752–758. doi:10.1001/jamaophthalmol.2013.71
33. Gunasekeran DV, Gupta B, Cardoso J, Pavesio CE, Agrawal R. Visual morbidity and ocular complications in presumed intraocular tuberculosis: an analysis of 354 cases from a non-endemic population. Ocul Immunol Inflamm. 2018;26(6):865–869. doi:10.1080/09273948.2017.1296580
34. Agrawal RM, Gunasekeran DVM, Agarwal AM, et al. Visual morbidity in ocular tuberculosis - Collaborative Ocular Tuberculosis Study (COTS)-1: report #6. Ocul Immunol Inflamm. 2020:1–9. doi:10.1080/09273948.2020.1774905.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
