Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Treatment of the Nasolabial Fold Using a Hyaluronic Acid-Based Filler with eXcellent Three-Dimensional Reticulation (XTR™) Technology: A Retrospective Study
Authors Abdulbaky AHI, Wong V
Received 26 September 2023
Accepted for publication 17 December 2023
Published 8 March 2024 Volume 2024:17 Pages 573—579
DOI https://doi.org/10.2147/CCID.S427736
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Rungsima Wanitphakdeedecha
Abdulrahman Hassan Imam Abdulbaky, Vincent Wong
TriVin Aesthetics, London, UK
Correspondence: Vincent Wong, TriVin Aesthetics, 10 Harley Street, London, W1G 9PF, UK, Tel +44 207 299 0380, Fax +44 207 467 8312, Email [email protected]
Purpose: The physicochemical characteristics of hyaluronic acid (HA) fillers can affect the outcome of minimally invasive aesthetic treatments. The effect of a novel HA-based filler manufactured with a novel 3D structure (XTRTM technology) was assessed in the nasolabial fold.
Patients and Methods: We performed a retrospective study of patients who underwent treatment of the nasolabial fold with a novel HA filler in our clinic. Treatment outcome was assessed at 3 and 6 months with wrinkle score, GAIS, and VAS.
Results: Eighteen patients were injected with the novel HA filler in the nasolabial fold. At six months, mean wrinkle scores were improved on both sides compared to baseline. GAIS and VAS were high at three and six months.
Conclusion: The HA-based filler manufactured with XTRTM technology is safe and effective in treating the nasolabial fold. Good aesthetic results were seen for up to 6 months. Patient satisfaction was high during the entire follow-up.
Keywords: filler injection, face rejuvenation, monophasic, crosslinking technology
Introduction
Hyaluronic acid (HA)-based fillers are a growing trend for minimally invasive rejuvenation. The physical properties of these hydrogels vary according to the chosen manufacturing approach allowing for a wider range of clinical applications. Indeed, commercially available HA-base fillers present distinct properties – HA concentration, degree of crosslinking, cohesivity levels, and rheology properties – depending on their formulation.1 In addition, some fillers also incorporate lidocaine with a dual effect: to reduce pain due to its anesthetic properties and to diminish bruising and swelling due to antihistamine action.2
Recently, the development of a new technology – eXcellent Three-dimensional Reticulation – has allowed to formulate a range of HA-based fillers with a particularly stable 3D structure.1 In brief, the innovative manufacturing process can be broken down into 3 phases: pretreatment, crosslinking, and purification. The pretreatment phase is a thermal-controlled fracturing step that forms skin-compatible HA chains of medium and high molecular weight. This mixture of HA chains is bound into a stable matrix using a crosslinking agent during the crosslinking phase. This step is optimized, meaning there is minimal presence of free HA chains in the final product. The most common molecule used during crosslinking is BDDE (1,4-butanediol diglycidyl ether). The last step is the purification of the product, that is, the elimination of unreacted BDDE and byproducts. Each batch of XTRTM fillers is set to have minimal levels of unreacted BDDE (<2 ppm, as per the recommendation of the US Food and Drug Administration).
The entire manufacturing process is developed to achieve optimal levels of size, crosslinking, and purity of HA fillers, critical to enhancing its biological effects on the skin, namely filling properties, and durability. In addition, the enhanced crosslinking step is particularly important to achieve stable viscoelastic properties that reduce extrusion during injection. The technology allows the manufacturing of high-quality gels with varying concentrations of HA and different G’ to better adapt to the patients’ needs.1 Fillers with higher HA concentration and storage modulus (G’) – such as Definisse core + lidocaine – are classified as volumizers, while Definisse restore + lidocaine is more suited for tissue projection and correction of facial defects with minimal amount of product.1,3,4
Here, we describe our experience with Definisse restore + lidocaine on the correction of the nasolabial fold by performing a retrospective analysis of patients who came to our clinic requesting this minimally invasive treatment.
Materials and Methods
Study Design
This is a single-center, observational, retrospective study conducted on patients who underwent treatment of the nasolabial fold with Definisse restore plus lidocaine (Relife srl, Italy) between 1st November 2021 and 28th February 2022. Follow-up was done at two weeks, three, and six months.
Patients
Patient eligibility criteria were the following: being female, aged between 25 and 65 years old, not pregnant/breastfeeding, without systemic illnesses, body dysphoria, or mental health concerns, with realistic expectations, and without fillers in the area for at least 18 months prior to enrollment.
Injection Technique
Injections were performed with Definisse restore plus lidocaine filler (Relife S.r.l., Italy): 0.2mL slow bolus to deep pyriform space (supraperiosteal), and 0.3mL to nasolabial fold (retrograde linear technique) in mid-dermis. Definisse restore plus lidocaine is classified according to the Medical Device Directive 93/42/EEC as a class III medical device. This novel filler is manufactured – using a unique XTR (Excellent Three-Dimensional Crosslinking) process previously described – as a matrix of medium and high molecular weight HA chains (medium: 2.5 × 106–3.2 × 106 Dalton, and high: 3.2 × 106–3.5 × 106 Dalton); the final concentration of HA is 23 mg/mL.1
Assessment
Wrinkle Score
Score is determined by a combination of depth, length, and width of wrinkle/fold in the region of interest. The score was obtained by comparing the subject against a population based on matching correspondence of age, gender, and skin type. Score of zero is average, +5 indicates top 16% of the population, +10 indicates top 2.5% of the population, −5 indicates bottom 16% of the population, −10 indicates bottom 2.5% of the population.
GAIS
The Global Aesthetic Improvement Scale (GAIS) assesses the level of improvement following treatment on a 5-point scale: exceptional improvement (1), moderate improvement (2), slight improvement (3), no change (4), or worse (5).5 Patients performed the self-assessment 3 and 6 months after the intervention.
VAS
Patient satisfaction with treatment was assessed with a visual analogue scale previously described.6 The assessments were done at baseline, 3 and 6 months after injection. The range of VAS scores was 0 to 10: minimum satisfaction corresponds to a score of 0 and maximum satisfaction is represented by a score of 10.
Statistical Analysis
SPSS software (version 17.00, SPSS, Chicago, USA) was used for statistical analysis. Data are presented as mean, SD, number, or percent. Comparisons between baseline and other time points were performed using unpaired-samples t-test. A value of P < 0.05 was considered statistically significant.
Results
We performed injection of the nasolabial fold with the novel HA-based filler in 18 patients (mean age, 40.3, Table 1, all female). Of note, patients presented a low wrinkle score both on the left and right sides (mean WSL −3.33 ± 1.28 and mean WSR −4.33 ± 1.91) at baseline (Table 1). The range of WSL and WSR positions all treated patients in the bottom 16% of the population as per WS definition. VAS confirmed that before treatment patients were unsatisfied with their appearance (mean VAS 4.83 ± 0.86).
 |
Table 1 Baseline Demographics and Characteristics |
The injection was successful in all patients. Figures 1 and 2 show representative images of pre- and post-treatment at the 6-month follow-up. No serious complications were seen: only one case of bruising which resolved without medical intervention.
After 6 months, WS were significantly increased on both sides (Figure 3). On the left side, mean WSL was rated as 3.17 ± 1.54 compared to −3.33 ± 1.28 registered at baseline (p < 0.0001); similarly, on the right side, mean WSR was 4.50 ± 2.04 vs −4.33 ± 1.91 seen at baseline (p < 0.0001). Considering the definition of the scale used, this result positions patients at the top 16% of the population on the right side and between average and top 16% on the left side.
 |
Figure 3 Treatment outcome assessed with wrinkle score scale. ****p < 0.0001. Abbreviations: WSL, wrinkle score left; WSR, wrinkle score right. |
Self-assessment with the GAIS showed that 17 out of 18 patients felt their aesthetic improvement was exceptional or moderate at 3 and 6 months (Table 2). Only one patient rated aesthetic improvement as slight at 3 months and one failed to see change at 6 months.
 |
Table 2 Aesthetic Improvement as Assessed by the Global Aesthetic Improvement Scale (GAIS) at Three and Six Months (Self-Assessment) |
Regarding the VAS assessment, a statistically significant increase in VAS scores was reported both at the 3- and 6-month follow-up, which demonstrates an increase in patient satisfaction. Mean VAS scores were 8.33 ± 0.77 at 3 months, 7.83 ± 0.86 at 6 months compared to 4.83 ± 0.86 at baseline (p < 0.0001; Figure 4). No statistically significant differences were seen between 3-month and 6-month VAS scores.
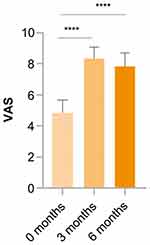 |
Figure 4 Treatment satisfaction assessed with visual analog scale (VAS). ****p < 0.0001. |
Discussion
This retrospective study describes the outcome of nasolabial fold correction with a novel HA-based filler in 18 patients with a follow-up of 6 months. To our knowledge, this is the first study to report the results of performing nasolabial correction with the monophasic HA-based filler formulated with XTRTM technology (Definisse restore plus lidocaine). Recently, a study investigated the effect of another XTR-manufactured filler – Definisse core +lidocaine – on facial volume loss and skin quality. This prospective study involving 50 female subjects showed a significant improvement in midface volume deficit and skin quality at the 6-month follow-up.7 However, this study differs from ours since the filler used is formulated with higher HA concentration and higher storage modulus G’; the injection site and technique used were also different (midface with 6-point injection versus nasolabial).
No safety concerns arose following treatment with Definisse restore plus lidocaine throughout the entire study duration, as seen by the absence of major complications. Indeed, only one minor complication was seen in one patient (bruising), which resolved without medical intervention.
The novel Definisse restore plus lidocaine was injected in the nasolabial fold with good aesthetic results. At six months, mean wrinkle scores were improved on both sides compared to baseline. Patients were very pleased with the intervention, as seen by high scores on the GAIS and with VAS as early as three months, which persisted at the latest follow-up (6 months).
Results from our study agree with the aesthetic improvement and safety profile of HA fillers in the nasolabial fold reported previously.8–10 However, a recent meta-analysis highlighted the importance of choosing a suitable filler to achieve better outcomes.11 Indeed, the authors show that physical differences between fillers can affect the clinical efficacy of the intervention. In particular, monophasic fillers – such as the one used in this study – show better aesthetic results than biphasic fillers. The filler under investigation was also formulated with lidocaine to reduce inflammation and pain during injection, which seems to have benefited patients’ experience, as seen by the almost entire absence of complications. It is expected that the more robust filler structure – given by the novel manufacturing technology – will extend the aesthetic effect of this treatment. Still, a longer follow-up is needed to draw more definite conclusions.
Limitations of this study include study design, relatively low number of subjects, and short follow-up. Larger studies with a control group would allow to better evaluate the efficacy of the novel HA-based filler in the correction of the nasolabial fold. However, the high patient satisfaction achieved and the favorable safety profile that emerged from this study suggest that the novel filler under investigation is a good alternative for correcting the nasolabial fold.
Conclusion
The HA-based filler manufactured with XTRTM technology is safe and effective in treating the nasolabial fold. Good aesthetic results were seen for up to 6 months. Patient satisfaction was high during the entire follow-up.
Ethical Approval
The study was conducted according to the Declaration of Helsinki. The authors confirm that ethical approval from the local institutional review board is not required as the data presented refers to standard routine clinical practice without deviating from the instructions for use of the CE-marked medical device. All patients signed an informed consent form. Patients in Figures 1 and 2 gave written permission for image publication.
Acknowledgments
Editorial assistance was provided by ERA ms S.r.l. and funded by Relife S.r.l.
Disclosure
V.W. discloses personal fees (key opinion leader/speaker) from Relife. A. H. I. A. has nothing to disclose for this work.
References
1. Salti G, Fundarò SP. Evaluation of the rheologic and physicochemical properties of a novel hyaluronic acid filler range with eXcellent Three-Dimensional Reticulation (XTRTM) technology. Polymers. 2020;12(8):1644. doi:10.3390/polym12081644
2. Smith L, Cockerham K. Hyaluronic acid dermal fillers: can adjunctive lidocaine improve patient satisfaction without decreasing efficacy or duration? PPA. 2011:133. doi:10.2147/PPA.S11251
3. RELIFE S.R.L. DefinisseTM Core Filler + Lidocaine. Instructions for Use (IFU).
4. RELIFE S.R.L. DefinisseTM Restore Filler + Lidocaine. Instructions for Use (IFU).
5. Muti GF. Midface 3D restoration with an innovative high G’ filler. J Cosmet Dermatol. 2021;20(S2):7–11. doi:10.1111/jocd.14282
6. Brumfitt SM, Sheeran P. The development and validation of the Visual Analogue Self-Esteem Scale (VASES) 1. Br J Clin Psychol. 1999;38(4):387–400. doi:10.1348/014466599162980
7. Salti G, Siquier-Dameto G, Rharbaoui S, Hernandez Malgapo DM, Innocenti S, Manni M. An interim 6-month analysis of the dermatologic effects and midface volume correction with XTRCL filler in a Prospective, Single-Center Study. Dermatol Surg. 2023;49(10):943–948. doi:10.1097/DSS.0000000000003878
8. Ehlinger‐David A, Gorj M, Braccini F, et al. A prospective multicenter clinical trial evaluating the efficacy and safety of a hyaluronic acid-based filler with
9. Lee JH, Kim SH, Park ES. The efficacy and safety of HA IDF plus (with lidocaine) versus HA IDF (without lidocaine) in nasolabial folds injection: a Randomized, Multicenter, Double-Blind, Split-Face Study. Aesth Plast Surg. 2017;41(2):422–428. doi:10.1007/s00266-016-0769-8
10. Hu Y, Liu Z, Wang Y, et al. Efficacy and safety of two hyaluronic acid fillers with different injection depths for the correction of moderate-to-severe nasolabial folds: a 52-week, prospective, randomized, double-blinded study in a Chinese population. J Cosmet Dermatol. 2022;21(3):940–948. doi:10.1111/jocd.14744
11. Peng T, Hong W, Fang J, Luo S. The selection of hyaluronic acid when treating with the nasolabial fold: a meta-analysis. J Cosmet Dermatol. 2022;21(2):571–579. doi:10.1111/jocd.14710
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


