Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Treatment of Striae Distensae with Filler Injection: A Systematic Review
Authors Alsharif SH, Alghamdi AS , Alhumaidi WA, AlRobaish OA, Al Hamoud MH , Alruwaili AS, AlQefari GB, Almutairi RT
Received 22 January 2023
Accepted for publication 21 March 2023
Published 2 April 2023 Volume 2023:16 Pages 837—845
DOI https://doi.org/10.2147/CCID.S405715
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Sahar Hasan Alsharif,1,* Asail Saeed Alghamdi,2,* Wareef Abdulkarim Alhumaidi,3 Omar Abdulaziz AlRobaish,4 Mohammad Hassan Al Hamoud,5 Asma Saleh Alruwaili,6 Ghaida B AlQefari,7 Rahaf Tayi Almutairi8
1Department of Dermatology, Ministry of Health, Makkah, Saudi Arabia; 2Department of Medicine, Albaha University, Albaha, Saudi Arabia; 3Department of Medicine, Taif University, Taif, Saudi Arabia; 4Department of Dermatology, King Fahad Specialist Hospital, Buraidah, Saudi Arabia; 5Department of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 6Department of Medicine, Northern Border University, Arar, Saudi Arabia; 7Department of Medicine and Surgery, Qassim University, Qassim, Saudi Arabia; 8Department of Medicine, King Faisal University, Al Ahsaa, Saudi Arabia
*These authors contributed equally to this work
Correspondence: Sahar Hasan Alsharif, Department of Dermatology, Ministry of Health, Makkah, Saudi Arabia, Tel +966555539141, Email [email protected]
Background: Stretch marks, also known as striae cutis distensae (SD), are visible linear scars that occur in regions of dermal damage due to skin stretching. Stretch marks are not serious health issues, but they may have a major psychological effect on patients. Due to poor skin color improvement or prolonged skin atrophy, there is no standard treatment for SD. Fillers have been studied for their effectiveness in the treatment of SD.
Objective: This systematic review aims to determine the efficacy of fillers on SD.
Methods: This systematic review is reported following PRISMA guidance. We included all relevant articles published up to November 2022 in the following electronic databases: Science Direct, Midline, the Web of Science, CINAHL, and Google Scholar. The initial search yielded 119, of which seven were included after applying inclusion and exclusion criteria.
Results: The systematic review included a total of 184 female participants who were over the age of 18 years old. Three studies used jet volumetric remodeling (JVR) to inject HA pneumatically. One study injected polycaprolactone filler. One study used calcium hydroxylapatite, micro-needling, and ascorbic acid. MFU-V and CaHA were given in one study. One study delivered MFU-V using micro-focused ultrasound. All studies showed that it reduces SD with only mild, temporary side effects. More favor was given to combining CaHA and MFU-V, which had the fewest side effects compared to other dermal fillers.
Conclusion: As monotherapy or combination therapy, injectable dermal fillers may treat SD with minimal adverse effects. We suggest that more RCTs look into injectable dermal filler to find out what is best for patients with SD and compare it to other treatment methods in terms of results, costs, and side effects to provide satisfactory practice and basic guideline interventions for these cases.
Keywords: striae distensae, Fillers, SD, Efficacy
Introduction
Stretch marks, also known as striae cutis distensae (SD), are visible linear scars that form in areas of dermal damage as a result of stretching of the skin.1 Stretch marks do not represent a serious health issue, but they can have a profound psychological impact on patients, especially on young, healthy women who are frequently affected by this problem.2 The breasts, upper arms, abdomen, buttocks, and thighs are the areas most frequently affected by this condition.2
SD is frequently seen in adolescents (incidence: 6–86%), pregnant women (43–88%), people who have gained excessive weight (43%), and people who have experienced negative effects from topical steroid use.3 The disorder initially manifests as a pink or purple band (SD rubrae), which may itch, but later turns into a white, atrophic dermal scar (SD alba[SDA]). Although the precise process is unknown, it is believed to have multiple causes, including hormonal, hereditary, lateral stretching, and prolonged steroid use.4
For the treatment of SDA, numerous techniques have been tried that work to stimulate the formation of collagen. These include topical creams, chemical peels, microdermabrasion, pulse dye laser, diode laser, ablative and nonablative lasers, intense pulse light, micro-needling, fractionated microneedle radiofrequency, dermal filler injections, and others.5 None is advised as standard therapy due to inadequate skin color improvement or persistent skin atrophy.6
Many authors have proposed the efficacy of using fillers in the treatment of SD.6–10 However, there are multiple recommendations with varying levels of evidence. The purpose of this systematic review is to determine the efficacy of fillers on SD.
Materials and Methods
The review was planned and conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance.
Literature Search
Systematic literature research was undertaken to identify relevant articles published up to November 2022 in the following electronic databases: Science Direct, Midline, Web of Science, CINAHL, and Google Scholar. The following Medical Subject Headings (MeSH) terms and keywords were used alone or in combination: (efficacy OR Effectiveness) AND (Calcium hydroxylapatite OR CaHA OR Radiesse OR filler OR Hyaluronic Acid) AND (striae alba OR stretch marks OR striae OR strai alba OR striae cutis distensae). All titles and abstracts of publications identified during the primary search were reviewed for relevance and eligibility. The reference lists of the selected papers and the reviewed articles were also searched manually for articles that may have been missed in the initial search.
Study Selection
Inclusion: All articles about the efficacy of injectable dermal filler as monotherapy or in combination with other treatment modalities for striae alba.
Exclusion: All articles about the treatment of striae alba other than injectable dermal filler, other uses for injectable dermal filler other than striae alba, and irrelevant articles.
Data Extraction
Two reviewers extracted the data from the chosen studies. The following categories of data were extracted: study characteristics (first author, title, publication year, study design, countries/regions, sample size, mean participant age, gender, and type of striae), treatment of striae with dermal fillers (whether they were used as monotherapy or in combination with other treatment modalities, delivery mode, dose, duration of treatment, and follow-up), and outcomes (Results, methods of measuring the improvement, and side effects). Any disagreement was resolved by consensus or the decision of a third reviewer.
Quality Assessment
Using the modified Newcastle-Ottawa Scale for eligible of cohort studies and the JBI Critical Appraisal Checklist for case reports, two reviewers independently assessed the methodological quality of each study. Disagreements were resolved by consensus.
Results
In this review process, following the above-detailed selection [Figure 1], seven articles were selected, all meeting the inclusion criteria. All these articles were published in English. From a timing standpoint, out of these seven selected articles, three were published in 2021,8,11,12 two in 20197,10, and only two in the period from 2017 to 2018.6,9 No article was published before 2017. The agreement among the authors for the title selection was high (κ = 90%), and the one for the full text assessment (κ = 100%) was unanimous.
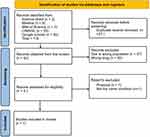 |
Figure 1 Flowchart of literature identification, screening, eligibility, and inclusion process. |
Study Characteristics
The main characteristics of the included studies are presented in Table 1. The studies were published in the period between 2017 and 2021. A total of 184 female participants who were over the age of 18 years were included. Among these seven studies, there was one from each of the following countries: Brazil, Singapore, South Korea, Spain, Italy, Canada, and the USA. Of the included studies, three were case reports, three were retrospective studies, and one was a prospective study. The selected studies included all types of SD: striae Alba in two studies; the remaining studies were red and white striae.
 |
Table 1 Study Characteristics |
Pneumatic injection of hyaluronic acid (HA) using a novel technology called jet volumetric remodeling (JVR) was delivered in three studies. Polycaprolactone filler injection was delivered in one study. Calcium hydroxylapatite combined with micro-needling and ascorbic acid was delivered in one study. Combined MFU-V and CaHA was delivered in one study. In addition, micro-focused ultrasound with MFU-V was delivered in one study. However, these studies differ in the dose, duration, and side effects.
Quality Assessment
For case reports, research quality and risk of bias were assessed using JBI Critical Appraisal Checklist in Figure 2. A case report by Hong (2019) lacked patient demographics, history, and how the patient was assessed, which could alter the validity of the results.10 Cassuto and Gupta displayed high quality in all aspects except for not providing the case’s history.9
 |
Figure 2 Assessment of quality of the included case reports. |
The Modified Newcastle-Ottawa scale in Table 2 was used to determine the quality of cohort studies after omitting the nonexposed cohort and the comparability elements. Low-quality scores were considered less than 3; moderate-quality scores were considered 4, and high-quality scores were considered 5–6. Accordingly, all studies were considered high quality, scoring 5.6–8,12
 |
Table 2 Assessment of the Quality of Included Cohort Studies |
The Effect of the Intervention on the Outcome
Combining Calcium Hydroxylapatite with Micro-Needling and Ascorbic Acid
Micro-needling is one treatment option for SD as it stimulates collagen production and allows for trans-epidermal drug delivery. Casabona conducted a retrospective study on 35 patients with SD, in which patients received calcium hydroxylapatite injections, followed by micro-needling and topical application of 20% ascorbic acid. The Manchester Scar Scale, patient satisfaction, and photography were used to assess progress. The mean baseline of the Manchester Scar Scale decreased from 12.0 (0.8) to 7.1 (1.4) after 1 month of treatment. Most (69.9%) of the patients were very satisfied, 22.9% were satisfied, 11.4% were neither satisfied nor dissatisfied, and 2.8% were unsatisfied. Also, skin biopsies were taken from the patients and revealed increased elastin and collagen.
Postinflammatory hyperpigmentation was one of the side effects experienced by two patients with Fitzpatrick skin type III; both were treated with whitening cream and completely resolved. Erythema (n = 32) and bruising (n = 35) were also reported, both of which resolved within 7 days. Furthermore, some patients reported mild pain that subsided in 2 days. Serious side effects were not observed.6
Combining Micro-Focused Ultrasound with Visualization and Calcium Hydroxylapatite
Micro-focused ultrasound with visualization and calcium hydroxylapatite both induce neocollagenesis and neoelastogenesis. Two studies were conducted to evaluate the effectiveness of combining them in the treatment of SD.
Lim conducted a prospective study on ten patients using a 5-point quartile grading scale, striae distensae alba (SDA) scoring scale, global aesthetic improvement scale, and 10-point visual analog score. The SDA scoring scale has shown great improvement, with 11.6 being the overall mean baseline, 11.1 at 1 month, 7.9 at 3 months, and 6.2 at 6 months. The patients were assessed at 3 and 6 months using the global aesthetic improvement scale; all of them showed improvement, four patients were much improved, and three patients were very much improved at 6 months. At 6 months, physicians evaluated the patients using a 5-point quartile grading scale and found that eight patients improved moderately, while two patients showed good improvement. The 10-point visual analog score was done at the end of the study, which revealed a mean reduction of 2.7.8
Furthermore, Casabona conducted a non-randomized retrospective study using micro-focused ultrasound with visualization on 20 patients who had previously been treated with calcium hydroxylapatite, topical 20% ascorbic acid solution, and micro-needling. After 3 months, the results were evaluated using The Manchester Scar Scale Score and patient satisfaction. The mean baseline of the Manchester Scar Scale Score was 9.35 (±1.18), and it dropped to 6.30 (±1.26). The mean baseline of the patient satisfaction scale score was 3.75 (±0.44) and increased to 4.70 (±0.47); 70% of the patients were very satisfied with their results.7
Micro-focused ultrasound with visualization had no side effects, whereas calcium hydroxylapatite fillers caused bruises in four patients in Lim’s study, which all resolved without intervention.
High-Velocity Pneumatic Injection of Hyaluronic Acid
Three studies have been conducted evaluating high-velocity pneumatic injection of hyaluronic acid. Which is the delivery of hyaluronic acid through a jet blast intradermally without a needle.
MacGillis conducted a retrospective study in Canada. 115 patients were reviewed. 26 had distinct striae. After administering pneumatic injections, they were evaluated three months later. They used two scoring systems for evaluation: a satisfaction score of 5 and a GIAS score of 5. After three months, the average GIAS score was 2, and the average satisfaction score was 4.2.12
Similarly, a case series was conducted on two female patients by Gupta in India. Using the Manchester scale with improvement noticed after 2 months of follow-up, re-evaluation found 45.71% improvement after 6 months and 42.88% after 15 months.11 Lastly, in a case report by Cassuto in Italy, a female patient found improvement in color, texture, and contour at 3 months, 8 months, and 3 years follow-up.9
All these studies established the safety and efficacy of high-velocity pneumatic injection of hyaluronic acid for SD with improvement from baseline in color, texture, and contour. Initial side effects of localized erythema, edema, and mild pain resolved in a few days. No serious adverse effects were recorded, such as infection or allergic reaction.
Polycaprolactone Filler Injection
Polycaprolactone filler is one of the Biodegradable collagen stimulatory fillers. They are on the latest, next-generation dermal fillers with characteristics capable of inducing neocollagenesis.
In a South Korean study, Hong (2019) injected part SD with 0.1 cc of polycaprolactone filler 5 mm apart and leaving part of SD as a control for comparison. They evaluated the depression volume using a 3D camera and detected a significant reduction in the volume of the depression. The pain from the injection was barely noticeable. The day following therapy, the immediate erythema and nodularity at the injection locations vanished.10
Discussion
In this systematic review, we assessed the results of studies that investigated the utilization of injectable dermal filler as monotherapy or in combination with other treatment modalities to treat SD. The review found that CaHA was effective when combined with micro-needling and ascorbic acid or MFU-V. Furthermore, monotherapy with high-velocity pneumatic hyaluronic acid and polycaprolactone fillers injections was also effective. Compared to the other treatments, combining CaHA and MFU-V had the fewest side effects, while combining CaHA with micro-needling and ascorbic acid had the most side effects. According to our systematic study, injectable dermal fillers were well tolerated as monotherapy or in combination with other treatments for SD alba, with only a small number of patients experiencing temporary side effects. Minor side effects were reported, including self-resolved bruising, temporary local edema, and mild pain during injection. Also, two patients with Fitzpatrick skin type III treated with CaHA combined with micro-needling and ascorbic acid developed postinflammatory hyperpigmentation. This postinflammatory hyperpigmentation was treated for 30 days with a whitening cream (Kligman’s formula) and completely disappeared.6 Injectable dermal fillers were one of the treatments used in the treatment of SD alone or combination with other treatment modalities. Our systematic review showed that it effectively reduces SD with only mild, temporary side effects and that it should be considered a potential treatment for SD. Despite dermal filler’s effectiveness in treating SD, its effectiveness could have been undermined by different follow-up periods, different doses, and either monotherapy or in combination with other modalities.
To our knowledge, this is the first systematic review that examines the effectiveness of injectable dermal filler for SD alba. This study has several strengths, including reviewing the current literature, the strict inclusion and exclusion criteria, and the reporting format according to PRISMA guidelines. Also, because there were insufficient randomized control trials, we included all types of studies (cohort studies, retrospective studies, and case reports). This is a strength of our review. And for an accurate assessment of our articles, different types of tools were used according to the type of study: the JBI critical appraisal tool for case reports and the Newcastle-Ottawa Scale with some modifications for the non-randomized trials. A limitation of our systematic review is the limited number of published articles regarding filler injection in the treatment of SD.
Conclusion
Some different treatments and modalities are effective in treating SD with various results. In this review, we concluded that injectable dermal fillers are a potential treatment for SD with minor side effects, either as monotherapy or combined therapy. Also, the review has revealed that combining CaHA and MFU-V had the fewest side effects compared to other dermal fillers. Because the number of studies included was insufficient, we recommend providing further information about the therapeutic differences between various dermal fillers and treatment concentration, dosage, and co-therapy. We thus advise that more future large-scale RCTs focus on investigating the utilization of injectable dermal filler to provide what is best for patients with SD and to compare it with other existing treatment modalities by outcomes, costs, and side effects to create satisfying practice and basic guideline interventions for such cases.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lokhande AJ, Mysore V. Striae distensae treatment review and update. Indian Dermatol Online J. 2019;10(4):380–395. doi:10.4103/idoj.IDOJ_336_18
2. Salter SA, Kimball AB. Striae gravidarum. Clin Dermatol. 2006;24(2):97–100. doi:10.1016/j.clindermatol.2005.10.008
3. Oakley AM, Patel BC. Stretch marks (striae). In: StatPearls. Treasure Island, Fla.: StatPearls Publishing; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK436005/.
4. Elsaie ML, Baumann LS, Elsaaiee LT. Striae distensae (stretch marks) and different modalities of therapy: an update. Dermatol Surg. 2009;35(4):563–573. doi:10.1111/j.1524-4725.2009.01094.x
5. Ross NA, Ho D, Fisher J. Striae distensae: preventative and therapeutic modalities to improve aesthetic appearance. Dermatol Surg. 2017;43(5):635–648. doi:10.1097/DSS.0000000000001079
6. Casabona G, Marchese P. Calcium hydroxylapatite combined with microneedling and ascorbic acid is effective for treating stretch marks. Plast Reconstr Surg Glob Open. 2017;5(9):e1474. doi:10.1097/GOX.0000000000001474
7. Gasa Casabona G. Microfocused ultrasound with visualization for the treatment of stretch marks. J Clin Aesthet Dermatol. 2019;12(2):20–24.
8. Lim JT. Treating striae distensae alba in Asians: efficacy and safety of combined MFU-V and CaHA. Plast Reconstr Surg Glob Open. 2021;9(2):e3429. doi:10.1097/GOX.0000000000003429
9. Cassuto D. Case report: effective treatment of striae distensae using pneumatic injection of hyaluronic acid. Available from: elogioasia.com.
10. Hong JY, Han HS, Kwon TR, et al. Remarkable improvement of striae distensae with polycaprolactone filler injection. J Eur Acad Dermatol Venereol. 2019;33(11):e399–e400. doi:10.1111/jdv.15702
11. Gupta A, Kroumpouzos G. A novel treatment of striae distensae with pneumatic injection of hyaluronic acid. J Appl Cosmetol. 2021;39(1):17–24.
12. MacGillis D, Vinshtok Y. High-velocity pneumatic injection of non-crosslinked hyaluronic acid for skin regeneration and scar remodeling: a retrospective analysis of 115 patients. J Cosmet Dermatol. 2021;20(4):1098–1103. doi:10.1111/jocd.14002
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
