Back to Journals » Clinical Ophthalmology » Volume 16
Treatment of Severe Dry Eye in Stevens-Johnson Syndrome with Umbilical Cord Serum Eye Drops
Authors Susiyanti M, Kurnia DA, Fasha I, Irawati Y , Rachmadi L, Liem IK, Artini W
Received 2 September 2022
Accepted for publication 25 November 2022
Published 10 December 2022 Volume 2022:16 Pages 4089—4095
DOI https://doi.org/10.2147/OPTH.S385078
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Made Susiyanti,1 Denisa Anggi Kurnia,1 Iqbal Fasha,2 Yunia Irawati,1 Lisnawati Rachmadi,3 Isabella Kurnia Liem,2,4 Widya Artini1
1Department of Ophthalmology, Faculty of Medicine, Universitas Indonesia – Cipto Mangunkusumo General Hospital, Jakarta, Indonesia; 2Cell Medical Technology Integrated Service Unit, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia; 3Department of Anatomical Pathology, Faculty of Medicine, Universitas Indonesia – Cipto Mangunkusumo General Hospital, Jakarta, Indonesia; 4Department of Anatomy, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Correspondence: Widya Artini, Department of Ophthalmology, Faculty of Medicine, Universitas Indonesia – Cipto Mangunkusumo General Hospital, Jl. Kimia No. 8-10, RT.10/RW.1, Pegangsaan, Menteng, Jakarta Pusat, Jakarta, 10320, Indonesia, Tel +62 811841010, Email [email protected]
Purpose: To evaluate the efficacy and safety of umbilical cord serum eye drops for dry eyes in ocular Stevens-Johnson Syndrome (SJS).
Patients and Methods: A pre-post test study with umbilical cord serum (UCS) eye drop for ocular SJS patient with moderate to severe dry eyes. Study was conducted at Kirana Cipto Mangunkusumo General Hospital from June 2020 to December 2020. A total of five patients (five eyes) with a diagnosis of SJS more than 6 months, dry eye symptoms, and abnormal tear stability test results were included in the study. Each patient was asked to instill UCS drop into the affected eye six times daily. Evaluation of ocular symptoms with ocular surface disease index (OSDI) questionnaires, non-invasive tear break-up time (NIBUT), Schirmer I, and keratoepitheliopathy scores was administered before applying UCS drop and at week 2 and 4 of eye drop use.
Results: From June 2020 to December 2020, five eyes of five patients were evaluated in this study. Patients were aged from 22 to 71 years old with history of SJS over periods from 1 to 35 years. Three patients underwent ocular surgeries prior to the study. After four weeks of treatment, symptoms score, Schirmer I, and keratoepitheliopathy scores improved significantly, while NIBUT scores improved insignificantly. No side effects were noted during treatment.
Conclusion: Administration of UCS eye drop was effective in improving symptoms and signs of dry eye in chronic SJS patients.
Keywords: umbilical cord serum eye drop, ocular Stevens-Johnson syndrome, dry eye
Introduction
Stevens-Johnson syndrome (SJS) is an autoimmune disease characterized by mucocutaneous reactions with mortality rates of up to 35%. The ocular sequelae of SJS cause visual disturbance due to chronic inflammatory changes on the ocular surface.1 Ocular manifestations have been found in 60–90% of cases and dry eye is the most common of these.2–4 Dry eye is mainly caused by impaired tear film stability and chronic inflammation combined with reduced aqueous secretion and increased evaporation.5 Patients with dry eyes may complain of foreign body sensation, pain, photophobia, visual disturbances, and difficulty opening their eyes.1,6–8 These complaints may disrupt patients’ daily activities, thus reducing their quality of life. In ocular SJS, dry eye with damage to the ocular surface and surrounding tissue may cause complications such as persistent corneal defects with vision-threatening corneal scarring.
Umbilical cord serum (UCS) eye drops have recently been used to treat patients with dry eye disease. Higher growth factors components have been reported by several studies.9–11 These components such as epidermal growth factor, nerve growth factor, insulin-like growth factor (IGF), Substance P, vitamin A, and transforming growth factor (TGF)-β are essential for ocular surface health and are common constituents of tear film. Umbilical cord serum eye drops are considered effective and safe in treating patients with dry eye disease. However, their use has not been tested specifically in SJS patients with severe dry eye.
This study aims to evaluate subjective and objective outcome of treatment with UCS eye drops in SJS patients with severe dry eye.
Materials and Methods
A pre-post prospective intervention study to evaluate UCS eye drop for chronic ocular SJS patients with severe dry eyes was conducted at Kirana Cipto Mangunkusumo General Hospital from June 2020 to December 2020. Inclusion criteria were as follows: minimum age of 18 years; a diagnosis of SJS more than 6 months prior to recruitment; dry eye symptoms, namely: foreign body sensation, photophobia, stinging, and pain requiring lubrication prior to the study; abnormal tear stability test results (Schirmer I test without topical anesthetic ≤ 10 mm in 5 minutes with or without other associated ocular surface abnormalities such as keratoepitheliopathy or symblepharon); good cognitive abilities; willing to participate in the study and having signed the informed consent form. We excluded subjects who had used topical steroids within one month of the study, with a history of ocular surface surgery within three months of the study, active ocular infection, a history of immunocompromised, pregnant or chronic ocular SJS with severe symptoms (tears, symblepharon in all quadrants, or keratinization over the entire ocular surface). Subjects were withdrawn if they showed severe side effects (such as allergic reactions and/or ocular surface infections), were lost to follow-up, or did not use drops for two consecutive days, and could voluntarily withdraw during the study. If both eyes met the inclusion and exclusion criteria, the worse eye based on tear stability was included in this study.
Evaluation of NIBUT, Schirmer I, keratoepitheliopathy scores, and ocular surface disease index (OSDI) questionnaires were carried out before administration of UCS drop and at week 2 and 4 of drop use. Subjective symptoms were graded using the OSDI.12 Non-invasive tear breakup time (NIBUT) and Schirmer test I were performed according to Dry Eye Workshop (DEWS) guidelines.13 Keratoepitheliopathy was scored by multiplying the 1% fluorescein stained area score (0–3) by the density score (0–4).14
A total of five patients (five eyes) were included in the study. Written informed consent was obtained from every patient. Each patient received one bottle of UCS drop weekly and was asked to instill it into the affected eye six times daily. The UCS (20% concentration) was prepared in accordance with the standard protocol in collaboration with the Integrated Service Installation (IPT) of Stem Cell Medicine, Faculty of Medicine, Universitas Indonesia-Cipto Mangunkusumo Hospital. Umbilical cord serum was obtained from normal or cesarean section delivery with informed consent. Donors were screened for hepatitis B, hepatitis C, syphilis and HIV/AIDS at 38 weeks gestation. Immediately after delivery, 100 mL of blood was drawn from the umbilical cord vein and was placed into a blood bag without anticoagulant. It was later placed in a plasma extractor at room temperature for 60 minutes to sediment then was centrifuged at 1850 rpm for 10 minutes without breaks at 15° Celsius. Serum was then diluted with 20% normal saline in a plasma bag before being poured into a 5 mL bottle to be used as eye drops. The remaining umbilical cord serum underwent microbiology testing within seven days. The bottles were stored at −20℃ and were used only if the microbiology test showed no bacterial contamination.
Every patient was asked to store their drops in a refrigerator and to look for presence of any thread like objects in the serum each time before instilling the drops and to discard the bottle if such contaminations occurred.
Results
The characteristics of SJS patients with dry eyes treated with UCS are presented in Table 1. Three of the patients had undergone eye surgery more than three months prior to treatment, including one penetrating keratoplasty, one cataract surgery, and one symblepharon release with amnion graft. Every patient has open puncta and continues their previous lubricants after using UCS for 4 weeks.
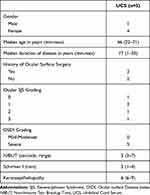 |
Table 1 Characteristics of SJS Patients Who Were Treated with Umbilical Cord Serum Shown in Median (Min-Max) |
While the ocular SJS grading are varied, all patients experienced severe dry eye symptoms at the beginning of the study. Ocular SJS grading systems based on corneal, conjunctiva, and eyelid involvement that has been mentioned in previous study.7 Patient that had undergone symblepharon release with amnion graft and penetrating keratoplasty had grade 1 and 2 based on ocular SJS grading system. One patient with grade 3 ocular SJS had severe corneal opacification and neovascularization with moderate irregularity of the mucocutaneous junction.
Changes in symptoms, tear film characteristics, and ocular surface features after two and four weeks of UCS use in SJS patients with dry eye are shown in Table 2. Significant improvements were found in symptoms scores (P = 0.04), Schirmer I (P = 0.05), and keratoepitheliopathy score (P = 0.04) after four weeks. Improvements in OSDI scores, NIBUT, Schirmer I, and Keratoepitheliopathy were apparent after two weeks but were not statistically significant. Three out of five eyes that were treated with UCS drops gained complete corneal reepithelization after four weeks. Keratoepitheliopathy improvement after four weeks of UCS treatment is shown in Figure 1. No side effects were observed in this study.
 |
Table 2 Changes of Symptoms and Signs After Umbilical Cord Serum Drops in SJS Patients with Dry Eyes |
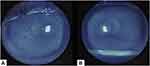 |
Figure 1 Keratoepitheliopathy improvement. (A) Fluorescein staining in UCS before treatment. (B) Fluorescein staining in UCS after four weeks. |
Discussion
Stevens-Johnson syndrome is an autoimmune disease with an incidence varying between 1.2 and 6 per million patients, which attacks conjunctival and other mucosa throughout the body and has a mortality rate of up to 35%.8 Fifty-seven of the SSJ/Toxic Epidermal Necrolysis cases treated at Hasan Sadikin Hospital in Bandung, Indonesia in 2009–2013 had ocular involvement.15 Toxic epidermal necrolysis is a severe form of SJS that also caused by autoimmune disease.7 The pathophysiology of SJS found in the acute phase includes apoptosis of keratinocytes, loss of conjunctival epithelium, and secondary inflammation.1 These three processes occur continuously so that several clinical manifestations arise including pseudomembrane formation, symblepharon, necrosis of the eyelid margin, recurrent meibomitis, shortened fornix, corneal degeneration, cornea ulcer progressing to perforation, and ultimately permanent blindness.16–18
The primary pathogenesis of SJS has not been fully elucidated to date, but previous studies and literature point to immunological and genetic factors.1,8 The acute phase of SJS is the result of a type IV hypersensitivity reaction mediated by cytotoxic T cells. The occurrence of severe ocular SJS has been reported to be associated with abnormalities in the innate immune system8,19 Chung20 reported the role of granulysin as a key factor in the apoptosis of keratinocytes, the involvement of cytotoxic T lymphocytes and natural killer cell-mediated cytotoxicity. Williams21 reported a cytokine-mediated response contributing to the development of SJS, and found that neutrophils are involved in the inflammation of the conjunctival mucosa. Sotozono et al22 reported better outcomes after administration of topical steroid than non-steroid in ocular SJS. Topical anti-inflammatory drugs are mostly used in the acute phase of SJS. Systemic oral immunosuppressants such as cyclosporine, azathioprine, cyclophosphamide, methotrexate, mycophenolate, and infliximab are considered to provide better results in moderate to severe persistent ocular inflammation than in mild cases.23 Regular use of non-preserved artificial tears can reduce complaints of dry eye-related ocular surface discomfort.1
Several other studies have reported the beneficial effects of topical autologous serum as an adjunctive treatment in ocular SJS patients with reduced symptoms and inflammation.24,25 Yoon et al compared the components of plasma serum and UCS with those of tears, and found higher levels of tear components in UCS than in plasma serum that gave better results. Therefore, application of UCS is currently being developed.26
In this case series report, the cases had chronic SJS with potential vision with moderate to severe ocular surface abnormalities accompanied by moderate dry eye. Abnormalities of the entire conjunctival surface, including the goblet cells which produce mucin to maintain ocular surface moisture, arise due to inflammation, but these patients did not report symptoms of severe dry eye.
In the present study, improvements in NIBUT, Schirmer I, keratoepitheliopathy, and OSDI scores were found in patients with SJS after UCS treatment. Subjective improvement was evaluated using OSDI scores. The symptoms scores were significantly improved after four weeks of UCS treatment, but improvement was insignificant at two weeks. This study found average OSDI reduction score of 24.8 (6.8–47.2). As reported by Wolffsohn, OSDI reduction of at least than 7.3 points is clinically significant.13 Other studies have also reported OSDI improvement after UCS therapy in non SJS dry eyes; Versura27 reported statistically significant OSDI improvement after UCS in patients with severe epithelial defect and Yoon28 reported OSDI improvement after one month in dry eye disease.
Objective improvement reflecting ocular surface restoration after severe SJS-related inflammation was found in this study. For example, tear film abnormality, especially in the lipid layer, was evaluated using NIBUT score and was improved. This result is consistent with those of Yoon28 and Versura27 which also showed NIBUT improvement in UCS-treated patients. One hypothetical explanation for this improvement is meibomian gland restoration after UCS treatment. Umbilical cord serum contains a rich mix of vitamin A and growth factors including insulin-like growth factor (IGF-1). Liu reported that IGF-1 modulates the morphology and size of meibomian tissue.29 Growth factors in UCS have restorative and soothing effects on the ocular surface, and improve adnexa gland productivity. UCS also promotes reepithelization of the cornea and improves expression of cell surface-associated mucin that will increase the NIBUT score.
Schirmer I test evaluates the aqueous layer of tear film, and our study showed that this is statistically significantly improved after two weeks of treatment. Previous studies have also reported Schirmer I improvement after UCS therapy.27 UCS includes a high level of nerve growth factor, and this may explain previously reported improvement in corneal sensitivity after UCS use in a neurotrophic keratitis patient.11 This result suggests that corneal nerve endings were repaired after UCS therapy, thus improving positive feedback to and increased aqueous secretion by the lacrimal gland.30 Unfortunately, our study did not include a corneal sensitivity test.
In the present study, the keratoepitheliopathy score was significantly improved after four weeks of treatment and complete reepithelization was found in three out of five patients. This result seems consistent with other previous studies. Vajpayee reported epithelial defect closure after UCS at day 21 in persistent corneal defect patients, and Yoon reported corneal defect improvement after two weeks of UCS therapy.31 UCS includes high levels of P substance, epithelial growth factor, transforming growth factor-β and IGF-1. These factors are involved in epithelial cell proliferation and migration and act as anti-apoptotic agents.9,26,32–34 All these effects contribute to the epithelial defect closure that we found in this study.
In this study, we have not found ocular surface inflammation while patients were taking the UCS eye drop, as chronic inflammation was a part of the suggested pathophysiology in SJS patient. A previous study reported no side effects after four weeks of UCS treatment8 suggesting that UCS anti-inflammatory effect contributes to its biological properties as reported by Buzzi,35 and other studies on the safety of UCS eye drops.27,28,31
From the previous reports cited above suggest that UCS eye drop can be used as an adjunctive topical treatment for ocular surface defects and have the advantages that a large amount of serum can be obtained during one delivery and can be supplied to numerous patients. However, they are limited by the possibility of transmission of blood-borne infectious disease from the pregnant donor, thus serological testing has to be performed several times to prevent transmission.
This is the first study to evaluate the efficacy and safety of UCS drop in dry eye patients in chronic SJS. The limitations of this study are its low sample size and short follow-up time. Further research using a larger sample and longer follow-up period should evaluate the long-term effect of UCS drop for SJS patients, including a range of tear film characteristics and ocular surface features.
Conclusion
Umbilical cord serum eye drop provided improvement in subjective discomfort, tear film stability, and corneal epithelialization of dry eyes in SJS patients with no apparent side effects.
Data Sharing Statement
Authors confirm that they have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis as well as the decision to submit for publication.
Ethical Approval
This research was conducted in accordance with the principle of the Declaration of Helsinki and was approved by the Standing Committee of Ethical Health Medical Research of Universitas Indonesia (reference KET-329/UN2.F1/ETIK/PPM.00.02/2020). Written informed consent was obtained from each patient. The patients understand that anonymity cannot be guaranteed.
Consent for Publication
Each patient gave consent for their images and other clinical information to be reported in the journal with no identifying information.
Acknowledgments
This study was made possible with help funding from Universitas Indonesia through Publikasi Terindeks Internasional Sains Teknologi dan Kesehatan (PUTI Saintekes) 2020 grant.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was made possible by funding from Universitas Indonesia through Publikasi Terindeks Internasional Sains Teknologi dan Kesehatan (PUTI Saintekes) 2020 grant with contract number NKB-2172/UN2.RST/HKP.05.00/2020. The sponsor had no involvement in any of the stages from study design to submission of the paper for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kohanim S, Palioura S, Saeed HN, et al. Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis – a comprehensive review and guide to therapy. Ii. Ophthalmic disease. Ocul Surf. 2016;14(2):168–188. doi:10.1016/j.jtos.2016.02.001
2. Morales ME, Purdue GF, Verity SM, Arnoldo BD, Blomquist PH. Ophthalmic manifestations of Stevens-Johnson syndrome and toxic epidermal necrolysis and relation to SCORTEN. Am J Ophthalmol. 2010;150(4):505–510. doi:10.1016/j.ajo.2010.04.026
3. Yip LW, Thong BY, Lim J, et al. Ocular manifestations and complications of Stevens–Johnson syndrome and toxic epidermal necrolysis: an Asian series*. Allergy. 2007;62(5):527–531. doi:10.1111/j.1398-9995.2006.01295.x
4. Gueudry J, Roujeau JC, Binaghi M, Soubrane G, Muraine M. Risk factors for the development of ocular complications of Stevens-Johnson syndrome and toxic epidermal necrolysis. Arch Dermatol. 2009;145(2):157–162. doi:10.1001/archdermatol.2009.540
5. Sotozono C, Ueta M, Yokoi N. Severe dry eye with combined mechanisms is involved in the ocular sequelae of SJS/TEN at the chronic stage. Invest Ophthalmol Vis Sci. 2018;59(14):DES80–DES86. doi:10.1167/iovs.18-24019
6. Di Pascuale MA, Espana EM, Liu DTS, et al. Correlation of corneal complications with eyelid cicatricial pathologies in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis syndrome. Ophthalmology. 2005;112(5):904–912. doi:10.1016/j.ophtha.2004.11.035
7. Sotozono C, Ang LPK, Koizumi N, et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens-Johnson Syndrome. Ophthalmology. 2007;114(7):1294–1302. doi:10.1016/j.ophtha.2006.10.029
8. Jain R, Sharma N, Basu S, et al. Stevens-Johnson syndrome: the role of an ophthalmologist. Surv Ophthalmol. 2016;61(4):369–399. doi:10.1016/j.survophthal.2016.01.004
9. Yoon K-C, Im S-K, Park Y-G, Jung Y-D, Yang S-Y, Choi J. Application of umbilical cord serum eyedrops for the treatment of dry eye syndrome. Cornea. 2006;25(3):268–272. doi:10.1097/01.ico.0000183484.85636.b6
10. Murri MS, Moshirfar M, Birdsong OC, Ronquillo YC, Ding Y, Hoopes PC. Amniotic membrane extract and eye drops: a review of literature and clinical application. Clin Ophthalmol. 2018;12:1105. doi:10.2147/OPTH.S165553
11. Yoon KC, You IC, Im SK, Jeong TS, Park YG, Choi J. Application of umbilical cord serum eyedrops for the treatment of neurotrophic keratitis. Ophthalmology. 2007;114:1637–1642. doi:10.1016/j.ophtha.2006.12.014
12. Özcura F, Aydin S, Helvaci MR. Ocular surface disease index for the diagnosis of dry eye syndrome. Ocul Immunol Inflamm. 2007;15:389–393. doi:10.1080/09273940701486803
13. Wolffsohn JS, Arita R, Chalmers R, et al. The ocular surface TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15:539–574. doi:10.1016/j.jtos.2017.05.001
14. Miyata K, Amano S, Sawa M, Nishida T, Novel Grading A. Method for superficial punctate keratopathy magnitude and its correlation with corneal epithelial permeability. Arch Ophthalmol. 2003;121(11):1537–1539. doi:10.1001/archopht.121.11.1537
15. Suwarsa O, Yuwita W, Dharmadji HP, Sutedja E. Stevens-Johnson syndrome and toxic epidermal necrolysis in Dr. Hasan Sadikin General Hospital Bandung, Indonesia from 2009–2013. Asia Pac Allergy. 2016;6(1):43. doi:10.5415/apallergy.2016.6.1.43
16. Isawi H, Dhaliwal DK. Corneal melting and perforation in Stevens Johnson syndrome following topical bromfenac use. J Cataract Refract Surg. 2007;33(9):1644–1646. doi:10.1016/j.jcrs.2007.04.041
17. Sachdev R, Bansal S, Sinha R, Sharma N, Titiyal JS. Bilateral microbial keratitis in highly active antiretroviral therapy-induced Stevens-Johnson Syndrome and toxic epidermal necrolysis: a case series. Ocul Immunol Inflamm. 2011;19(5):343–345. doi:10.3109/09273948.2011.601389
18. Saeed HN, Chodosh J. Ocular manifestations of Stevens-Johnson syndrome and their management. Curr Opin Ophthalmol. 2016;27(6):522–529. doi:10.1097/ICU.0000000000000312
19. Ueta M, Kinoshita S. Ocular surface inflammation is regulated by innate immunity. Prog Retin Eye Res. 2012;31(6):551–575. doi:10.1016/j.preteyeres.2012.05.003
20. Chung WH, Hung SI, Yang JY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14(12):1343–1350. doi:10.1038/nm.1884
21. Williams GP, Mudhar HS, Leyland M. Early pathological features of the cornea in toxic epidermal necrolysis. Br J Ophthalmol. 2007;91(9):1129–1132. doi:10.1136/bjo.2006.113241
22. Sotozono C, Ueta M, Koizumi N, et al. Diagnosis and treatment of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis with ocular complications. Ophthalmology. 2009;116(4):685–690. doi:10.1016/j.ophtha.2008.12.048
23. De Rojas MV, Dart JKG, Saw VPJ. The natural history of Stevens–Johnson syndrome: patterns of chronic ocular disease and the role of systemic immunosuppressive therapy. Br J Ophthalmol. 2007;91:1048–1053. doi:10.1136/bjo.2006.109124
24. Phasukkijwatana N, Lertrit P, Liammongkolkul S, Prabhasawat P. Stability of epitheliotrophic factors in autologous serum eye drops from chronic Stevens-Johnson syndrome dry eye compared to non-autoimmune dry eye. Curr Eye Res. 2011;36(9):775–781. doi:10.3109/02713683.2011.587935
25. Tsubota K, Higuchi A. Serum application for the treatment of ocular surface disorders. Int Ophthalmol Clin. 2000;40:113–122. doi:10.1097/00004397-200010000-00009
26. Yoon KC. Use of umbilical cord serum in ophthalmology. Chonnam Med J. 2014;50(3):82. doi:10.4068/CMJ.2014.50.3.82
27. Versura P, Profazio V, Buzzi M, et al. Efficacy of standardized and quality-controlled cord blood serum eye drop therapy in the healing of severe corneal epithelial damage in dry eye. Cornea. 2013;32:412–418. doi:10.1097/ICO.0b013e3182580762
28. Yoon KC, Heo H, Im SK, You IC, Kim YH, Park YG. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am J Ophthalmol. 2007;144:86–92. doi:10.1016/j.ajo.2007.03.016
29. Liu Y, Knop E, Knop N, et al. Growth hormone influence on the morphology and size of the mouse meibomian gland. J Ophthalmol. 2016:2016. doi:10.1155/2016/5728071
30. Vajpayee RB, Mukerji N, Tandon R, et al. Evaluation of umbilical cord serum therapy for persistent corneal epithelial defects. Br J Ophthalmol. 2003;87(11):1312. doi:10.1136/BJO.87.11.1312
31. Yoon KC, Heo H, Jeong IY, Park YG. Therapeutic effect of umbilical cord serum eyedrops for persistent corneal epithelial defect. Kor J Ophthalmol. 2005;19(3):174–178.
32. Nishida T, Nakamura M, Ofuji K, Reid T, Mannis MJ, Murphy CJ. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. J Cell Physiol. 1996;169(1):159–166. doi:10.1002/(SICI)1097-4652(199610)169:1<159::AID-JCP16>3.0.CO;2-8
33. Yoon K, Choi W, You I, Choi J. Application of umbilical cord serum eyedrops for recurrent corneal erosions. Cornea. 2011;30(7):744–748. doi:10.1097/ICO.0b013e31820d850f
34. Yoshino K, Garg R, Monroy D, Ji Z, Pflugfelder SC. Production and secretion of transforming growth factor beta (TGF-β) by the human lacrimal gland. Curr Eye Res. 1996;15(6):615–624.
35. Buzzi M, Versura P, Grigolo B, et al. Comparison of growth factor and interleukin content of adult peripheral blood and cord blood serum eye drops for cornea and ocular surface diseases. Transfus Apher Sci. 2018;57(4):549–555. doi:10.1016/j.transci.2018.06.001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
