Back to Journals » International Medical Case Reports Journal » Volume 13
Treatment of Radiation Retinopathy with Intravitreal Injection of Ranibizumab (Lucentis®)
Authors Horowitz SA , Damasceno NP, Damasceno EF
Received 21 October 2018
Accepted for publication 5 June 2019
Published 11 February 2020 Volume 2020:13 Pages 27—32
DOI https://doi.org/10.2147/IMCRJ.S191654
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Soraya Alessandra Horowitz, 1 Nadyr P Damasceno, 1 Eduardo F Damasceno 2
1Department of Ophthalmology, Hospital Naval Marcilio Dias, Rio de Janeiro, Brazil; 2Department of Ophthalmology, Universidade Federal Fluminense, Niteroi, Brazil
Correspondence: Soraya Alessandra Horowitz; Nadyr P Damasceno
Department of Ophthalmology, Hospital Naval Marcílio Dias (HNMD), 185 Rua Cesar Zama, Lins, Rio de Janeiro, Brazil
Email [email protected]; [email protected]
Abstract: To report a treatment of radiation retinopathy in a patient exposed to ionizing radiation for a period of 2 years. A 26-year-old female patient with no comorbidities diagnosed with myelodysplasia confirmed by bone marrow biopsy. She presented a complaint of bilateral progressive visual acuity reduction. At the ophthalmologic examination, she presented alterations suggestive of radiation retinopathy as well as macular thickness to optical coherence tomography (OCT) of over 500 μm. The patient underwent intravitreal injection (0.05 mL) of ranibizumab (Lucentis®) monthly in both eyes and follow-up through visual acuity and OCT examination. She presented reduction of macular edema as well as a slight improvement of visual acuity. In this case, the treatment of radiation retinopathy with intravitreal injection of ranibizumab (Lucentis) was relatively useful, with a slight improvement of visual acuity, due to the regression of macular edema, not being curative.
Keywords: retinopathy abnormalities, retina/radiation effects, intravitreal injection, angiogenesis inhibitors/therapeutic use, macular edema, improvement of visual acuity
Introduction
Radiation retinopathy is an important complication of orbital radiotherapy or radiation exposure. The literature describes cases of radiotherapy treatment such as plaque radiotherapy for pathologies such as Graves’ ophthalmopathy, uveal or orbital tumors, and ocular metastases.1
Radiation can damage cells and affect genetic material, with two types of radiation: electromagnetic radiation and particle emission radiation. Ionizing radiations are electromagnetic waves or particles that propagate with high speed and carrying energy, possibly electric and magnetic charge, and that, when interacting, can produce varied effects on matter (National Nuclear Energy Commission, 2009). Radiation is considered ionizing when it has the ability to ionize, the most known type of ionizing radiation is X-rays. The effect of radiation depends on factors such as total amount of radiation received, range of exposure, other associated damages, region affected and individual predisposition.
In radiation retinopathy, alterations occur that are dose dependent, occurring between 6 months to 3 years of exposure, with retinal damage and vasculopathy (exudate, retinal hemorrhage, intraretinal microangiopathy, retinal edema, neovascularization). Macular edema is a common cause of low visual acuity due to radiation retinopathy.
Vascular endothelial growth factor (VEGF) is produced by retinal cells such as Muller, glia and Retinal Pigmented Epithelium cells (EPR). It is a stimulator of proliferation and endothelial migration, responsible for neovascularization, intraretinal edema due to vascular extravasation and vascular injury.
Medications with anti-VEGF action have been used as a treatment for vascular pathology retininians, such as ranibizumab, which is a humanized antibody fragment that acts to inhibit all VEGF-A isoforms.2–4
Case Report
A 26-year-old female, brown patient with a history of exposure to ionizing radiation for a period of 2 years as an X-ray technique, despite all protective measures regulated by law, developed myelodysplasia confirmed by Bone marrow biopsy and excluding other causes such as autoimmune diseases, neoplasia, viral, drug and exposure to other metals or chemical substances. She was referred for ophthalmologic evaluation for visual turbidity complaint. The patient had visual acuity of 20/80 in the right eye and 20/50 in the left eye.
The funduscopy showed bilateral pale papilla, with physiological digging, vessels with reduced caliber and some phantom vessels, macula with decreased foveal brightness, pale retina, RPE hyperplasia in the middle periphery (see Figures 1 and 2). The macular optical coherence tomography (StratusOCT) showed retinal thickening and cysts formation (see Figures 3–5). Visual field showed island of bilateral central vision (see Figures 6 and 7).
 |
Figure 1 Color fundus photograph – right eye. |
 |
Figure 2 Color fundus photograph – left eye. |
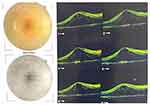 |
Figure 3 SD-OCT topcon – incidences and macular view. Right eye (previous anti-VEGF therapy). |
 |
Figure 4 SD-OCT topcon – incidences and macular view. Right eye (previous anti-VEGF therapy). |
 |
Figure 5 SD-OCT topcon – central macular thickness (CMT). Right eye = 703 µm, left eye = µm. Previous Anti-VEGF therapy. |
 |
Figure 6 Octopus 900 – perimeter – visual field – right eye. |
 |
Figure 7 Octopus 900 – perimeter – visual field – left eye. |
The multifocal ERG (electroretinogram)/PEV (visual evoked potential)/EOG (electrooculogram) examinations was generally affected in both eyes according to the low acuity.
It was performed continuous intravitreal injection with ranibizumab (0.3mg/0,05mL) in 4- week intervals and monitoring with visual acuity and OCT. After performing nine monthly injections the visual acuity was improved for 20/40 in both eyes and decreased macular edema (see Figures 8 and 9).
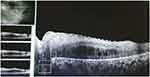 |
Figure 8 Zeiss cirrus – SD-OCT – left eye. CMT = 387 µm (after anti-VEGF therapy). |
 |
Figure 9 SD-OCT topcon – right eye. CMT = 319 µm (after anti-VEGF therapy). |
A case of systemic and ophthalmological manifestation related to tolerable exposure to ionizing radiation in both eyes is rare, not found in the literature similar reports. The improvement of macular edema with the use of intravitreal injection of anti-VEGF as observed was not permanent and did not have a determined number of intravitreal procedures necessary to stabilize the macular condition. Continuous treatment is necessary to maintain acuity improvement. Intravitreal Dexamethasone treatment was not performed due to pallor of the optic nerve and ischemic neuropathy of increased intraocular pressure risk of further injury.5–9
Conclusion
There is still no curative treatment for pathologies related to exposure to ionizing radiation, requiring more studies with a larger number of patients and a longer follow-up. The patient maintains follow up in hematology and ophthalmology. Aplastic anemia and myelodysplasia are rare diseases with ophthalmic manifestations like vascular changes, retinal hemorrhages, and peripheral retinal ischemia. The systemic changes combined with radiation retinopathy explain the quickly progressing to low vision and concentric impairment of visual field.10–12 The macular edema treatments with anti-VEGF (Ranibizumab) is promising, and the results depends on continuity what can increase the side effects.13–17 The mechanisms of radiation protection are still the most effective in preventing radiation retinopathy, which do not have curative treatment.
Ethics Committee in Research
Supported by Hospital Naval Marcílio Dias.
Patient Consent
The patient consented to publication of the case, and this report does not contain any personal information that could lead to the identification of the patient. The patient provided written informed consent for publication of this case.
Disclosure
The authors have no financial or proprietary interest in the materials presented herein.
References
1. Finger PT, Chin KJ, Semenova EA. Intravitreal anti-VEGF therapy for macular radiation retinopathy: a 10-year study. Eur J Ophthalmol. 2016;26(1):60–66. doi:10.5301/ejo.5000670. Epub 2015 Sep 19.
2. Gupta A, Muecke JS. Treatment of radiation maculopathy with intravitreal injection of bevacizumab (Avastin®). Retina. 2008;28(7):964–968. doi:10.1097/IAE.0b013e3181706302
3. Mason JO, Albert MA, Persuad TO, Vail RS. Intravitreal bevacizumab treatment for radiation macular edema after plaque radiotherapy for choroidal melanoma. Retina. 2007;27(7):903–907. doi:10.1097/IAE.0b013e31806e6042
4. Stacey AW, Demirci H. Serial intravitreal bevacizumab injections slow the progression of radiation maculopathy following iodine-125 plaque radiotherapy. Open Ophthalmol J. 2016;10:103–110. doi:10.2174/1874364101610010103
5. Hykin PG, Shields CL, Shields JA, Arevalo JF. The efficacy of focal laser therapy in radiation-induced macular edema. Ophthalmology. 1998;105(8):1425–1429. doi:10.1016/S0161-6420(98)98023-X
6. Kinyoun JL, Zamber RW, Lawrence BS, Barlow WE, Arnold AM. Photocoagulation treatment for clinically significant radiation macular oedema. Br J Ophthalmol. 1995;79(2):144–149. doi:10.1136/bjo.79.2.144
7. Bakri SJ, Beer PM. Photodynamic therapy for maculopathy due to radiation retinopathy. Eye (Lond). 2005;19(7):795–799. doi:10.1038/sj.eye.6701637
8. Reid GA, Sahota DS, Sarham M. Observed Complications from Dexamethasone Intravitreal Implant for Treatment of Macular Edema in Retinal Vein Occlusion over 3 Treatment Rounds. Retinal. 2015;35(8):1647–1655.
9. Shields CL, Demirci H, Dai MBP, et al. Intravitreal triamcinolone acetonide for radiation maculopathy after plaque radiotherapy for choroidal melanoma. Retina. 2005;25(7):868–874. doi:10.1097/00006982-200510000-00009
10. Metelitsina TI. Peripheral retinopathy associated with aplastic anemia. Retin Cases Brief Rep. 2017;11(2):108–110. doi:10.1097/ICB.0000000000000302
11. Mansour AM, Lee JW, Yahng SA, et al. Ocular manifestations of idiopathic aplastic anemia: retrospective study and literature review. Clin Ophthalmol. 2014;17(8):777–787. doi:10.2147/OPTH
12. Mansour AM, Salti HI, Han DP, et al. Ocular findings in aplastic anemia. Ophthalmologica. 2000;214(6):399–402. doi:10.1159/000027532
13. Russo A, Avitabile T, Uva M, et al. Radiation macular edema after Ru-106 plaque brachytherapy for choroidal melanoma resolved by an intravitreal dexamethasone 0.7-mg implant. Case Rep Ophthalmol. 2012;3(1):71–76. doi:10.1159/000337144
14. Damasceno NP, Horowitz SA, Damasceno EF. Leuconostoc as cause of endophthalmitis post-intravitreal injection of ranibizumab. Ocul Immunol Inflamm. 2016;24(1):118–119. doi:10.3109/09273948.2014.898073
15. Damasceno NP, Horowitz SA, Damasceno EF. Anterior uveitis after treatment od age-related macular degeneration with ranibizumab and bevacizumab: uncommon complication. Clin Ophthalmol. 2012;6:1201–1205. doi:10.2147/OPTH.S31239
16. Reichstein D. Current treatments and preventive strategies for radiation retinopathy. Curr Opin Ophthalmol. 2015;26(3):157–166. doi:10.1097/ICU.0000000000000141
17. Roelofs K, Larocque MP, Murtha A, Weis E. The use of intravitreal anti-VEGF and triamcinolone in the treatment of radiation papillopathy. Ocul Oncol Pathol. 2018;4(6):395–400. doi:10.1159/000487543. Epub 2018 Jun 8.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
