Back to Journals » Clinical Ophthalmology » Volume 15
Treatment of Open-Angle Glaucoma with iStent Implantation Combined with Phacoemulsification in Polish Caucasian Population
Authors Kozera M , Konopińska J , Mariak Z, Rękas M
Received 28 November 2020
Accepted for publication 26 January 2021
Published 10 February 2021 Volume 2021:15 Pages 473—480
DOI https://doi.org/10.2147/OPTH.S293637
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Milena Kozera,1 Joanna Konopińska,2 Zofia Mariak,2 Marek Rękas1
1Department of Ophthalmology, Military Institute of Medicine, Warsaw, Poland; 2Department of Ophthalmology, Medical University of Bialystok, Białystok, Poland
Correspondence: Joanna Konopińska
Department of Ophthalmology, Medical University of Białystok, M. Sklodowska-Curie 24A STR, Białystok, 15-276, Poland
Tel +48-857468372
Fax +48-857468604
Email [email protected]
Purpose: The aim of this investigation was to evaluate the impact of iStent (the first-generation trabecular bypass) implantation with phacoemulsification on the intraocular pressure (IOP) and glaucoma medication in subjects with mild to moderate open-angle glaucoma (OAG) and cataract in a Polish Caucasian population.
Patients and Methods: This prospective case series covered 78 eyes of (57 Polish Caucasian patients) that had undergone iStent implantation in conjunction with cataract surgery. Patients were surveyed preoperatively and at postoperative day 1, week 1, and months 1, 3, 6, 12, and 24. Pre- and postoperative outcome measurements included visual acuity, IOP, and medication burden. Intraoperative and postoperative complications were noted for the safety profile. For effective treatment, an IOP reduction ≥ 20% was assumed, regardless of the use of IOP-lowering drops. Complete surgical success was defined as an IOP ≤ 15 mmHg, medications free, and a qualified surgical success as IOP ≤ 15 mmHg with or without medications.
Results: Post-operatively at two years, mean IOP reduced from 18.5 mmHg to 16.1 mmHg. The mean medication burden dropped from 1.8 to 0.4 at the end of follow-up. Preoperatively, 2 (2.6%) eyes were medication free, but by postoperative month 24, 53 (68%) eyes were medication-free (p < 0.05). Effective treatment was achieved in 50 cases (64%) at the end of follow-up period. Kaplan–Meier cumulative incidence of qualified success was 51.9% after 24 months, CI95 [41.9%; 64.4%], while cumulative incidence of complete success after 2 years of observation was 35.1%, CI95 [25.9%; 47.5%].
Conclusion: The iStent device combined with a cataract surgery served to decrease, significantly and positively, both IOP and medication use in the 24-months follow-up in patients with coexistent OAG and cataract in Polish patients.
Keywords: iStent first generation, trabecular by-pass, medication burden, microinvasive glaucoma surgery, open-angle glaucoma
Introduction
The goal in treating glaucomatous neuropathy is to reduce the intraocular pressure (IOP), which is the fundamental and only modifiable risk factor of glaucoma onset and progression.1,2
In open-angle glaucoma (OAG), the source of an elevated IOP is the expanded resistance to aqueous humor drainage through the trabecular meshwork. Changes in the trabecular meshwork’s extracellular matrix are considered to be a potential cause of this heightened resistance.3
Trabeculectomy was the first choice surgery in glaucoma surgical treatment for decades.4 Notwithstanding, owing to the danger of serious adverse events, as well as a decline in its effectiveness over time after surgery,4,5 the quest for more secure meticulous strategies is progressing persistently. For more than 10 years, extended exploration has been led on the utilization of low-intrusive glaucoma medical procedure strategies, named as microinvasive glaucoma medical procedure (MIGS). These devices have created an increasing interest, especially during the recent years.6 Ahmed and Saheb7 described MIGS group of treatments having the following properties: Ab interno approach through a clear corneal entry which spares the cutting of the conjunctiva; an insignificantly traumatic procedure to the tissues; an acceptable effective IOP reducing procedure; a high well-being safety profile in contrast to other glaucoma medical procedures, and a quick recuperation with negligible effect on the patient’s personal compliance.7 In 2014, the American Glaucoma Society and the US Food and Drug Administration (FDA) acknowledged that MIGS was described by the implantation of surgical devices either by an ab interno or an ab externo approach, in conjunction with very little or no scleral dismemberment.8 These devices are suitable to be performed either as a standalone procedure or in conjunction with phacoemulsification.9,10
The iStent (Glaukos Corporation, San Clemente, CA) is a heparin-covered L-formed device made of highly biocompatible titanium. It has a length of 1.0 mm and height of 0.33 mm, and a snorkel with a measurement of approximately 120 µm, and it is preloaded on a single-use injector. At the junction when embedded via ab interno approach, the snorkel is situated in the anterior chamber angle and the open half-pipe lumen is anchored in the Schlemm’s canal (SC). This appliance allows for reducing the increased resistance the aqueous humor encounters at the trabecular meshwork, and re-establishing a stable physiologic flow through the SC. A substantial number of studies have demonstrated significant long-term IOP reduction and medication reduction following the implantation of the iStent.11–16 Our previous study13 was focused on physiology of aqueous outflow and was intended to confirm the conclusions of work of Batista et al.17 Current manuscript is a typical clinic research, and similar to recent population studies18–20 its aim is to confirm the results of iStent implantation combined with a cataract surgery taking into consideration a Caucasian Polish population.
The main objective of the current examination was to evaluate the postoperative IOP and medication reduction after combined trabecular micro-bypass stent implantation and cataract phacoemulsification in an essentially Polish patient population experiencing mild-to-moderate OAG with coexisting cataract.
Patients and Methods
This is a prospective case series of 78 eyes (of 57 patients) aged 40 years or older with mild-to-moderate glaucoma, who qualified for the iStent first-generation implantation in conjunction with phacoemulsification. The study followed the tenets of the Declaration of Helsinki, and was approved by the Bioethics Committee at the Military Institute of Medicine (16/WIM/2013) and Bioethics Committee of Medical University of Bialystok (UMB/21/2014). Written informed consent was sought from all patients before they were enrolled into the study. The protocol adhered to the Methodology Sub-Committee and European Glaucoma Society (EGS) recommendations.21 The study was registered at ClinicalTrials.gov under the number NCT03807869.
Inclusion criteria were as follows: the cataract which had a significant effect on the visual acuity of the patients and one of the following conditions: progression of glaucoma in visual field (VF) confirmed by two consecutive examinations by the Humphrey visual field analyzer (Carl Zeiss AG, Germany) with SITA Standard 24–2 algorithm in the course of primary open-angle glaucoma (POAG) as well as pseudoexfoliative glaucoma (PXG), despite the use of IOP-lowering medications (1 to 4 active ingredients) or patients not tolerating or adhering to the treatment.
The glaucoma staging was described as a mean deviation (MD) 0 to −6.0 dB (mild glaucoma) and MD from −6.01 to −12.00 dB (moderate glaucoma). The IOP result was obtained using the Goldmann applanation tonometry based on the Glaucoma Intervention Study. The mean of two consecutive measurements was used to determine the IOP.
Exclusion criteria were as follows: lack of consent for study participation, severe proliferative diabetic retinopathy, cloudy cornea, advanced macular degeneration, advanced glaucoma, closed or narrow-angle glaucoma, history of glaucoma surgical interventions (as well as trabeculoplasty), and use of more than 4 IOP-lowering medications.
Surgical Technique
Surgeries were performed at two centres (Department of Ophthalmology, Military Medical Institute in Warsaw and Department of Ophthalmology Medical University in Bialystok) by two surgeons (MR and JK) under topical anesthesia. Cataract phacoemulsification with implantation of an artificial posterior-chamber lens insertion into the capsular bag was first performed in all patients, and subsequently, the implantation of the by-pass into the SC was undertaken. A single first-generation iStent was entered into the nasal quadrant of the SC through the existing clear corneal temporal incision. A Swan-Jacobs gonioscope was used to visualize the angle with the technique described previously.22,23 At the end of the surgery, an intracameral injection of cefuroxime was made. The eyes were treated with post-operative moxifloxacin 0.5% one drop four times a day for two weeks, steroids: loteprednol 0.5% one drop, twice daily for four weeks. The follow-up visits took place on Day 1, Week 1, Months 1, 3, 6, 12 (M12), and 24 (M24).
Study Procedures
Detailed data on the patient demographics (age, sex), previous treatment, and surgical procedures were collected at the preoperative visit. All the patients underwent a basic examination, ie, IOP measurement, best-corrected visual acuity (BCVA) obtained with the Snellen chart and converted into decimal, and biomicroscopic examination of the anterior segment and the fundus with detailed assessment of the retina and optic nerve disc.
Gonioscopy (Goldman 3 mirror gonioscopy lens) was assessed by the Schaffer classification.
Postoperatively: BCVA, IOP, and the number of IOP-lowering medications were assessed, and the anterior segment and the fundus were examined at each control visit. VF tests were performed at M12 and M24. Surgery-related complications were noted on each visit. No IOP-lowering medications were allowed from the day of the surgery. When the target IOP was not obtained, the medications were re-administered based on the EGS recommendations.21 The amount of medication used was determined by the number of active ingredients. Medication burden was described by the mean number of medications taken, and by proportional analyses.
Surgical Success
For effective treatment, an IOP reduction ≥20% was assumed, regardless of the use of IOP-lowering drops. Complete surgical success was defined as an IOP ≤ 15 mmHg, medications free, and a qualified surgical success as IOP ≤ 15 mmHg with or without medications.
Statistical Analysis
The statistical analysis was performed using the R program, version 3.5.1. The studied variables were presented using descriptive statistics. The normality of the distribution of the quantitative variables was assessed using the Shapiro–Wilk test, the indicators of skewness and kurtosis of the data, and the visual assessment of the histograms. Equality of variance was checked with the Leven test. The comparison of changes in individual parameters between individual observation periods was performed using the paired t-test or the Wilcoxon sum rank test, depending on the fulfillment of the assumptions of the parametric tests. The mean/median difference (MD) with 95% confidence level was also calculated as appropriate. The significance level of α = 0.05 was used; all tests were two-tailed.
Results
Demographic Data
A total of 78 eyes (of 57 patients) diagnosed with POAG (81%) or PXG (19%) and concomitant cataract in the mean age of 72.48 ± 8.70 years were included into the trial (Table 1).
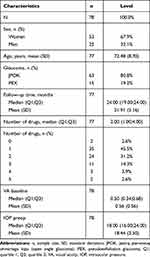 |
Table 1 Characteristics of the Patients |
Intraocular Pressure
Before surgery, the mean IOP was 18.44 ± 3.5 mmHg. The IOP reduction in comparison to the preoperative measurements was −2.33 ± 3.81 mmHg (−12,65 ± 19%) and −2.70 ± 4.18 mmHg (−14.06 ± 21%) at M12 and M24, respectively. The preoperative IOP values were statistically significantly and different from the IOP values at M12 and M24 (p < 0.0001). Preoperatively, no eyes had an IOP ≤15 mmHg, increasing to 23.4% post-op. Confidence interval at 95% (CI95) [13.8%, 35.7%] eyes at M12 and 32.9%, CI95 [22.5%, 44.6%] at M24. IOP data are summarized in Tables 2 and 3.
 |
Table 2 Change of Intraocular Pressure (IOP), Visual Acuity (VA) and Number of Medications After 12 Months and 24 Months of Observation |
 |
Table 3 Change of Intraocular Pressure (IOP) After 12 Months and 24 Months of Observation |
Medication Burden
A statistically significant reduction in the medication burden from preoperative to M24 was seen with p < 0.0001. In 8 patients (10.8%) there was a reduction of 3 medications, in 16 (21%) patients there was a reduction of 2 medications, in 39 (49%) cases, there was a reduction of 1 medication, there were 10 patients (12.8%) in whom no change in the amount of medication was observed. In 5 cases (6.4%) the amount of IOP lowering drops had increased by 1 versus preoperatively. In 68 patients (87.2%), the IOP and the number of medications were reduced at M24 compared to the preoperatively values. In 73 patients (94.7%) the IOP values were reduced or remained the same and the number of medications was reduced or remained the same compared to the preoperative values (Table 4).
 |
Table 4 The Amount of Intraocular Pressure (IOP) Lowering Medications Surgery and 24 Months Post-Op |
Preoperatively, 2 (2.6%) eyes were medication free, but by the postoperative M24, 53 (68%) eyes were medication-free (p < 0.0500).
Success Rate
Effective treatment was achieved in 50 cases (64%) at the end of follow-up period. Kaplan–Meier cumulative incidence of qualified success was 51.9% after 24 months, CI95 [41.9%; 64.4%], while cumulative incidence of complete success after 2 years of observation was 35.1%, CI95 [25.9%; 47.5%] (Figure 1).
Safety
Safety included the BCVA and intraoperative and postoperative complications. There were no reports of allergy or inflammation related to the iStent device.
We did not observe any significant intraoperative complications regarding the iStent implantation or the cataract surgery. In the first postoperative visit, microhyphema in the anterior chamber, and corneal edema with Descemet’s folds, were observed in seven eyes. All these cases spontaneously resolved within a week, without any additional treatment. Five cases of BCVA deterioration unrelated to the iStent device were reported. These eyes developed posterior capsular opacification and were treated with Nd: YAG capsulotomy. Following the treatment, the BCVA was restored.
Discussion
The study aims to contribute to the existing literature of MIGS while emphasizing its role in treating a Polish Caucasian population with mild to moderate glaucoma with concomitant cataract. In this trial, iStent implantation in combination with phacoemulsification resulted in statistically significant IOP reduction. Considering that every 1 mmHg of IOP reduction results in an average 10% declined risk of glaucoma progression, the additional 2.7 mmHg of IOP lowering provided by the combined iStent and cataract surgery is valuable, in addition to its coexisting good safety profile.24,25 This study adds some clinical knowledge to our previous study conclusions,13 in which we analyzed the physiology of aqueous humor outflow and confirmed the role of distal outflow tracts in regulation of IOP.17
The greatest advantage of iStent surgery is the reducing or elimination of the medication burden, which is advisable, since protracted use of IOP-lowering medications may lead to ocular surface impairment, hypersensitivity to the formulation,26 or may even reduce the success rate of a future trabeculectomy.27 Adherence to medication treatment regimens is known to be poor in patients diagnosed with OAG, with a reported non-adherence of up to 80%.28 In addition to the IOP reduction, a substantial medication burden reduction was observed in our study. A total of 68% of the eyes were medication-free at M24.
Our results are consistent with other population studies. In the prospective case series of the Manchester iStent study in 40 patients of the United Kingdom population in the mean age of 76.8 years, Patel et al29 reported similar preoperative IOP levels (21.1 mmHg). Six months after surgery the mean IOP dropped to 16.7 mmHg. The mean medication burden before surgery was 2.3 and decreased to 0.6 at the end of follow-up. Sixty-six percent of the patients was medication free at the end of the observation period. Gallardo et al19 reported an IOP reduction of 31% after three years in a mainly Hispanic population of 167 patients with a mean age of 74.6 ± 8.9 years. In his study, the mean preoperative IOP was 16.5 mmHg and it declined to 12.9 mmHg 12 months postoperatively. The mean medication burden decreased from 2.3 to 0.9 at the end of follow-up.
In the research performed in Asian populations, the initial IOP was lower than in our study, since in these nations the prevalence of normal-tension glaucoma (NTG) is higher compared to the European population.30 Kim and Lim31 conducted a retrospective research on the Korean population of iStent standalone procedure (group Solo) versus iStent combined with phacoemulsification (group Combo) with results reported up to 12 months, showing no statistically significant difference between these groups. In the Combo group, preoperatively IOP dropped from 15.8 ± 2.5 mmHg to 13.5 ± 1.4 mmHg, and the mean medication burden declined from 1.8 ± 1.0 to 0.3 ± 0.7 medications at 12 months. The mean age of the population at the time of surgery was 73.7 ± 4.5 years. Another study evaluating the iStent effectiveness on the Asian population was the one conducted by Nitta et al20 carried on a Japanese glaucoma population, which has a significantly greater incidence of NTG than most other populations. They enrolled 74 patients with a mean age of 73 years for implantation of the iStent combined with phacoemulsification. Among them, there were 30% of NTG. Mean baseline IOP was 16.5 ± 3.4 mmHg, and the mean number of IOP-lowering medication was 1.96 ± 0.98. After surgery, the mean IOP dropped to 13.2 mmHg at M12 and 13.6 mmHg at M24, and the mean number of medications dropped to 0.37 ± 0.74 at 24 months postop. They noted the reduction of IOP accounted to 20%, 11%, and 19% in POAG, NTG, and PXG, respectively, versus baseline IOP (18% mean). This number was similar to our outcomes however we did not include NTG patients in our group.
Recently Al Habash and Khan18 released interventional case series on 33 Arabic patients. The mean baseline IOP was 17.47 ± 5.44 mmHg and the mean number of IOP lowering medication was 2.69 ± 0.92. Subsequently, at 12 months postoperatively, the IOP dropped to 13.44 ± 1.99 mmHg (23.1% reduction) and the number of medication to 1.47 ± 1.13 (45.4% reduction). The mean age of this population at the time of surgery (66.34 ± 6.12 years) was lower than in the other aforementioned studies.
The safety profile is similar among all analyzed studies regardless of the population. None of the authors observed serious complications like choroidal detachment, retinal detachment, hypotony, and endophthalmitis. In the Gallardo study, 2 patients required additional glaucoma surgery within the first 12 months postoperatively; however, the study included moderate-to-severe glaucoma stages patients for which lower IOP is desirable.19 There were no major post-surgery complications related to the iStent and no vision deterioration noted in any eye. The mean BCVA was significantly improved in the majority of cases. There were no data of inflammation or allergy to the device up to date. Most common complications in the early postoperative period were corneal edema, inflammation, microhyphema, and transient IOP spikes.
The main strength of our study is that it is the first to our knowledge to report iStent implantation as a treatment for glaucoma in the Polish population. However, this study is not without limitations. First, it did not proceed with a wash-out period, so the unmedicated values of the IOP postoperatively were compared with the medicated IOP preoperatively. That might be the reason, for the unsubstantial drop of IOP given in mmHg. However, we believe that it is more important to assess the effectiveness of antiglaucoma procedures is the population efficacy rather than the IOP drop. For this reason, we have highlighted the number of patients meeting the surgical success criteria, and those who are medication-free. Despite the limitations, we believe that the study is relevant in providing evidence for the performance and safety of the iStent in the Polish population.
Conclusion
The results of our study showing a successful iStent surgery in a Polish Caucasian population which is consistent with the knowledge reported in the data for other populations. The iStent in conjunction with the phacoemulsification procedure decreased the medication burden and reduced the IOP in glaucoma patients. These changes were stable within the 24 months of the observation period. The study also demonstrates the high safety profile of the iStent implantation with concomitant phacoemulsification in patients with mild to moderate glaucoma. Further research could examine the iStent usage combined with a cataract surgery in a much larger, prospective setting. Additionally, safety and performance of the iStent in a long-term, practical setting in Poland may be explored.
Data Sharing Statement
All the materials and information will be available upon an e-mail request to the corresponding author. Names and exact data of the participants of the study may not be available owing to patient confidentiality and privacy policy.
Ethics Approval and Informed Consent
This study was performed in line with the principles of the Declaration of Helsinki. The protocol was approved by the Bioethics Committee at the Military Institute of Medicine. Written informed consent to participate in the study for at least 24 months was sought from all patients.
Consent for Publication
The participants have consented for the submission of results of the study to the journal.
Acknowledgments
We would like to thank Editage for English language editing.
Author Contributions
MK worked on the main text and worked on figures. JK worked on the main text. ZM worked on the main text. MR operated the patients and worked on the main text. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chauhan BC, Mikelberg FS, Balaszi AG, LeBlanc RP, Lesk MR, Trope GE. Canadian Glaucoma Study: 2. risk factors for the progression of open-angle glaucoma. Arch Ophthalmol. 2008;126(8):1030–1036. doi:10.1001/archopht.126.8.1030
2. Actis AG, Versino E, Brogliatti B, Rolle T. Risk factors for primary open angle glaucoma (POAG) progression: a study ruled in Torino. Open Ophthalmol J. 2016;10:129–139. doi:10.2174/1874364101610010129
3. Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47(1):226–234. doi:10.1167/iovs.05-1060
4. Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789–803.e782. doi:10.1016/j.ajo.2011.10.026
5. Soltau JB, Rothman RF, Budenz DL, et al. Risk factors for glaucoma filtering bleb infections. Arch Ophthalmol. 2000;118(3):338–342. doi:10.1001/archopht.118.3.338
6. SooHoo JR, Seibold LK, Radcliffe NM, Kahook MY. Minimally invasive glaucoma surgery: current implants and future innovations. Can J Ophthalmol. 2014;49(6):528–533. doi:10.1016/j.jcjo.2014.09.002
7. Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96–104. doi:10.1097/ICU.0b013e32834ff1e7
8. Caprioli J, Kim JH, Friedman DS, et al. Special commentary: supporting innovation for safe and effective minimally invasive glaucoma surgery: summary of a joint meeting of the American Glaucoma Society and the food and drug administration, Washington, DC, February 26, 2014. Ophthalmology. 2015;122(9):1795–1801. doi:10.1016/j.ophtha.2015.02.029
9. Craven ER, Katz LJ, Wells JM, Giamporcaro JE. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339–1345. doi:10.1016/j.jcrs.2012.03.025
10. Arriola-Villalobos P, Martínez-de-la-Casa JM, Díaz-Valle D, Fernández-Pérez C, García-Sánchez J, García-Feijoó J. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: a long-term study. Br J Ophthalmol. 2012;96(5):645–649. doi:10.1136/bjophthalmol-2011-300218
11. Ferguson TJ, Berdahl JP, Schweitzer JA, Sudhagoni RG. Clinical evaluation of a trabecular microbypass stent with phacoemulsification in patients with open-angle glaucoma and cataract. Clin Ophthalmol. 2016;10:1767–1773. doi:10.2147/OPTH.S114306
12. Fea AM, Consolandi G, Zola M, et al. Micro-bypass implantation for primary open-angle glaucoma combined with phacoemulsification: 4-year follow-up. J Ophthalmol. 2015;2015:795357.
13. Konopinska J, Kozera M, Krasnicki P, Mariak Z, Rekas M. The effectiveness of first-generation iStent microbypass implantation depends on initial intraocular pressure: 24-month follow-up-prospective clinical trial. J Ophthalmol. 2020;2020:8164703.
14. Ferguson TJ, Swan R, Ibach M, Schweitzer J, Sudhagoni R, Berdahl JP. Trabecular microbypass stent implantation with cataract extraction in pseudoexfoliation glaucoma. J Cataract Refract Surg. 2017;43(5):622–626. doi:10.1016/j.jcrs.2017.02.029
15. Fea AM, Belda JI, Rękas M, et al. Prospective unmasked randomized evaluation of the iStent inject (®) versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol. 2014;8:875–882. doi:10.2147/OPTH.S59932
16. Le JT, Bicket AK, Wang L, Li T. Ab interno trabecular bypass surgery with iStent for open-angle glaucoma. Cochrane Database Syst Rev. 2019;3(3):Cd012743. doi:10.1002/14651858.CD012743.pub2
17. Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49(12):5346–5352. doi:10.1167/iovs.08-1707
18. Al Habash A, Khan O. Outcomes of combined iStent trabecular micro-bypass and cataract surgery for the treatment of open-angle glaucoma in a Saudi population. Clin Ophthalmol. 2020;14:1573–1580. doi:10.2147/OPTH.S249261
19. Gallardo MJ, Supnet RA, Giamporcaro JE, Hornbeak DM. Outcomes of combined trabecular micro-bypass and phacoemulsification in a predominantly Hispanic patient population. Clin Ophthalmol. 2016;10:1931–1937. doi:10.2147/OPTH.S117403
20. Nitta K, Yamada Y, Morokado S, Sugiyama K. iStent trabecular micro-bypass stent implantation with cataract surgery in a Japanese glaucoma population. Clin Ophthalmol. 2020;14:3381–3391. doi:10.2147/OPTH.S274281
21. Mathew DJ, McKay BR, Basilious A, Belkin A, Trope GE, Buys YM. Adherence to World Glaucoma Association guidelines for surgical trials in the era of microinvasive glaucoma surgeries. Ophthalmol Glaucoma. 2019;2(2):78–85. doi:10.1016/j.ogla.2019.01.007
22. Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. doi:10.1016/j.ophtha.2010.07.007
23. Kozera M, Konopinska J, Mariak Z, Rekas M. Effectiveness of iStent trabecular micro-bypass system combined with phacoemulsification vs. phacoemulsification alone in patients with glaucoma and cataract depending on the initial intraocular pressure. Ophthalmic Res. 2020. doi:10.1159/000511456
24. Heijl A, Leske MC, Hyman L, Yang Z, Bengtsson B. Intraocular pressure reduction with a fixed treatment protocol in the Early Manifest Glaucoma Trial. Acta Ophthalmol. 2011;89(8):749–754. doi:10.1111/j.1755-3768.2009.01852.x
25. Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi:10.1001/archopht.120.10.1268
26. Baudouin C, Pisella PJ, Fillacier K, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999;106(3):556–563. doi:10.1016/S0161-6420(99)90116-1
27. Hoang TKH, Kim YK, Jeoung JW, Park KH. Relationship between age and surgical success after trabeculectomy with adjunctive mitomycin C. Eye (Lond). 2018;32(8):1321–1328. doi:10.1038/s41433-018-0071-x
28. Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(Suppl1):S57–S68. doi:10.1016/j.survophthal.2008.08.002
29. Patel I, de Klerk TA, Au L. Manchester iStent study: early results from a prospective UK case series. Clin Exp Ophthalmol. 2013;41(7):648–652. doi:10.1111/ceo.12098
30. Kim KE, Park KH. Update on the prevalence, etiology, diagnosis, and monitoring of normal-tension glaucoma. Asia Pac J Ophthalmol (Phila). 2016;5(1):23–31. doi:10.1097/APO.0000000000000177
31. Kim HJ, Lim SH. Clinical outcomes of trabecular microbypass stent (iStent) implantation in medically controlled open-angle glaucoma in the Korean population. Medicine (Baltimore). 2020;99(33):e21729. doi:10.1097/MD.0000000000021729
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

