Back to Journals » Clinical Ophthalmology » Volume 15
Treatment of Keratoconus with WaveLight Contoura and Corneal Cross-Linking Combined
Authors Motwani M
Received 4 March 2021
Accepted for publication 10 May 2021
Published 14 June 2021 Volume 2021:15 Pages 2455—2472
DOI https://doi.org/10.2147/OPTH.S303559
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Manoj Motwani
Motwani LASIK Institute, San Diego, CA, 92121, USA
Correspondence: Manoj Motwani
Motwani LASIK Institute, 4520 Executive Dr., Suite 230, San Diego, CA, 92121, USA
Tel +1 858 554-0008
Email [email protected]
Purpose: To investigate the outcomes of the treatment of keratoconus/corneal ectasia utilizing topographic-guided ablation (WaveLight Contoura) followed by corneal cross-linking.
Methods: Thirty-six eyes of 21 patients were treated for keratoconus/corneal ectasia utilizing topographic guided ablation photorefractive keratectomy (PRK) for treatment of corneal higher-order aberrations and refractive error followed immediately by 15-minute cross-linking were examined retrospectively. Six-month results were analyzed via measurement of vision, refraction, residual higher-order aberrations (HOAs), residual lower-order and higher-order aberrations, as well as for loss or gains of lines of best-corrected visual acuity.
Results: All eyes save one had reduction in K1, K2, K Max, K Mean. All eyes had reduction in manifest astigmatism, Contoura-measured astigmatism, higher-order aberrations, higher-order aberrations grouped with lower-order aberrations (Grouped). Four eyes had lost 1– 2 lines of vision, mainly to corneal haze formation, 17 eyes gained lines of vision, and 15 eyes equaled their pre-op best-corrected visual acuity. Eight eyes from four sample patients have their data included in this manuscript to demonstrate the procedure and the outcomes.
Conclusion: Treatment with WaveLight Contoura combined with 15-minute corneal cross-linking is an effective and safe treatment for keratoconus and should be considered a primary treatment to prevent corneal transplant as well as improve vision and corneal irregularity.
Keywords: astigmatism, best-corrected visual acuity, corneal cross-linking, higher-order aberrations, keratoconus, topographic-guided ablation
Two Letters to the Editor have been received and published for this article
A Response to Letter has been published for this article.
Introduction
Corneal ectasia, of which keratoconus is one, is a progressive corneal ectatic degeneration that causes progressive corneal protrusion, irregular astigmatism, and corneal thinning.1 The progressive corneal irregularity can cause severe impairment of vision necessitating visual and surgical intervention. Historically in the United States (US) treatment has utilized contact lenses (soft/rigid gas permeable (RGP)/scleral), spectacles, Intacs corneal implants to attempt to modify the shape, and corneal transplant.2
Dr Theo Seiler in 2003 invented the procedure of corneal cross-linking which used riboflavin with ultraviolet light that catalyzed a reaction that increased the number of bonds between keratocytes increasing corneal rigidity slowing and stopping progression of the corneal irregularity.3–5 In approximately 2003 topographic guided ablation was first used on WaveLight lasers to treat corneal irregularity with excimer laser by segmental ablation driven by placido topographic images. Dr John Kanellopoulos combined the two procedures of decreasing, or “normalizing,” corneal irregularity with immediate subsequent increase of corneal rigidity with corneal cross-linking, essentially “freezing” the newly normalized cornea into place.6 Dr Kanellopoulos called this the Athens Protocol.7
In the United States, we now have US Food and Drug Administration (FDA) approval of both topographic guided ablation (WaveLight Contoura, WaveLight Laser Technologie AG, Erlangen, Germany) and corneal cross-linking.8–10 In this study, we combine the two procedures utilizing the Athens Protocol, with some modifications from The San Diego Protocol for repairing and reconstructing corneas to perform keratoconus correction.11
The goals of the procedure, in order of importance, are:
- Stop progression of keratoconus and strengthen the cornea to avoid cornea transplant.
- Normalize the cornea by decreasing the irregularity of the anterior cornea improving the optics of the cornea, as well as increasing the ability of further refractive correction via spectacles, soft contact lenses, and RGP/scleral lenses.
- In cases where sufficient tissue is available, also correct the sphere and cylinder to get as close to plano correction as possible.
Materials and Methods
We retrospectively examined patients who had keratoconus/corneal ectasia treatment utilizing Contoura and corneal cross-linking, and also had at least 6 months of follow-up results. As many of our patients come from out of town, we do not have full long-term results on many of our treatments; therefore, we included all eyes in this study where at least 6-month results were obtained at our clinic. We were able to analyze data from 36 eyes of 21 patients.
Epithelium removal was performed via 20% alcohol solution placed on the corneal surface via 8-mm corneal well for 30 seconds. Manual removal was performed with a cellulose sponge and corneal epithelial scraper. WaveLight Contoura was used to treat the corneal higher-order aberrations and irregular astigmatism of the anterior cornea.11 Surgical planning utilized the measured astigmatism up to the maximum 3 diopters (D) allowed in the Contoura FDA approval, with the sphere adjusted by the spherical equivalent of the difference between the manifest and measured astigmatism, if possible. Treatment parameters were designed to remove approximately 60–70 µm of tissue leaving a corneal bed of 350 µm to maintain safety parameters for corneal cross-linking.
Unfortunately, the 5- and 5.5-mm optical zones for Contoura are not available in this country so the smallest zone that can be used is 6 mm. This limits the amount of normalization and correction possible as the smaller zones allow for a greater amount of treatment. It is also more difficult to get 6-mm placido topography images on an irregular cornea with the WaveLight Topolyzer Vario (Alcon Inc, Ft. Worth, TX, USA) as opposed to 5-mm images. The unavailability of the 5-mm optical treatment zone in Contoura does impact the difficulty of surgical planning and the potential candidacy of a patient.
If enough tissue is available, “Full Correction” was performed. This amounted to correcting Contoura-measured sphere and cylinder by 70%, allowing the remainder to be corrected by the flattening of the cornea from the corneal cross-linking. “Partial correction” was performed in patients where less than 70% of the sphere and cylinder was corrected during the ablation. “Normalization ablation” was performed to reduce only the higher-order aberration, and some portion of the Contoura-measured astigmatism, usually between 0 D and 2 D. These patients usually had more severe corneal irregularity which required more tissue to reduce the HOA in cornea that had been thinned significantly by the keratoconus.
If keratoconus were severe enough that only performing HOA reduction would still reduce the corneal thickness to less than 350 µm, but still over 300 µm, the cornea was swollen to 400+ µm with hypotonic saline, ie sterile water.
Once corneal ablation is complete, 0.2 mg/cc mitomycin C (MMC) was applied for 20 seconds or 40 seconds to each laser treated eye utilizing an 8-mm corneal shield. Corneal irrigation was performed after MMC placement with balanced salt solution.
Corneal saturation with riboflavin was performed immediately after laser treatment for 2 minutes as this was “epi-off.” As corneal cross-linking (CXL) treatment was done on the right eye first as a rule, the left eye would be re-saturated for another minute before UV treatment.
Dr. Kanellopoulos has found that the 15-minute cross-linking regimen is the most stable long-term and the preferred one.6 The US only has FDA approval for the Avedro 30-minute procedure, so 15 minute cross-linking was performed as an off-label procedure.
15-minute cross-linking parameters: 5.4 J/cm2; 6 mW/cm2; 9-mm spot size of homogenous UV beam
Riboflavin formulation used was the same as Dr. Kanellopoulos prefers in The Athens Protocol, utilizing 0.1% riboflavin w/Carboxymethyl Cellulose pH Balance (no dextran). Dr. Kanellopoulos prefers and recommended a non-dextran solution. This is also off-label in the US as the FDA approved riboflavin solution has dextran.
All LASIK procedures were performed on the WaveLight EX500 excimer laser. All procedures were performed by one surgeon (MM) at one center in San Diego, California. All topographies were obtained utilizing the Topolyzer Vario (Alcon Surgical, Fort Worth, TX, USA). All epithelial thickness maps (ETM) were obtained with the Optovue iVue (6-mm ETM) or Avanti (9-mm ETM) devices (Optovue, Fremont, CA, USA).
Post-operative care consisted of prednisolone acetate 1% qid for 1 week, ofloxacin qid x 1 week, and Prolensa qd PRN for pain during epithelial healing. Bandage contact lenses were removed with healing of the corneal abrasion which was between 4 and 7 days on average.
FML was used qid x 5 weeks after the first week to prevent haze. This has now been changed to Lotemax qid x 2 weeks, then bid x 5 weeks after the first week.
All patients signed written informed consent forms allowing their data to be used in this study and published including sample cases 1–4. This study falls under the exemption of the Health and Human Services (HHS) Policy for the Protection of Human Research Subjects 45 CFR 46.104 for retrospective studies and for exempt research and thus, no Institutional Review Board approval was required. This study also conforms to the Declaration of Helsinki guidelines. There were no safety-related incidents that occurred or were reported to Alcon Inc. or WaveLight concerning patients involved in this study.
Results
Thirty-six eyes of 21 patients are included in this study, 14 men and 7 women. The average age was 35 years, range 20–74 years. All eyes had diagnoses of corneal ectasia: keratoconus (33 eyes), LASIK-induced ectasia (2), or pellucid marginal degeneration (1). All results from eyes that had at least 6 months of post-operative follow-up.
Table 1 shows K values from the Topolyzer pre- and post-op, as well as K Max and K Mean. It also shows the reduction in manifest and Contoura-measured cylinder. Table 1 displays this same information for each of the three groups: Normalization/+, Partial Correction, Full Correction. Only eye had an increase in K MAX of 0.2 D and increase of K Mean of 2.4 D, but this eye gained 1 line of best-corrected vision post-operatively. All other eyes demonstrated a decrease in all of these values. Note the decreasing levels of manifest and Contoura-measured astigmatism in both pre- and post-op values in the three cohorts as the amount of correction increases. This correlates with extensive disease having greater pre-operative values and allowing the least amount of correction.
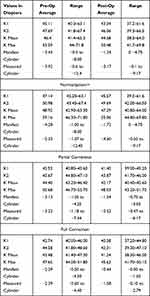 |
Table 1 Topolyzer Analysis Results |
In Table 2 Zernike polynomials are presented for fourth-order higher-order aberrations (HOA), and Grouped polynomials include lower-order aberrations (does not include tilt or constant height/piston). Overall reduction of aberrations for all eyes was 31.87% for HOA and 25.5% for the Grouped values. Six eyes had an increase of HOA with an average increase of 0.123 µm, and all polynomials (except tilt and constant height/piston) increased 0.094 µm. Note that the only cohort where the post-operative Grouped values were actually higher than the HOA reduction value was in the Full Correction cohort as would be expected.
 |
Table 2 Zernicke Polynomial Results |
Two tailed T tests were performed to look for statistical significance. Comparisons of pre-op to post-op HOA values and Grouped values were statistically significant, with P less than 0.05. This test was also performed comparing pre-op HOA and Grouped values to the three different subgroups. The values were statistically significant comparing the overall group to the Normalization group and the Full Correction group with P values less than 0.05, but the Partial Correction group values were not statistically significant with a P value of over 0.05.
Improvements in lines of vision (Supplementary Figure S1): Four eyes lost 1–2 lines of best-corrected vision (2 eyes lost one line, 2 eyes lost 2 lines). Two of these eyes had increased post-op HOA values, and all had haze. Seventeen eyes gained 1–4 lines of vision (9 eyes gained 1 line, 4 eyes gained 2 lines, 3 eyes gained 3 lines, and 1 eye gained 4 lines). Fifteen eyes achieved best-corrected visual acuity (BCVA) post-operatively equal to pre-op.
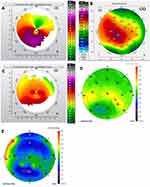
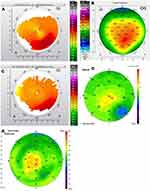
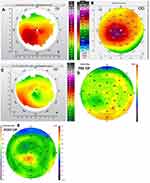
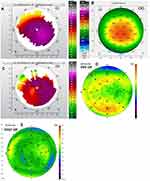
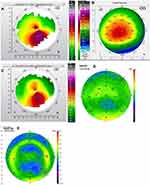
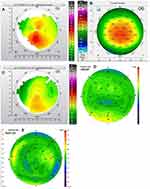
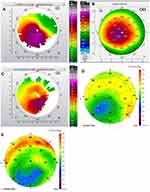
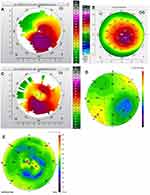
Figures 1–8 and Tables 3–18 show visual acuity, K MAX, and higher-order aberration results for the right and left eyes of 4 cases.
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Table 17 CASE 4. Left Eye Visual Acuity (Figures 8A–E) |
 |
Table 18 CASE 4. Left Eye K MAX and HOA (Figures 8A–E) |
Discussion
Keratoconus as a disease can be very debilitating for the patient and can lead to a lifetime of vision problems. Currently, patients often receive RGP lenses or scleral lenses,2 but fitting highly irregular corneas as keratoconus progresses is difficult, frustrating, expensive, and does not prevent progression. As keratoconus worsens and even scleral lenses are hard to fit, many surgeons perform corneal transplants. Unfortunately, these can lead to irregular astigmatism and a new set of visual problems for the patient.
Corneal cross-linking has added a new tool to the treatment of keratoconus (KC), but although increasing corneal rigidity and preventing progression, it does little to increase visual quality and decrease irregularity that can make fitting of even scleral lenses very difficult and uncomfortable for the patient.12 Furthermore, many surgeons performing CXL perform it “epithelium on”, which simply does not have the corneal penetration and depth of corneal stiffening as “epi-off.” This would theoretically leave a certain number of patients still at risk of progression.
Intacs have been used for years by some surgeons, but they are hand placed and not actually reconstructing the irregularity but physically compressing the cornea to grossly remove irregularity. This, by its hand-placed nature, is a less specific method to treat the irregularity of the cornea, does not prevent progression, and in our experience does not always improve vision or visual quality. We have removed Intacs from keratoconus patients and found little benefit after removal to vision or decrease of corneal irregularity/astigmatism. We realize this may be due to the self-selection of patients who did not do well with Intacs. On the other hand, topographic-guided excimer laser ablation selectively removes specific tissue to reduce the corneal irregularity, and combined with CXL is in theory a far more accurate treatment than Intacs. Self-evidently, the type of HOA present in keratoconus patients are high levels of coma caused by the corneal cone, and usually peri-pupillary.
Utilizing topographic guided ablation to actually address the corneal higher-order aberrations and to normalize the corneal shape directly treats the visual quality and optical aberrations,13 and the more regularly shaped cornea makes the fitting of scleral lenses, if necessary, afterwards much easier and more comfortable for the patient. Since the epithelium is already removed after Contoura treatment, “epi-off” CXL is performed to stiffen the cornea which produces deeper penetration of the cross-linking.
When performing this procedure, we separated the groups depending on the extent of treatment the tissue available allowed. This was in an attempt to further understand and analyze how these different treatment choices impacted reduction of corneal irregularity by measurement of the Zernike polynomials. We termed eyes where enough tissue was available to only reduce the corneal higher-order aberrations “Normalization.” If we had enough tissue to also remove some of the Contoura-measured astigmatism we termed “Normalization+.” These were performed on the most severely irregular corneas, and although most ended up with significant refractive errors post-operatively, we found some patients actually had fairly large decreases in refractive error as the cornea and corneal epithelium healed over the 6 months. We surmise this was likely due to a combination of healing and a manifest refraction that was artificially higher due to the multi-focal nature of the irregular cornea.
If enough tissue available, we would maximize the treatment of astigmatism (unfortunately only up to 3 D in US-approved Contoura), and then treat as much sphere as possible. A partial correction of sphere we termed “Partial Correction”. If the patient were fortunate enough to have a refraction that could be fully treated in the limitations of tissue removal, “Full Correction” was performed which incorporated 70% of the sphere and 70% of the astigmatism. The residual 30% is theoretically treated by flattening of the cornea from the CXL. The astigmatism used for treatment was always the Contoura-measured astigmatism, and not the manifest astigmatism.
Table 1 lists topographic K values pre- and post-operatively. Reduction of K1, K2, K Mean, and K Max were achieved in all cases except 1. Reduction in manifest cylinder was significantly greater than for Contoura-measured cylinder, which may be due to greater capacity for cortical compensation of residual higher-order aberration/astigmatism due to the more regular cornea created as have described in a past manuscript.14 Notably, one eye that increased in K Max by 0.2 D and K Mean by 2.4 D also resulted in an eye with a UCVA of 20/40 and an increase in 1 line in BCVA. This eye has not increased in K Max or K Mean since the 6-month post-op, and at approximately a year out this patient shows no further sign of progression.
Table 2 shows pre-and post-operative higher-order aberration values in micrometers for sixth-order HOA and also for the Grouped category which includes lower-order values but does not include tilt or constant height/piston. These values are presented for the three different groups of treatment, normalization, partial correction, and Full Correction groups, as well as for the overall cohort of patents. This shows an overall reduction in HOA of about 30%. The least reduction HOA percentage occurred in the “Full Correction” group, ostensibly as these were the eyes with the mildest disease. The eyes with the largest amount of reduction in Zernike values were the “Partial Correction”, as these were eyes with more advanced disease but enough tissue available to perform more treatment, ie, HOA and lower-order astigmatism than simply normalization. Unfortunately, the Partial Correction group was not deemed statistically significant from the pre-op total group, so the conclusion from this analysis is somewhat limited.
Case 1 shows the treatment for right (Figure 1) and left (Figure 2) eyes. This patient had more severe keratoconus on the right eye vs the left but was able to achieve 20/20 and 20/15 vision uncorrected post-operatively even with the presence of haze in both eyes. The right eye is notable for a significant percentage reduction of HOA, more so than the left, but the left achieves better vision. This tells us that reduction of HOA is important, but other factors also play a part such as haze and the regularity of the cornea through the central visual axis as is noted in comparing the post-operative topographies of the right and left eye which show the more regularly shaped left cornea.
Case 2 shows the treatment for right (Figure 3) and left (Figure 4) eyes. This case is notable for severe disease in the left eye with a large pre-operative manifest refraction that over time surprisingly decreased to plano with a 20/40 UCVA and BCVA. We had counseled this patient that he would likely be significantly myopic post-operatively which did not occur. It is evident that the patient was looking through a flat area of his multi-focal cornea pre-operatively, and as the corneal epithelium renormalized post-procedure over time the myopia decreased. Also remarkable was the high level of residual HOA (90%) and yet this patient comfortably achieved 20/40 vision. This also may be due to a cornea that is still highly aberrant but regular enough through the central visual axis to allow for good vision.
Case 3 shows the treatment for right (Figure 5) and left (Figure 6) eyes. This patient is notable for a 20/20 result with plano correction in the left eye, and an uncorrected 20/40 vision in the more severe right eye. This patient may continue to improve as these measurements were on the 6-month visit. This patient is also notable for only having a 40-second MMC treatment and having trace haze. As mentioned below, we are re-evaluating the use of MMC, but that this time we were attempting to decrease haze by increasing MMC time on the cornea.
Case 4 shows the treatment for right (Figure 7) and left (Figure 8) eyes. This patient had severe disease in both eyes and had a remarkable reduction in refractive error in both eyes after receiving only normalization laser ablation with no refractive/astigmatic correction at all. Surprisingly, they achieved BCVA of 20/20 and 20/25, respectively, with only a small refractive error. This patient had significant haze in the 20/25 eye which was also notable for the approximately 50% reduction in corneal HOA.
Note in all of these cases the epithelium thickness map is abnormal in all cases pre- and post-operatively, with multiple cases of epithelial compensation of the cone denoted by thinner corneal epithelium over the cone. This compensation would reduce the level of aberration measured by the Topolyzer for Contoura processing. The use of excimer laser to remove the epithelium with depth set by the average epithelial thickness would reduce the aberration being masked by the epithelium and likely increase the percentage of corneal HOA reduction. It is this transepithelial Contoura PRK procedure that we are now performing utilizing a Nidek EC-5000 excimer laser for epithelium removal via PTK.
We found that in virtually all cases as the epithelium renormalized and became smoother and the cornea healed patients reported better vision and quality of vision, even those that had a loss of a line of vision. Some eyes with severe pre-operative disease were further helped via scleral lens fitting, which was much easier and more comfortable post-operatively as the cornea was more regular.
The increase in visual quality reported from these patients was quite remarkable, and even patients with residual refractive errors were happy enough that few wore corrective lenses for smaller amounts of refractive error. We found that patients that achieved good uncorrected vision in one eye were often uninterested in wearing a scleral lens or correction for the other eye.
Our biggest issues were with haze. Attempting to increase steroid strength by switching from fluorometholone to loteprednol 0 post-operatively resulted in only limited effectiveness. We attempted to increase MMC treatment time to 40 seconds. Since the switch to 40 seconds of MMC time, we seemed to have a decrease in haze overall, but this has been a limited sample with incomplete data. In the sample cases, Cases 1, 2, and 4 had 20 seconds of MMC treatment time, while Case 3 had a 40-second treatment. The only eye without haze was the OD of Case 4. Since the first version of this manuscript was submitted for publication, a study published by Awwad et al has demonstrated that MMC can increase haze after cross-linking, and the haze also develops at deeper levels of the cornea instead of superficial as in PRK.15 Mild levels of haze have not been inconsistent with good vision as Case 1 shows, and PRK excimer laser ablation mainly causes more superficial haze which is easier to treat than the deeper haze described as being caused by MMC, so it may well be advisable to eliminate MMC from the procedure altogether. This will be a point of further study.
Some patients did have epithelial healing issues. There may be some relation to corneal cross-linking, as we have found patients with prior epi-on CXL did seem to have epithelium that healed more slowly. For this reason, we have now switched to minimal steroid treatment in all patients during initial epithelial abrasion healing to prevent steroid response from slowing epithelial healing.
Due to the large amount of epithelial compensation of aberration in KC patients preventing accurate and full measurement of the central corneal aberration, we have also now started utilizing transepithelial PTK as an epithelial removal. By utilizing OCT epithelial thickness measurements, we are able to determine the areas and depth of epithelial compensation. By utilizing excimer laser PTK, we remove tissue to a not only epithelial tissue but also stromal tissue that the epithelium has compensated which would not be measurable via topography.
It is notable that in many patients the highest amount of corneal tissue removal during topographic-guided ablation was often not centrally, but in the superior periphery to normalize the superior flat area. It was essential during surgical planning to only use the central tissue removal depth, and not the overall ablation depth during calculations to leave a corneal bed of 350 µm to ensure safe corneal cross-linking. We calculated these this utilizing pachymetry from scheimpflug imaging, OCT pachymetry, OCT epithelial thickness maps, as well as the optical pachymetry on the WaveLight EX500.
The lack of the 5.0-mm zone optical treatment zone in the US version of WaveLight EX500 Contoura is an unfortunate limitation. The option of a smaller optical zone would allow for less tissue removal allowing for a greater amount of cornea irregularity and refractive correction in an available amount of corneal tissue. It is unknown why the 5.0-mm optical zone is locked out here in the US, when non-FDA approved optical treatment zones are allowed in Wavefront Optimized treatments with a disclaimer in the software. Allowing the use of the 5.0-mm optical zone off-label would be a great help in treating these patients and would allow some patients to become candidates for treatment where the 6.0-mm ablation zone removes too much tissue, or when the cornea is so irregular that the Topolyzer Vario is unable to obtain 6.0 mm scans.
A note on corneal cross-linking alone. There seems to be a belief among some cornea surgeons that treatment with CXL can be performed as a primary therapy for keratoconus, and topographic guided excimer laser treatment can be performed at a later time if the patient wishes. Unfortunately, the laser ablation will remove the cross-linked tissue, and the cornea must be cross-linked again, especially if the original CXL was “epi-on.” Treatment with CXL will stop progression, but will not do anything to help the vision, the irregularity, corneal optics, or aid in the fitment of scleral/RGP/soft lenses as CXL mainly stiffens the cornea overall and does not specifically treat corneal irregularity (although some limited decrease in irregularity can occur due to the stiffening of the cornea).
Keratoconus can be an extremely debilitating disease, and the procedure of topographic guided ablation combined with CXL holds great promise in treating keratoconus patients and should be considered a front-line primary treatment for those with keratoconus. We believe that utilizing transepithelial PRK will decrease corneal HOA and create a more regular cornea further decreasing residual HOA, but care must be taken to ensure total stromal tissue removal to prevent excessive resultant thinning of the corneal stroma prior to CXL. Finally, we continue to develop our MMC and topical steroid treatment protocols to find the right balance in between healing and haze prevention.
Abbreviations
BCVA, best-corrected visual acuity; CXL, corneal cross-linking; D, diopters; ETM, epithelial thickness maps; FDA, Food and Drug Administration; HHS, Health and Human Services; HOAs, higher-order aberrations; LASIK, laser-assisted in situ keratomileusis; K MAX, maximum keratometry value; MMC, mitomycin C; OCT, optical coherence tomography; OD, right eye; OS, left eye; PRK, photorefractive keratectomy; PRN, pro re nata or as needed; PTK, phototherapeutic keratectomy; qd, daily; qid, 4 times daily; RGP, rigid gas permeable; UCVA, uncorrected visual acuity; US, United States; UV, ultraviolet.
Acknowledgments
The author would like to thank Julie Crider, PhD, for editing contributions.
Disclosure
Dr Manoj Motwani reports a patent issued (U.S. patent nos. 10,857,032 and 10,857,033). The author reports no other conflicts of interest in this work.
References
1. Sorkin N, Varssano D. Corneal collagen crosslinking: a systematic review. Ophthalmologica. 2014;232(1):10–27. doi:10.1159/000357979
2. Boyd K. Keratoconus diagnosis and treatment. Am Acad Ophthalmol. 2020. Available from: https://www.aao.org/eye-health/diseases/keratoconus-diagnosis. Accessed January 22, 2021.
3. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a–induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627. doi:10.1016/S0002-9394(02)02220-1
4. Munir WM. Corneal crosslinking. Am Acad Ophthalmol. 2016. Available from: https://www.aao.org/current-insight/corneal-crosslinking. Accessed January 22, 2021.
5. Koller T, Seiler T. [Therapeutic cross-linking of the cornea using riboflavin/UVA]. Klin Monbl Augenheilkd. 2007;224(9):700–706. German. doi:10.1055/s-2007-963492
6. Kanellopoulos AJ. Long term results of a prospective randomized bilateral eye comparison trial of higher fluence, shorter duration ultraviolet A radiation, and riboflavin collagen cross-linking for progressive keratoconus. Clin Ophthalmol. 2012;6:97–101. doi:10.2147/OPTH.S27170
7. Kanellopoulos AJ, Binder PS. Management of corneal ectasia after LASIK with combined, same-day, topography-guided partial transepithelial PRK and collagen cross-linking: the Athens protocol. J Refract Surg. 2011;27(5):323–331. doi:10.3928/1081597X-20101105-01
8. Motwani M, Pei R. The use of WaveLight Contoura to create a uniform cornea: 6-month results with subjective patient surveys. Clin Ophthalmol. 2018;12:1559–1566. doi:10.2147/OPTH.S175661
9. Mathew J, Stonecipher K. Wavefront optimized versus topography guided corneal ablations with WaveLight® Platform: a summary of visual outcomes. White paper; 2020. Available from: https://us.alconscience.com/wp-content/uploads/2020/10/Wavefront-Optomized-Versus-Topography-Guided-Corneal-Ablations-With-WaveLight-Platform_A-Summary-Of-Visual-Outcomes-US-ALL-2000003.pdf.
10. Vimont C. Corneal collagen cross-linking approved to treat keratoconus in U.S. Am Acad Ophthalmol. 2016. Available from: https://www.aao.org/eye-health/news/cross-linking-approved-keratoconus-united-states. Accessed January 22, 2021.
11. Motwani M. A protocol for topographic-guided corneal repair utilizing the US Food and Drug Administration-approved wavelight contoura. Clin Ophthalmol. 2017;11:573–581. doi:10.2147/OPTH.S127855
12. Seiler TG, Fischinger I, Senfft T, Schmidinger G, Seiler T. Intrastromal application of riboflavin for corneal crosslinking. Invest Ophthalmol Vis Sci. 2014;55(7):4261–4265. doi:10.1167/iovs.14-14021
13. Lin DT, Holland SR, Rocha KM, Krueger RR. Method for optimizing topography-guided ablation of highly aberrated eyes with the ALLEGRETTO WAVE excimer laser. J Refract Surg. 2008;24(4):S439–S445. doi:10.3928/1081597X-20080401-22
14. Motwani M. The use of WaveLight((R)) contoura to create a uniform cornea: the LYRA protocol. Part 1: the effect of higher-order corneal aberrations on refractive astigmatism. Clin Ophthalmol. 2017;11:897–905. doi:10.2147/OPTH.S133839
15. Awwad ST, Chacra LM, Helwe C, et al. Mitomycin C application after corneal cross-linking for keratoconus increases stromal haze. J Refract Surg. 2021;37(2):83–90. doi:10.3928/1081597X-20201124-01
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.