Back to Journals » Clinical Ophthalmology » Volume 16
Treatment of Epithelial Basement Membrane Dystrophy to Optimize the Ocular Surface Prior to Cataract Surgery
Authors Yeu E , Hashem O , Sheha H
Received 30 December 2021
Accepted for publication 23 February 2022
Published 15 March 2022 Volume 2022:16 Pages 785—795
DOI https://doi.org/10.2147/OPTH.S356421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Elizabeth Yeu,1,2 Omar Hashem,3 Hosam Sheha3– 5
1Virginia Eye Consultants, Norfolk, VA, USA; 2Eastern Virginia Medical School, Norfolk, VA, USA; 3Research Institute of Ophthalmology, Cairo, Egypt; 4Florida International University Herbert Wertheim College of Medicine, & Glaucoma Research Organization, Miami, FL, USA; 5Manhattan Eye, Ear and Throat Hospital, Hofstra Northwell School of Medicine, New York, NY, USA
Correspondence: Hosam Sheha, Department of Ophthalmology, Manhattan Eye, Ear and Throat Hospital, 210 E 64 Street, New York, NY, 10065, USA, Tel +1 917-810-9555, Email [email protected]
Purpose: To assess the effectiveness of cryopreserved amniotic membrane (CAM) after debridement in treating epithelial basement membrane dystrophy (EBMD) prior to cataract surgery.
Methods: This pilot study included 2 treatment groups: a prospective study group of 9 subjects with significant EBMD who received debridement followed by self-retained CAM, and a retrospective, control group of 10 consecutive subjects who received debridement followed by a bandage contact lens (BCL). Slit-lamp photography after fluorescein staining were used to monitor healing. Corneal topography and IOL calculation were compared at baseline and 1 month after the procedure. Refraction and ocular surface stability were also compared after cataract surgery.
Results: Corneal reepithelialization after debridement occurred in 4.6 ± 0.8 days in the study group and 6.8 ± 0.6 days in the control (p < 0.05). Corneal topography showed changes in curvature from 43.5 ± 1.2D at baseline to 44.6 ± 1.2D at 1 month in the study group and from 45.0 ± 0.6D to 45.7 ± 0.8D in the control (p = 0.38). Average change in IOL calculation was 1.56 D in the study group, compared to 0.95 D in control (p = 0.29). Post-cataract refraction in both groups was within ± 0.5 Diopter of the anticipated, and corneal surface remained stable without EBMD recurrence.
Conclusion: Management of ocular surface disorders prior to cataract surgery stabilizes IOL calculation and reduces postoperative refractive surprises. CAM relatively accelerated healing after debridement; however, it was not better than BCL in stabilizing the ocular surface and improving visual outcome. The use of CAM in cases of EBMD remains speculative.
Keywords: amniotic membrane, cataract, EBMD, ocular surface
Introduction
The ocular surface is the first refractive interface of the eye that plays a major role in refractive cataract surgery planning and outcome. Despite the advances in technology and techniques, overlooked ocular surface disorders are common and usually induce postoperative surprises. In this sense, epithelial basement membrane dystrophy (EBMD) may represent a challenge, as up to 75% of subjects over the age of 50 may be affected.1–4 Consequently, overlooked EBMD may lead to improper intraocular lens calculation and postoperative corneal healing problems. Proper diagnosis and management before and after surgery is critical to provide the best care and to meet patient’s expectations.
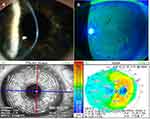
EBMD is characterized by an abnormal basement membrane (BM) which may result in defective adhesions and recurrent breakdowns of the epithelium (Figure 1).5–15 EBMD is also associated with increased levels of matrix metalloproteinase (MMP) enzymes, which further dissolve the BM and its anchoring components.16,17 The goal of treating EBMD is to remove the poorly adherent irregular epithelium and restore a healthy and smooth ocular surface. In order to achieve this goal, common practice is to perform mechanical debridement or superficial keratectomy to allow a new, healthy epithelium to grow.18–24 However, these procedures can often be accompanied with postoperative pain, delayed epithelial healing, haze and recurrent epithelial erosion.25,26
Cryopreserved amniotic membrane (CAM) is known to have potent anti-inflammatory, anti-scarring and pro-epithelial adhesion actions that promote faster healing.27 In addition, CAM is rich in BM anchoring components such as integrins, laminins, and collagens and other growth factors essential for regenerative healing.28 It is also rich in MMPs inhibitors which can prevent the dissolution of the BM.29 Therefore, we anticipated that using CAM after debridement of EBMD may accelerate healing,30 and stabilize the ocular surface prior to cataract surgery.
Methods
This pilot study evaluated the use of self-retained CAM (PROKERA® Slim, Biotissue, Miami, FL) after debridement in treating EBMD in an attempt to restore ocular surface regularity and stabilizing IOL calculation before cataract surgery. There were 2 treatment groups: a prospective study group that received debridement followed by self-retained CAM, and a retrospective control group who received bandage contact lens (BCL) after debridement. The study was approved by the Quorum Institutional Review Board (QIRB, Seattle, WA), and conducted at Virginia Eye Consultants, Norfolk, VA, in accordance with the Health Insurance Portability and Accountability Act (HIPAA) and Declaration of Helsinki. The study is registered at clinicaltrials.gov with trial identifier: NCT02766907.
After obtaining a written informed consent, subjects in the study group underwent a detailed medical and ocular history followed by complete ophthalmic examination to determine their eligibility. Inclusion criteria included subjects of both genders, aged 50 yEars or older who had significant EBMD and were intending to undergo cataract surgery. Based on clinical exam, EBMD was considered clinically significant if located within the central 6 mm of the cornea, as this would induce greater inaccuracies in topography and keratometry measurements (Figure 2). Exclusion criteria included eyelid abnormality, symblepharon, known intolerances to CAM, recent ocular infection within 14 days, recent ocular surgery or injury within 3 months, history of non-healing epithelial defect, or subjects unwilling to give consent or follow the investigational plan.
After meeting the eligibility criteria, subjects enrolled in the study group underwent baseline slit lamp examination/photography without and with Fluorescein staining, corneal topography using Atlas topographer (Carl Zeiss Meditec, Inc, Dublin, CA), and IOL calculation using Holladay I formula were performed.
Under topical anesthesia of proparacaine 0.5%, a lid speculum was applied, and corneal epithelium debridement was performed using a spatula to remove the central epithelium, leaving a peripheral rim of 1–1.5 mm of peripheral corneal epithelium intact. Topical antibiotic, moxifloxacin ophthalmic solution 0.5% (Vigamox; Alcon Laboratories, Inc., Fort Worth, TX), was instilled to the eye at the end of the procedure. Self-retained CAM was thawed at room temperature, retrieved aseptically, rinsed with sterile saline, then placed on the ocular surface behind the upper and lower lids under the speculum. The speculum was then carefully removed to avoid dislodging the device, followed by the topical antibiotic and a steroidal difluprednate ophthalmic emulsion 0.05% (Durezol; Alcon Laboratories, Fort Worth, TX). The upper eyelid was partially closed using Steri-strips to allow for the use of postoperative topical antibiotic and steroids three times daily. For postoperative pain control, each subject was prescribed Ketorolac Tromethamine (Toradol, Roche Laboratories Inc. New Jersey, NJ) 10 mg tablet daily for 3 days only, and Tramadol hydrochloride (Tramadol, Janssen Pharmaceuticals, Inc., Titusville, NJ) 50 mg tablets to be used every six to 8 hours as needed.
The primary outcome measure was corneal reepithelialization. Subjects were examined daily for the first postoperative week and slit lamp photography with fluorescein staining was used to evaluate closure of the epithelial defect while the self-retaining CAM was in place. After complete epithelialization, the self-retaining CAM was removed and a therapeutic BCL was placed for one week to mechanically protect the newly healed corneal surface. Then, subjects discontinued antibiotics eye drops and tapered topical steroids over 3 weeks. Secondary outcome measures included changes in corneal topography and IOL calculation which were evaluated at 1 month, and again two weeks afterwards for interval comparison. Tertiary outcome measures included ocular pain and discomfort using the Visual Analog Scale (VAS), corneal clarity, and post-debridement complications. In addition, refraction and ocular surface stability were evaluated after cataract surgery.
For the retrospective control group, the medical records of similar number of consecutive subjects with significant EBMD who received debridement followed by BCL (without self-retaining CAM) were reviewed. Postoperative pain, concomitant medications and corneal epithelialization time were assessed and compared to the study group. Corneal topography and IOL calculation were also compared before and 1 month after treatment. Refraction and ocular surface stability were also evaluated after cataract surgery and compared to the study group.
Descriptive statistics for continuous variables were reported as mean ± SD and were analyzed using SPSS software, version 24.0 (SPSS Inc., Chicago, Illinois, USA). Differences between parameters before and after treatment were analyzed by Student’s t-test and ANOVA test. A p value less than 0.05 was considered statistically significant.
Results
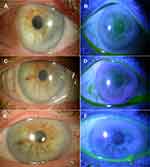
The study was approved to include 20 subjects in each of the study and control groups but was terminated after the interim analysis that showed no statistical difference between groups. Therefore, a total of 9 subjects (5 females, 4 males) with an average age of 74.0 ± 7.5 years completed the required follow-up visits in the study group. All subjects had bilateral significant EBMD (Figures 2, 3A and B) and were treated with epithelial debridement in one eye followed by a self-retained CAM (Figure 3C). The control group included 10 consecutive EBMD subjects (7 females, 3 males) with an average age of 66.5 ± 5.9 years, and comparable baseline measurements. All subjects in both groups reported similar post-debridement ocular pain and discomfort which were relieved by Tramadol 50mg as needed.
Relatively faster corneal epithelialization was observed in the study group (4.6 ± 0.8 days- range 2–5 days) compared to the control group (6.8 ± 0.6 days- range 4–10 days) and the difference was statistically significant (p<0.05). Self-retained CAM was removed after 5.4 ± 1.3 days (range 4 to 8 days) and the AM was still intact within the holding ring (eg not ruptured or dissolved). Upon self-retained CAM removal, the cornea was clear with no fluorescein staining in 6 subjects (67%) (Figure 3D). The remaining 3 corneas (33%) showed residual superficial punctate staining that vanished within one week of using BCL. In the control group, BCL was kept in place for an average of 2 weeks. All subjects discontinued antibiotics eye drops and tapered topical steroids over 3 weeks.
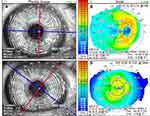
One month after debridement, corneas remained clear with no residual EBMD in both groups. The stability of refractive change was reaffirmed 2 weeks thereafter in both groups to confirm biometry and topographic readings before cataract surgery. Corneal topography showed changes in corneal curvature (Mean K) from 43.5 ± 1.2D at baseline to 44.6 ± 1.2D at 1 month in the study group (Figure 4), and from 45.0 ± 0.6D to 45.7 ± 0.8D in the control group (Figure 5), however, the difference between groups was not statistically significant (p=0.38) (Table 1). Corneal topography also showed decreased irrigular astigmatism with concurrent axis deviation in both groups from baseline to one month (p<0.05). The average change in axis was 114° to 81° in the study group (Figure 4) compared to 106° to 118° in the control group (Figure 5), but there was no statistically significant difference between groups (p=0.91). Biometry also showed a similar absolute average change of 1.56 D in IOL calculation in the study group, compared to 0.95 D in the control group, with no statistically significant difference (p= 0.29). There were no adverse events, recurrence or haze reported in the early post-debridement period in either group.
 |
Table 1 Corneal Topograpy and Biometry Readings Before and 1 Month After Debridement in the Study and Control Groups |
The patients in both groups were followed up one month after cataract surgery, and the refractive error was within ±0.5 Diopter of the anticipated measurement. In addition, the corneal surface remained stable without EBMD recurrence for at least 1 year in both groups (Figure 3E and F).
Discussion
With more than 24 million Americans affected by cataracts, there is an increased need to ensure preoperative evaluations encompass all aspects that may have a deleterious effect on postoperative outcomes. As suggested by the American Academy of Ophthalmology, current strategies for achieving best outcome include the early diagnosis and management of ocular surface disorders before surgery.31,32 Although EBMD is often asymptomatic and underdiagnosed, the severity of symptoms usually increases after ocular surgeries and may lead to postoperative wound healing problems.33 This may be due to transection of corneal nerves, elevation of pro-inflammatory factors, intense irrigation of the tear film during operation, and/or damage to the corneal epithelium from use of topical anesthesia and preservative-containing eye drops.34
It is then of vital importance to effectively diagnose and treat patients with significant EBMD prior to cataract surgery to ensure a satisfactory surgical outcome. This is becoming ever more important as we implant more advanced technologies, such as premium IOLs. Detection of EBMD is often challenging being extremely variable from patient to patient and diagnostic tests are feebly associated with patient symptoms.31,35 Based on our experience, EBMD can be suspected when keratometry measurements are inconsistent amongst diagnostic devices. While corneal topography is a widely used diagnostic tool to highlight the irregularities of the mires, EBMD can be detected clinically by sways in the Placido disc images within the central 6 mm of the cornea. Negative fluorescein staining is also a simple test that can delineate EBMD either central or peripheral (Figure 1).
To our knowledge, this is the first study to evaluate the use of self-retaining CAM after debridement in EBMD. The goal was to remove all the irregular epithelium and to promote regeneration of a new, smooth epithelium with strong adherence to the basement membrane. Hence, we debrided most of the epithelium as opposed to just the “visually-involved area” as the diseased epithelium tends to extend further. Then, we placed self-retaining CAM after debridement to accelerate corneal epithelialization, stabilize the corneal surface and reduce complications such as corneal haze and recurrence. We compared the results with subjects who received debridement followed by BCL.
Early post-debridement all subjects experienced moderate to severe ocular pain that was controlled with Tramadol in both groups. Painkillers also assisted in reducing discomfort induced by CAM holding rings in the study group. This ring-induced discomfort was much less in our study than the previously reported in the literature. Suri et al36 reported 17% ring induced pain and discomfort in a series of 35 patients and 6% required removal of the device as patients could not tolerate the associated pain. Pachigolla et al37 also reported 30% discomfort in a series of 20 patients, and the device was removed in 5% of cases. Vlasov et al38 reported greater discomfort from day 1 post debridement for PRK in a series of 40 patients of younger age (<40 years). In addition to painkillers, the reduced discomfort in our study can be explained by the reported decreased corneal sensitivity due to decreased corneal nerve density in EBMD,39 or the associated neurotrophic keratopathy in the relatively older patient population (>60 years) in our study. The latter adds another risk for poor epithelialization, and therefore emphasizes the concept of using CAM not only due to its known wound healing properties but also due to the abundant neurotrophic factors inherently present in CAM including brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), nerve growth factor (NGF), fibroblast growth factor-2 (FGF-2), and epidermal growth factor (EGF).40–43
Relatively faster corneal epithelialization occurred in the study group compared to the control group. However, this was not clinically significant and complete healing occurred within 2 weeks in both groups and required additional BCL in the study group. One month after treatment, corneal topography and biometry showed significant changes in both groups compared to baseline. However, these changes were similar in both groups which may be explained by the direct effect of debridement. The healed corneal surface was clear and stable with no morphological recurrence of EBMD for at least 1 year in both groups.
While CAM relatively accelerated healing of the ocular surface after debridement in the study group, it was not better than BCL in stabilizing the ocular surface and improving structural and visual outcome after complete healing. Hence, these results do not justify the additional cost of the self-retaining CAM.
There were no adverse events, recurrence or haze reported in the early post-debridement period in either group. Our results were better than the previously reported in the literature, for example, debridement with or without diamond-dusted burr polishing has shown a recurrence rate of 20–24% in some studies along with corneal haze evident in 25.5% of cases within 3 months.23,44 Other procedures such as PTK have shown a higher incidence of corneal haze and recurrence within 9 months.45 Apparently our assertive debridement technique was the most important factor in reducing the recurrence in our study.
In addition to the small sample size, the inclusion of a historic control arm is another limitation of this study. Although prospective control would have been beneficial, there was an ethical concern of the anticipated delayed healing in the control group. Although CAM is prepared under complete aseptic conditions and passed all the required screening and microbial testing, it is non-sterile and carries the potential risk of transmitting unrecognized infectious diseases including COVID-19. Currently, there is no data on the survival of SARS-CoV-2 on CAM.46 However, the Global Alliance of Eye Bank Association recommends ocular tissue donors with confirmed or suspected COVID-19 to be excluded for harvesting.47 Before more evidence is available, we suggest taking a more prudent approach in infectious control when conducting similar studies.48
In conclusion, EBMD is a very common yet often overlooked condition that may lead to improper intraocular lens calculation and postoperative wound healing problems. Proper diagnosis and management before ocular surgery is critical to provide the best outcome and avoid postoperative refractive error. Although the concept of using self-retaining CAM after debridement holds great promise to accelerate the healing and optimize the ocular surface prior to cataract surgery, its use in cases of EBMD remains speculative. It offers an alternative to an existing less expensive management options such as BCL without any distinct advantage over the others. Future research with strict infection control may undoubtedly reveal alternative modalities of amniotic membrane derived therapies to ocular surface and other ocular disorders to benefit from its true useful potential.
Data Sharing Statement
Study records are available at the following link “Optimizing the Ocular Surface Prior to Cataract Surgery - Full Text View - ClinicalTrials.gov”. No further data will be shared.
Acknowledgments
This study was partially presented as a poster at the American Academy of Ophthalmology (AAO) annual meeting, New Orleans, LA, November 2017. It was also presented at the American Society of Cataract and Refractive Surgery (ASCRS) annual meeting, Washington, DC, April 2018. Mr. Sean Tighe assisted in the statistical analysis and manuscript editing. Adam El Sheha and Ryan El Sheha assisted in manuscript editing. The content is the responsibility of the authors and does not represent the views of the acknowledged individuals.
Funding
This study was supported in part by a research grant from Tissuetech (Miami, FL).
Disclosure
Dr. Yeu served as a consultant or a speaker bureau for companies mentioned in this manuscript such as Alcon Laboratories (Farnworth, TX), Biotissue (Miami, FL), and Carl Zeiss Meditec (Dublin, CA). Dr. Hashem and Dr. Sheha report no conflicts of interest in this work.
References
1. Cogan DG, Donaldson DD, Kuwabara T, Marshall D. Microcystic Dystrophy of the Corneal Epithelium. Trans Am Ophthalmol Soc. 1964;62:213–225.
2. Guerry D. Observations on Cogan’s microcystic dystrophy of the corneal epithelium. Trans Am Ophthalmol Soc. 1965;63:320–334.
3. Rodrigues MM, Fine BS, Laibson PR, Zimmerman LE. Disorders of the corneal epithelium. A clinicopathologic study of dot, geographic, and fingerprint patterns. Arch Ophthalmol. 1974;92(6):475–482. doi:10.1001/archopht.1974.01010010489005
4. Werblin TP, Hirst LW, Stark WJ, Maumenee IH. Prevalence of map-dot-fingerprint changes in the cornea. Br J Ophthalmol. 1981;65(6):401–409. doi:10.1136/bjo.65.6.401
5. Fogle JA, Kenyon KR, Stark WJ, Green WR. Defective epithelial adhesion in anterior corneal dystrophies. Am J Ophthalmol. 1975;79(6):925–940. doi:10.1016/0002-9394(75)90674-1
6. Brown N, Bron A. Recurrent erosion of the cornea. Br J Ophthalmol. 1976;60(2):84–96. doi:10.1136/bjo.60.2.84
7. Kenyon KR, Berman M, Rose J, Gage J. Prevention of stromal ulceration in the alkali-burned rabbit cornea by glued-on contact lens. Evidence for the role of polymorphonuclear leukocytes in collagen degradation. Invest Ophthalmol Vis Sci. 1979;18(6):570–587.
8. Aitken DA, Beirouty ZA, Lee WR. Ultrastructural study of the corneal epithelium in the recurrent erosion syndrome. Br J Ophthalmol. 1995;79(3):282–289. doi:10.1136/bjo.79.3.282
9. Cogan DG, Kuwabara T, Donaldson DD, Collins E. Microcystic dystrophy of the cornea. A partial explanation for its pathogenesis. Arch Ophthalmol. 1974;92(6):470–474. doi:10.1001/archopht.1974.01010010484004
10. Ehlers N, Moller HU. Pathology and pathomechanisms of epithelial microcystic and basement membrane abnormalities of the cornea. Acta Ophthalmol (Copenh). 1988;66(3):318–326. doi:10.1111/j.1755-3768.1988.tb04604.x
11. Tripathi RC, Bron AJ. Cystic disorders of the corneal epithelium. II. Pathogenesis. Br J Ophthalmol. 1973;57(6):376–390. doi:10.1136/bjo.57.6.376
12. Laibson PR. Microcystic corneal dystrophy. Trans Am Ophthalmol Soc. 1976;74:488–531.
13. Brodrick JD, Dark AJ, Peace GW. Fingerprint dystrophy of the cornea. A histologic study. Arch Ophthalmol. 1974;92(6):483–489. doi:10.1001/archopht.1974.01010010497006
14. Kaufman HE, Clower JW. Irregularities of Bowman’s membrane. Am J Ophthalmol. 1966;61(2):227–230. doi:10.1016/0002-9394(66)90275-3
15. Laibson PR, Krachmer JH. Familial occurrence of dot (microcystic), map, fingerprint dystrophy of the cornea. Invest Ophthalmol. 1975;14(5):397–399.
16. Fini ME, Cook JR, Mohan R. Proteolytic mechanisms in corneal ulceration and repair. Arch Dermatol Res. 1998;290(14):S12–23. doi:10.1007/PL00007449
17. Chen YT, Huang CW, Huang FC, Tseng SY, Tseng SH. The cleavage plane of corneal epithelial adhesion complex in traumatic recurrent corneal erosion. Mol Vis. 2006;12:196–204.
18. Orndahl MJ, Fagerholm PP. Phototherapeutic keratectomy for map-dot-fingerprint corneal dystrophy. Cornea. 1998;17(6):595–599. doi:10.1097/00003226-199811000-00004
19. Tzelikis PF, Rapuano CJ, Hammersmith KM, Laibson PR, Cohen EJ. Diamond burr treatment of poor vision from anterior basement membrane dystrophy. Am J Ophthalmol. 2005;140(2):308–310. doi:10.1016/j.ajo.2005.01.036
20. Pogorelov P, Langenbucher A, Kruse F, Seitz B. Long-term results of phototherapeutic keratectomy for corneal map-dot-fingerprint dystrophy (Cogan-Guerry). Cornea. 2006;25(7):774–777. doi:10.1097/01.ico.0000214801.02195.d4
21. Buxton JN, Constad WH. Superficial epithelial keratectomy in the treatment of epithelial basement membrane dystrophy. Ann Ophthalmol. 1987;19(3):92–96.
22. Vo RC, Chen JL, Sanchez PJ, Yu F, Aldave AJ. Long-Term Outcomes of Epithelial Debridement and Diamond Burr Polishing for Corneal Epithelial Irregularity and Recurrent Corneal Erosion. Cornea. 2015;34(10):1259–1265. doi:10.1097/ICO.0000000000000554
23. Aldave AJ, Kamal KM, Vo RC, Yu F. Epithelial debridement and Bowman’s layer polishing for visually significant epithelial irregularity and recurrent corneal erosions. Cornea. 2009;28(10):1085–1090. doi:10.1097/ICO.0b013e3181a166b9
24. Bae SS, Chan CC. Superficial keratectomy: indications and outcomes. Can J Ophthalmol. 2018;53(6):553–559. doi:10.1016/j.jcjo.2018.01.030
25. Itty S, Hamilton SS, Baratz KH, Diehl NN, Maguire LJ. Outcomes of epithelial debridement for anterior basement membrane dystrophy. Am J Ophthalmol. 2007;144(2):217–221. doi:10.1016/j.ajo.2007.04.024
26. Sayegh RR, Kouyoumjian PB, Vedula GG, Nottage JM, Nirankari VS. Cocaine-assisted epithelial debridement for the treatment of anterior basement membrane dystrophy. Cornea. 2013;32(6):889–892. doi:10.1097/ICO.0b013e318288ad4d
27. Liu J, Sheha H, Fu Y, Liang L, Tseng SC. Update on amniotic membrane transplantation. Expert Rev Ophthalmol. 2010;5(5):645–661. doi:10.1586/eop.10.63
28. Tseng SC. HC-HA/PTX3 Purified From Amniotic Membrane as Novel Regenerative Matrix: insight Into Relationship Between Inflammation and Regeneration. Invest Ophthalmol Vis Sci. 2016;57(5):ORSFh1–8. doi:10.1167/iovs.15-17637
29. Heiligenhaus A, Li HF, Yang Y, Wasmuth S, Steuhl KP, Bauer D. Transplantation of amniotic membrane in murine herpes stromal keratitis modulates matrix metalloproteinases in the cornea. Invest Ophthalmol Vis Sci. 2005;46(11):4079–4085. doi:10.1167/iovs.05-0192
30. Huang YS, Sheha H, Tseng SG. Self-retained Amniotic Membrane Transplantation for Recurrent Corneal Erosion. J Clin Exp Ophthalmol. 2013;4:272.
31. Afsharkhamseh N, Movahedan A, Motahari H, Djalilian AR. Cataract surgery in patients with ocular surface disease: an update in clinical diagnosis and treatment. Saudi J Ophthalmol. 2014;28(3):164–167. doi:10.1016/j.sjopt.2014.06.013
32. Olson RJ, Braga-Mele R, Chen SH, et al. Cataract in the Adult Eye Preferred Practice Pattern(R). Ophthalmology. 2017;124(2):P1–P119. doi:10.1016/j.ophtha.2016.09.027
33. Cho YK, Kim MS. Dry eye after cataract surgery and associated intraoperative risk factors. Korean J Ophthalmol. 2009;23(2):65–73. doi:10.3341/kjo.2009.23.2.65
34. Kasetsuwan N, Satitpitakul V, Changul T, Jariyakosol S. Incidence and pattern of dry eye after cataract surgery. PLoS One. 2013;8(11):e78657. doi:10.1371/journal.pone.0078657
35. Bjerrum KB. Test and symptoms in keratoconjunctivitis sicca and their correlation. Acta Ophthalmol Scand. 1996;74(5):436–441. doi:10.1111/j.1600-0420.1996.tb00595.x
36. Suri K, Kosker M, Raber IM, et al. Sutureless amniotic membrane ProKera for ocular surface disorders: short-term results. Eye Contact Lens. 2013;39(5):341–347. doi:10.1097/ICL.0b013e3182a2f8fa
37. Pachigolla G, Prasher P, Di Pascuale MA, McCulley JP, McHenry JG, Mootha VV. Evaluation of the role of ProKera in the management of ocular surface and orbital disorders. Eye Contact Lens. 2009;35(4):172–175. doi:10.1097/ICL.0b013e3181a66a12
38. Vlasov A, Sia RK, Ryan DS, et al. Sutureless cryopreserved amniotic membrane graft and wound healing after photorefractive keratectomy. J Cataract Refract Surg. 2016;42(3):435–443. doi:10.1016/j.jcrs.2015.11.045
39. He J, Bazan HE. Corneal nerve architecture in a donor with unilateral epithelial basement membrane dystrophy. Ophthalmic Res. 2013;49(4):185–191. doi:10.1159/000345766
40. Touhami A, Grueterich M, Tseng SC. The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture. Invest Ophthalmol Vis Sci. 2002;43(4):987–994.
41. Uchida S, Inanaga Y, Kobayashi M, Hurukawa S, Araie M, Sakuragawa N. Neurotrophic function of conditioned medium from human amniotic epithelial cells. J Neurosci Res. 2000;62(4):585–590. doi:10.1002/1097-4547(20001115)62:4<585::AID-JNR13>3.0.CO;2-U
42. Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20(3):173–177. doi:10.1076/0271-3683(200003)2031-9FT173
43. Davis GE, Blaker SN, Engvall E, Varon S, Manthorpe M, Gage FH. Human amnion membrane serves as a substratum for growing axons in vitro and in vivo. Science. 1987;236(4805):1106–1109. doi:10.1126/science.3576223
44. Lee WS, Lam CK, Manche EE. Phototherapeutic keratectomy for epithelial basement membrane dystrophy. Clin Ophthalmol. 2017;11:15–22. doi:10.2147/OPTH.S122870
45. Sridhar MS, Rapuano CJ, Cosar CB, Cohen EJ, Laibson PR. Phototherapeutic keratectomy versus diamond burr polishing of Bowman’s membrane in the treatment of recurrent corneal erosions associated with anterior basement membrane dystrophy. Ophthalmology. 2002;109(4):674–679. doi:10.1016/S0161-6420(01)01027-2
46. Seah I, Su X, Lingam G. Revisiting the dangers of the coronavirus in the ophthalmology practice. Eye. 2020;34(7):1155–1157. doi:10.1038/s41433-020-0790-7
47. Global alliance of eye bank associations alert update on COVID-19 and ocular tissue donation. Available from: http://www.gaeba.org/2020/alert-coronavirus-2019-ncov-and-ocular-tissue-donation/.
48. Lai THT, Tang EWH, Chau SKY, Fung KSC, Li KKW. Stepping up infection control measures in ophthalmology during the novel coronavirus outbreak: an experience from Hong Kong. Graefes Arch Clin Exp Ophthalmol. 2020;258:1049–1055. doi:10.1007/s00417-020-04641-8
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.