Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Treatment of Discoid Lupus Erythematosus with Upadacitinib: A Case Report
Authors Hu W , Zhang S, Lian C
Received 29 April 2023
Accepted for publication 3 October 2023
Published 9 October 2023 Volume 2023:16 Pages 2793—2800
DOI https://doi.org/10.2147/CCID.S419344
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Wenting Hu, Si Zhang, Cuihong Lian
Department of Dermatology, Shenzhen Second People’s Hospital, Shenzhen, Guangdong, People’s Republic of China
Correspondence: Cuihong Lian, Department of Dermatology, Shenzhen Second People’s Hospital, No. 3002 Sungang West Road, Shenzhen, Guangdong, People’s Republic of China, Tel +86 755 83366388, Fax +86 755 83003435, Email [email protected]
Abstract: Cutaneous lupus erythematosus (CLE) is a group of diseases within the spectrum of lupus that primarily manifests with skin lesions. Discoid lupus erythematosus (DLE) is the most common subtype of CLE. Currently, there is no specific medication available for the treatment of CLE. Here, we reported the efficacy and safety of upadacitinib, a JAK1 selective inhibitor, in treating one DLE patient for 28 weeks. Upadacitinib 15mg QD alone improved DLE lesions significantly, while reduction of the drug to 15mg QOD led to a relapse of the skin lesions. Upadacitinib showed favorable safety in this DLE patient in the 28-week period, except for acne, which was controlled by topical application of benzoyl peroxide gel. In this case, we observed rapid and sustained improvement of DLE lesions using upadacitinib with favorable safety, which provided the opportunity to use upadacitinib as an alternative therapy for DLE.
Keywords: cutaneous lupus erythematosus, upadacitinib, JAK inhibitor, discoid lupus erythematosus
Introduction
Cutaneous lupus erythematosus (CLE) is a group of diseases within the spectrum of lupus that primarily manifests with skin lesions. Based on combinations of clinical, histopathological and serological features, CLE can be classified into four main types: acute CLE (ACLE), subacute CLE (SCLE), chronic CLE (CCLE), and intermittent CLE (ICLE), which can be further divided into different subtypes.1 62–80% of CLE patients were diagnosed with discoid lupus erythematosus (DLE),2,3 a subtype of CCLE. Classical DLE lesions are discoid erythematous plaques with adherent scales. Removal of scales shows follicular hyperkeratosis, the so-called “carpet tack sign”.4 Margins of the DLE lesions are often hyperpigmented, while centers of the lesions are usually hypopigmented and atrophic with depressed scars.4,5 However, atypical DLE mimicking other diseases such as rosacea, angiofibroma and blepharitis was also reported, leading to the difficulty in diagnosis occasionally.6,7 As stated in the Rook’s Textbook of Dermatology (the ninth edition, chapter 51),8 rosaceous pattern can be seen in 7.5% of DLE patients, and tumid or telangiectatic lesions may also be observed. Skin biopsy is required to distinguish atypical DLE from other diseases. Histopathology is similar between different LE subtypes, which is characterized by interface dermatitis and mononuclear cellular infiltration at the dermoepidermal junction and around the vessels and cutaneous appendages.9 DLE may demonstrate more pronounced follicular plugging and inflammation that extends into the dermis.9,10 Mild pruritus at the site of lesions may occur in some of the DLE patients, while systemic symptoms are absent or mild, such as mild joint pain.
Currently, there is no specific drug available for the treatment of CLE. Several guidelines recommend a stepwise treatment approach, using topical medications along with systemic therapy such as antimalarials, glucocorticoids or immunosuppressants based on the severity of the patient’s condition.11,12 The use of immunosuppressants, such as methotrexate (MTX) and mycophenolate mofetil, in CLE is considered as the second or third-line therapy, as there is a lack of reliable clinical trials to confirm their efficacy and safety. Glucocorticoids and immunosuppressants suppress the proliferation of T and B lymphocytes, thereby controlling the progression of the disease. However, their overall immunosuppressive effects can lead to a range of side effects. With a deepened understanding of CLE pathogenesis, increased targeted drugs emerged for the treatment of CLE, such as anti-LILRA4 antibody Daxdilimab, SYK inhibitor Lanraplenib and Janus kinase (JAK) inhibitors (eg, Tofacitinib, Delgocitinib and Filgotinib).1
Increasing evidence suggests that type I interferon and the JAK-STAT signaling pathway play crucial roles in the pathogenesis of CLE.13 However, limited clinical reports are available regarding the treatment of CLE with JAK inhibitors, and most of the reports used broad-spectrum JAK inhibitors. Here, we would like to share a case of DLE, the most common type of CLE, which improved significantly with upadacitinib, a selective JAK1 inhibitor.
Case Report
A 26-year-old female patient presented to our department with a two-year history of facial swelling and erythema. She was diagnosed with rosacea in one hospital and treated with successive doxycycline and isotretinoin, but the lesions did not improve. The patient was then diagnosed with CLE in another hospital and was treated with topical tacrolimus ointment and oral hydroxychloroquine (HCQ) combined with low-dose prednisone, which improved the rash. However, due to steroid-induced weight gain, the patient discontinued prednisone on her own and experienced a rash relapse.
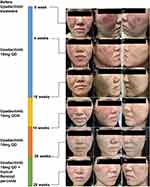
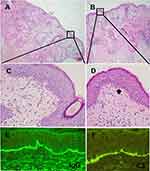
Physical examination showed erythema and swelling on both cheeks, particularly on the right side, with a relatively clear boundary (Figure 1A–C). Multiple depressed scars, telangiectasia, and a small amount of inflammatory papules were visible within the erythema, while adherent scales were absent. Laboratory tests showed normal complete blood count, negative autoantibodies, normal serum creatine kinase level, but positive hepatitis B virus (HBV) with a viral load of 1.86 × 108 IU/mL. Histopathological pictures of the skin lesion revealed hair follicle plugging, vacuolar degeneration of basal cells with Civatte bodies, indistinct interface, lymphocytic infiltration around superficial and deep dermal vessels and cutaneous appendages, edema in the superficial dermis with widened collagen bundle gap (Figure 2A–D). Direct immunofluorescence (DIF) test using IgG and C3 antibodies revealed a characteristic lupus band at the basement membrane zone (BMZ) (Figure 2E and F). The patient had a history of facial acne but no other chronic diseases and denied a family history of similar diseases. Based on the patient’s medical history, physical examination, and auxiliary examination results, we considered a diagnosis of DLE.
The patient refused to use steroids and immunosuppressants due to concerns about systemic side effects. She felt burning and pruritus when tacrolimus ointment was applied on the lesions, so she refused to continue using topical agents. As a result, we attempted to treat the patient with upadacitinib 15mg QD as a monotherapy, along with entecavir 0.5mg QD for antiviral therapy. At the 4-week follow-up visit, the patient’s skin lesions had significantly improved (Figure 1D–F). At the 10-week follow-up visit, the skin lesions had further improved (Figure 1G–I), and the HBV DNA quantitation was 1.49 × 105 IU/mL, with normal complete blood counts, liver and kidney function, high-sensitivity C-reactive protein, blood lipids, and creatine kinase level. We instructed the patient to reduce upadacitinib to 15mg every other day. The reduction of upadacitinib for 4 weeks resulted in a relapse of the lesions (Figure 1J–L). Therefore, upadacitinib 15mg QD was resumed in this patient. After 20 weeks of upadacitinib treatment, the patient’s facial erythema improved (Figure 1M–O). However, some inflammatory papules were observed, which may be the adverse drug reaction of upadacitinib to cause acne. Topical benzoyl peroxide gel was applied to treat acne. At the 28-week follow-up visit, the patient’s facial swelling and erythema showed sustained improvement with a satisfactory control of acne. The disease activity scores of Revised Cutaneous Lupus Erythematosus Disease Area and Severity Index (RCLASI)14 of the patient at week 0, 4, 10, 14, 20, 28-week after upadacitinib treatment are 5, 3, 2, 4, 2, 1, respectively. At the 28-week visit, the patient’s HBV DNA quantitation was 7.45 × 102 IU/mL, with normal complete blood counts, liver and kidney function, blood lipids, and creatine kinase level. No other adverse drug reactions have been reported by the patient at the time of manuscript submission. The patient’s written informed consent for the case details including images to be published were obtained. The study was approved by ethical review board of Shenzhen Second People’s Hospital.
Discussion
Attributed to the paucity of high-quality clinical trial evidence, CLE treatment lacks licensed therapy and often requires “off-label” application of topical and systemic medications.11 Both European and Asian guidelines for treatment of CLE recommend systemic corticosteroids in addition to antimalarials as the first-line therapy in patients with severe or widespread active CLE lesions.11,12 In spite of the optimal efficacy (94.3%) of corticosteroids in comparison to other systemic agents applied to CLE,15 long-term therapy with corticosteroids is not recommended for CLE patients without systemic involvement because of its well-known serious side effects including Cushing syndrome, osteoporosis and cataracts.11 Hydroxychloroquine (HCQ) was proven to be effective in 50% to 86.9% CLE patients in different studies15–17 with overall safety.18 The main side effect of HCQ is retinal toxicity but was not reported in CLE-related studies.16,17 Dermatologic adverse events related to HCQ treatment, including cellulitis, drug eruption, Stevens-Johnson syndrome and worsening of DLE, were reported in one of the aforementioned studies. Other immunosuppressants, including MTX, are suggested to be second- or third-line choices in CLE systemic treatment.11,12 MTX therapy was considered to be successful in 65.5% of CLE patients,15 which is perhaps equivalent to the efficacy of chloroquine.19 9.8% and 6.7% of patients receiving systemic MTX monotherapy were affected by transaminitis and neutropenia, respectively.20 Due to the potential liver toxicity of MTX, baseline liver diseases, including HBV/HCV infection, should be considered during therapy with MTX.12
The patient presented in this article experienced a rash relapse after discontinuation of oral prednisone, indicating HCQ alone was not effective in this patient as a systemic monotherapy. Given the comparable efficacy of MTX and antimalarials in CLE treatment19 and the potentially higher risk of liver toxicity of MTX in this patient with HBV infection, whether MTX was an appropriate option for this patient was questionable. There are currently no clinical trials comparing the efficacy and safety of immunosuppressants and JAK inhibitors in the treatment of CLE. A Phase 2 clinical trial (NCT03978520) evaluated the benefit and risk of elsubrutinib and upadacitinib given alone or in combination in SLE patients and the result was posted recently. Upadacitinib 30mg alone group displayed a significantly higher percentage of patients achieving 4 points reduction of SLE Responder Index (SRI) than the placebo group at week 24, demonstrating the favorable efficacy of upadacitinib in SLE treatment. However, skin manifestations were not posted alone in this trial, leading to the undefined function of upadacitinib in LE skin lesions. The safety profile of upadacitinib 30mg alone group seemed to be comparable to that of the placebo group, including serious infections, major cardiovascular events, and venous thromboembolic events, which are labeled warnings of upadacitinib. The risk of malignancies and hepatic toxicity were not mentioned in the analyses of adverse events in this clinical trial. Our patient had hepatitis B virus infection prior to upadacitinib treatment. Concurrent with upadacitinib therapy, we administered entecavir to the patient for antiviral treatment. Throughout the 28-week follow-up period, the patient exhibited a sustained decline in HBV DNA quantification, and liver function consistently remained within the normal range.
JAK-STAT signaling pathways mediate the function of many pro-inflammatory cytokines in CLE pathogenesis, including type I interferon. The level of pJAK1 was significantly increased in the dermal inflammatory cells of patients with CLE, while the levels of pJAK2, pJAK3, and pTyk2 did not change.21 In vitro experiments demonstrated that the JAK1 selective inhibitor downregulated the expression of CLE-related genes, including CXCL10.22 JAK1 selective inhibitors are promising to achieve therapeutic effects while avoiding the side effects of pan JAK inhibitors, such as anemia and thrombocytopenia. Limited clinical reports are available regarding the treatment of CLE with JAK inhibitors, and most of the reports have used broad-spectrum JAK inhibitors. Related studies are listed in Table 1.23–31 Some studies reported disease improvement after JAK inhibitor administration while others did not. Based on the mixed results of the clinical trials, the efficacy and safety of JAK inhibitors, especially selective JAK inhibitors, in the treatment of CLE still need to be further observed and evaluated.
 |
Table 1 Studies Using JAK Inhibitors for Treating SLE and CLE |
Upadacitinib is a selective JAK1 inhibitor, approved for several autoimmune diseases including atopic dermatitis, psoriatic arthritis, and ulcerative colitis. We reported the efficacy and safety of upadacitinib in treating one DLE patient for 28 weeks and the long-term observation is still going on. Upadacitinib 15mg QD alone improved DLE lesions, while reduction of the drug to 15mg QOD led to a relapse of the skin lesions. Upadacitinib showed favorable safety in this DLE patient in the 28-week period, except for one adverse drug reaction, acne, which was controlled by the topical use of benzoyl peroxide gel. Only one patient involvement and the relatively short-term observation are limitations of the current study. Further research, including randomized controlled trials, is still needed to provide reliable evidence of the efficacy, safety and optimal use of upadacitinib in CLE treatment.
Acknowledgments
The authors would like to thank Dr. Suchun Hou and Dr. Xikang Wu for their contributions in immunofluorescent staining, pathological image analysis and diagnosis.
Consent Statement
The patients in this manuscript have given written informed consent to the publication of the case details.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Niebel D, de Vos L, Fetter T, Brägelmann C, Wenzel J. Cutaneous lupus erythematosus: an update on pathogenesis and future therapeutic directions. Am J Clin Dermatol. 2023;24(4):521–540. doi:10.1007/s40257-023-00774-8
2. Petersen MP, Möller S, Bygum A, Voss A, Bliddal M. Epidemiology of cutaneous lupus erythematosus and the associated risk of systemic lupus erythematosus: a nationwide cohort study in Denmark. Lupus. 2018;27(9):1424–1430. doi:10.1177/0961203318777103
3. Grönhagen CM, Fored CM, Granath F, Nyberg F. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164(6):1335–1341. doi:10.1111/j.1365-2133.2011.10272.x
4. Worm M, Zidane M, Eisert L, et al. S2k guideline: diagnosis and management of cutaneous lupus erythematosus - Part 1: classification, diagnosis, prevention, activity scores. J Dtsch Dermatol Ges. 2021;19(8):1236–1247.
5. Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10(6):365–381. doi:10.2165/11310780-000000000-00000
6. Mokhtari F, Ganjei Z. A rare form of discoid lupus erythematosus as a rosacea and angiofibroma: a case report. Clin Case Rep. 2020;8(1):155–158. doi:10.1002/ccr3.2611
7. Jisha K, Rajesh PS, Kurien G, Sathish G, Vijayamma N. Chronic blepharitis like picture in patients with Discoid lupus erythematosis - Case series. Nepal J Ophthalmol. 2017;9(18):175–179. doi:10.3126/nepjoph.v9i2.19264
8. Goodfield MJD, Jones SK, Veale DJ. The ‘Connective Tissue Diseases’. In: Rook’s Textbook of Dermatology. John Wiley & Sons; 2010:1–138.
9. Hejazi EZ, Werth VP. Cutaneous lupus erythematosus: an update on pathogenesis, diagnosis and treatment. Am J Clin Dermatol. 2016;17(2):135–146. doi:10.1007/s40257-016-0173-9
10. Baltaci M, Fritsch P. Histologic features of cutaneous lupus erythematosus. Autoimmun Rev. 2009;8(6):467–473. doi:10.1016/j.autrev.2008.12.014
11. Lu Q, Long H, Chow S, et al. Guideline for the diagnosis, treatment and long-term management of cutaneous lupus erythematosus. J Autoimmun. 2021;123:102707. doi:10.1016/j.jaut.2021.102707
12. Kuhn A, Aberer E, Bata-Csörgő Z, et al. S2k guideline for treatment of cutaneous lupus erythematosus - guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2017;31(3):389–404. doi:10.1111/jdv.14053
13. Wenzel J. Cutaneous lupus erythematosus: new insights into pathogenesis and therapeutic strategies. Nat Rev Rheumatol. 2019;15(9):519–532. doi:10.1038/s41584-019-0272-0
14. Kuhn A, Meuth AM, Bein D, et al. Revised cutaneous lupus erythematosus disease area and severity index (RCLASI): a modified outcome instrument for cutaneous lupus erythematosus. Br J Dermatol. 2010;163(1):83–92. doi:10.1111/j.1365-2133.2010.09799.x
15. Sigges J, Biazar C, Landmann A, et al. Therapeutic strategies evaluated by the European society of cutaneous lupus erythematosus (EUSCLE) core set questionnaire in more than 1000 patients with cutaneous lupus erythematosus. Autoimmun Rev. 2013;12(7):694–702. doi:10.1016/j.autrev.2012.10.005
16. Ruzicka T, Sommerburg C, Goerz G, Kind P, Mensing H. Treatment of cutaneous lupus erythematosus with Acitretin and hydroxychloroquine. Br J Dermatol. 1992;127(5):513–518. doi:10.1111/j.1365-2133.1992.tb14851.x
17. Yokogawa N, Eto H, Tanikawa A, et al. Effects of hydroxychloroquine in patients with cutaneous lupus erythematosus: a multicenter, double-blind, randomized, parallel-group trial. Arthritis Rheumatol. 2017;69(4):791–799. doi:10.1002/art.40018
18. Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–28. doi:10.1136/ard.2008.101766
19. Fairley JL, Oon S, Saracino AM, Nikpour M. Management of cutaneous manifestations of lupus erythematosus: a systematic review. Semin Arthritis Rheum. 2020;50(1):95–127. doi:10.1016/j.semarthrit.2019.07.010
20. Li CK, Baker K, Jones T, Coulson E, Roberts A, Birrell F. Safety and tolerability of subcutaneous methotrexate in routine clinical practice. Arthritis Care Res. 2021;73(9):1306–1311. doi:10.1002/acr.24334
21. Alves de Medeiros AK, Speeckaert R, Desmet E, Van Gele M, De Schepper S, Lambert J. JAK3 as an emerging target for topical treatment of inflammatory skin diseases. PLoS One. 2016;11(10):e0164080. doi:10.1371/journal.pone.0164080
22. Fetter T, Smith P, Guel T, Braegelmann C, Bieber T, Wenzel J. Selective Janus kinase 1 inhibition is a promising therapeutic approach for lupus erythematosus skin lesions. Front Immunol. 2020;11:344. doi:10.3389/fimmu.2020.00344
23. Bonnardeaux E, Dutz JP. Oral tofacitinib citrate for recalcitrant cutaneous lupus. JAAD Case Rep. 2022;20:61–64. doi:10.1016/j.jdcr.2021.09.030
24. You H, Zhang G, Wang Q, et al. Successful treatment of arthritis and rash with tofacitinib in systemic lupus erythematosus: the experience from a single centre. Ann Rheum Dis. 2019;78(10):1441–1443. doi:10.1136/annrheumdis-2019-215455
25. Hasni SA, Gupta S, Davis M, et al. Phase 1 double-blind randomized safety trial of the Janus kinase inhibitor tofacitinib in systemic lupus erythematosus. Nat Commun. 2021;12(1):3391. doi:10.1038/s41467-021-23361-z
26. Ananthan L, Williams M, Morgan H, Patel GK. Cutaneous lupus erythematosus variants responsive to Janus kinase inhibition. Dermatol Ther. 2022;35(12):e15967. doi:10.1111/dth.15967
27. Morand EF, Vital EM, Petri M, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, Phase 3 trial (SLE-BRAVE-I). Lancet. 2023;401(10381):1001–1010. doi:10.1016/S0140-6736(22)02607-1
28. Petri M, Bruce IN, Dörner T, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 3 trial (SLE-BRAVE-II). Lancet. 2023;401(10381):1011–1019. doi:10.1016/S0140-6736(22)02546-6
29. Werth VP, Fleischmann R, Robern M, et al. Filgotinib or lanraplenib in moderate to severe cutaneous lupus erythematosus: a phase 2, randomized, double-blind, placebo-controlled study. Rheumatology. 2022;61(6):2413–2423. doi:10.1093/rheumatology/keab685
30. Wenzel J, van Holt N, Maier J, Vonnahme M, Bieber T, Wolf D. JAK1/2 inhibitor ruxolitinib controls a case of chilblain lupus erythematosus. J Invest Dermatol. 2016;136(6):1281–1283. doi:10.1016/j.jid.2016.02.015
31. Wallace DJ, Furie RA, Tanaka Y, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392(10143):222–231. doi:10.1016/S0140-6736(18)31363-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.