Back to Journals » Therapeutics and Clinical Risk Management » Volume 11
Treatment of degloving injury involving multiple fingers with combined abdominal superficial fascial flap, dorsalis pedis flap, dorsal toe flap, and toe-web flap
Authors Han F, Wang G, Li G, Ping J, Mao Z
Received 19 April 2015
Accepted for publication 27 May 2015
Published 23 July 2015 Volume 2015:11 Pages 1081—1087
DOI https://doi.org/10.2147/TCRM.S86948
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Deyun Wang
Fengshan Han, Guangnan Wang, Gaoshan Li, Juan Ping, Zhi Mao
Department of Microsurgery, PLA 205 Hospital, Jinzhou, People’s Republic of China
Background: Our aim was to summarize the treatment of degloving injury involving multiple fingers using combined abdominal superficial fascial flap, dorsalis pedis flap, dorsal toe flap, and toe-web flap.
Patients and methods: Each degloved finger was debrided under microscopic guidance and embedded in the superficial layer of the abdominal fascia. The abdominal skin was sutured to the skin on the back and side of the hand to promote circumferential healing. After removal, the only remaining injured region was on the flexor surface, and this was repaired by multiple dorsal toe flaps, toe-web flaps, and dorsalis pedis flaps to provide blood vessels and sensory nerves. All fingers had proper flap thickness 3–6 months after surgery, and required only lateral Z-plasty modification with web deepening and widening to narrow the fingers and extend their relative length.
Results: We completed flap-graft and finger narrowing for 25 fingers in eight patients. Abdominal skin flaps and dorsal toe flaps were grafted, and resulted in both firmness and softness, providing finger flexibility. The dorsal toe flap provided good blood circulation and sensory nerves, and was used to cover the finger-flexor surface to regain sensation and stability when holding objects. During the 1–8 years of follow-up, sensation on the finger-flexor side recovered to the S3–4 level, and patient satisfaction based on the Michigan Hand Outcomes Questionnaire was 4–5. Flap ulcers or bone/tendon necrosis were not observed.
Conclusion: Treatment of degloving injury involving multiple fingers with combined abdominal superficial fascial flap, dorsalis pedis flap, dorsal toe flap, and toe-web flap was effective and reliable.
Keywords: abdominal thin flap, combined dorsalis pedis flap, dorsal toe flap, toe-web flap, degloving fingers, graft
Introduction
Surgery to repair degloving injury involving multiple fingers (DIMF) caused by high-energy trauma remains a challenge, because of the relatively large area of injured skin and the lack of an ideal treatment.1,2 Currently, common repair methods include the use of abdominal flaps, fascial free flaps, anterolateral thigh-skin flaps, latissimus dorsi-skin flaps, medial arm free flaps, and cutaneous free flaps.3 However, these methods are suboptimal, functionally and cosmetically. Abdominal skin flaps are advantageous, as the abdominal area is large and the skin has a high survival rate. However, the obvious shortcoming is that the flap is thick and dense, and thus multiple plastic thinning surgeries are needed. The advantages of the cutaneous free flap are that the repair surgery is performed only once and that it provides good postoperative flap thickness and function; however, the tissue supply is limited and not suitable for large defects. Combined dorsalis pedis flap, dorsal toe flap, and finger web flap can cover the soft tissue of the hand with satisfactory function and appearance.4–9 However, this method cannot be used to repair DIMF because of the limited tissue supply.
Considering the advantages and disadvantages of current protocols, we used a combined dorsalis pedis flap, dorsal toe flap, finger web flap, and abdominal superficial fascial flap to treat DIMF with a goal to provide another surgical option for DIMF to retain fingers and reconstruct key functions. We describe our results and the important findings that we obtained treating DIMF using this method.
Patients and methods
Patients
From June 2001 to September 2005, we treated eight cases of DIMF: five male and three female. The age range was 6–34 years (mean 20.5 years), and all of the cases were caused by machine-related trauma. There were a total of 25 fingers involving the second to fifth fingers. Three cases involved the four fingers, three involved three fingers, and two involved two fingers. Accompanying fingertip-bone amputation or defects occurred in four cases. Soft-tissue injury included the following: one case extended from the wrist to the fingertip, and involved the superficial muscle layer on the palm and the aponeurotic layer on the back of the hand; injury in four fingers extended from the metacarpophalangeal joint to the fingertip; injury in six fingers extended from the metacarpophalangeal flexor-surface striation to the fingertip; and injury in 14 fingers extended from the proximal mid-finger to the fingertip (Table 1). Our study was approved by the 205 Hospital Committee for Clinical Research, and informed consent was obtained from the eight patients.
Surgical procedure
Epidural anesthesia combined with brachial plexus nerve block was used in five cases, and general anesthesia was used in three cases. Following sufficient cleaning and disinfection of the injured limb, general surface decontamination and necrotic fascial tissue removal were performed visually. Next, finger bones, fascial tissues surrounding the tendons, and blood circulation were examined under ×10 magnification. Necrotic soft tissues without blood circulation were removed, and soft tissues with blood circulation were retained using microscopic guidance. Direct anastomosis was performed for nine ruptured arteries in the center of the fascial tissue, and vascular graft anastomosis was performed for three arteries.
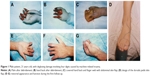
The injured hand was moved to the contralateral lower abdomen to allow the fingers to be naturally placed, and the mid-axis of each finger was marked on the skin. The incision site for each finger was chosen on the basis of the condition surrounding the injury site. In cases of multifinger proximal injuries, we made a combined incision from above the palm lateral striation to the superficial layer of the subcutaneous fascia, followed by selection of the placement site for each finger along its axis. If the injury edges were different among fingers, individual placement sites were created. After the finger was placed at the incision site, a mattress suture (to the abdominal aponeurosis layer through the skin) was placed along each side of the finger. The fingers were then stabilized from the lateral side to maintain the attachment between the skin and the hand back/side without affecting circulation to the skin flap or injured fingers. Four weeks after the surgery, when the abdominal flaps had covered the finger back, side, and web, the fingers and the abdominal flaps were removed simultaneously.
Prior to the surgery, Doppler ultrasound was used to detect blood flow and mark the dorsalis pedis arteries, lateral tarsal arteries, and the first dorsal metatarsal arteries. If the first dorsal metatarsal arteries were Gilbert type I–II, the flaps were obtained from the dorsal fibular side of the toe, interdigital web between the first and second toes, and the back and lateral side of the second and third toes. Flap-surface areas were based on the size of the hand injuries. The first dorsal metatarsal arteries were absent in three cases in the present study. To increase the blood supply to the dorsalis pedis flap, the toe web and toe back supplied by the dorsalis pedis arteries, dorsalis pedis main artery, lateral tarsal arteries, and the corresponding fascial vascular branches of the lateral tarsal arteries were simultaneously removed with the flap. The proximal lateral side of the dorsalis pedis flap was parallel to or higher than the level of the tarsometatarsal joint. In eight cases, the great saphenous vein and the corresponding branches were removed with the flap.
Flap-obtainment process
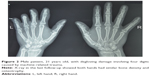
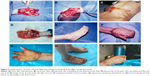
Separation of the toe-back flap, toe-web flap, and dorsalis pedis flap along the superficial layer of the toe-back tendon began 3 mm proximal to the dorsal nail and extended through the toe laterally midline to the distal edge of the toe web. Toes were connected by the dorsal side of the toe web and flap separation was extended toward the first and second metatarsal bones at the metatarsophalangeal joint level. When the separation was between the first and second metatarsal bones, the dorsal flap was removed simultaneously. The plantar deep branch of the dorsalis pedis artery was then ligated. Through an anterior ankle dorsal incision, the dorsalis pedis arteries, the lateral tarsal arteries, and dorsalis pedis skin were freed so that the neurovascular bundle was connected to the dorsalis pedis flap, toe flap, and toe-web flap. Next, the injured fingers were held together and the foot flaps grafted to the injured edge of the lateral side of the hand using an angled-suture pattern. A subcutaneous tunnel was created from the palm to the thumb-finger web, and the flap was placed at the incision site in the thumb-finger web. Anastomoses of the flap artery and radial artery deep branch, flap vein and radial vein, great saphenous vein and cephalic vein, and flap nerves and radial nerve superficial branch were then performed. The flap area was then covered by skin obtained from the thigh, and multiple Z-plasty modifications were performed along the finger sides approximately 3–6 months after the initial surgery with an embedded suture on the flap along the hand-flexor surface. The finger web was also deepened and widened to extend the finger length.
Results
One patient experienced necrosis of the distal half of the fourth to fifth toe-back flap (case 3, Table 1), and one had necrosis of the distal half of the fifth toe-back flap (case 6, Table 1). The necrotic portion of the flap was removed approximately 3 weeks after the abdominal flap-pedicle division. Next, the flap proximal edge and dorsalis pedis flap were partially separated, and one separated section was moved to the distal end. Slight flexion allowed the injured area at the fingertip to be covered. All of the flaps on the lateral flexor surface in the remaining 22 fingers survived. During the 12–96 months of follow-up, the regions covered by the flap were firm and soft, with a slim and satisfactory finger appearance. The interdigital joints were capable of flexion, extension, and touch, and pain sensation in the finger-flexor side flaps began to return 6 months after the surgery. Sensory nerves recovered to the S3–4 level (British Medical Research Council [MRC] classification) by the 1-year follow-up, and sensation on the hand back flap recovered to the MRC S0–1 level. The degree of patient satisfaction based on the Michigan Hand Outcomes Questionnaire was 4–5.10 None of the 25 fingers developed malnutrition ulcers or finger-bone/tendon infection, and necrosis and flap-donor areas healed during phase I without malfunction (Table 1). Representative case 1 is shown in Figures 1 and 2, and case 2 in Figure 3.
Discussion
Reconstruction of the DIMF area requires large amounts of tissue, and regaining flexor function is challenging.1 Previous studies have explored using abdominal flaps, and Nazerani et al used this flap in six DIMF cases.11 Yu et al12 reported using an anterolateral thigh free flap to treat degloving injury involving three digits in one case, and Ju et al8 used a combined dorsal great toe flap and dorsalis pedis artery flap to treat degloving injury of the thumb, with satisfactory results. Wang et al13 used a cross-finger flap and a composite-free flap from the dorsum of the second toe to treat 18 cases of distal degloving injuries, and Kim et al3 used a thigh free flap to treat hand degloving injury. However, most DIMF cases have large areas of injured soft tissue, and abdominal skin covers a large area, but is much thicker and denser than normal finger soft tissues. Also, the antifriction and sensory functions of abdominal skin flaps are not satisfactory. Among all free flaps, dorsalis pedis free flaps are best adapted to the hand; however, their donor area is limited and incapable of meeting the demand of DIMF. Therefore, we designed a protocol using combined abdominal skin flaps and dorsalis pedis free flaps.
We used an embedded graft that included combined abdominal skin flaps, dorsalis pedis flaps, toe flaps, and toe-web flaps, which were naturally integrated. Abdominal skin flaps are elastic and soft, whereas foot-derived flaps are low in fatty tissues and do not roll or slide after healing with deep tissues, permitting good holding capacity in the grafted fingers. The two types of flaps integrated well, and resulted in satisfactory firmness and softness. The donor area of the abdominal skin flap was large and wide, providing enough capacity to cover the injured area on the hand. In the present study, the degloved fingers were embedded into the superficial layer of the skin fascia and laterally stabilized by abdominal tendons via the skin. This allowed one-time repair of most of the circumferential injuries of the hands. The abdominal thin skin flap was used mainly on the finger back and side, which have no critical function, and the dorsalis pedis flap, dorsal toe flap, and toe-web flaps, which were of good quality with good blood circulation and resulted in rapid return of skin sensation, were used to cover the finger-flexor side. Therefore, covering the common injured regions and reconstructing the key functions were effectively combined, so that flap resources were fully utilized. We followed our cases for 1 year, and found that sensation recovery in the palm reached an MRC level of S3–4, whereas functional recovery of the abdominal skin flap on the hand back only reached S0–1, confirming that coverage of the palm by the dorsalis pedis flap maximized hand-function recovery.
We treated the degloving injuries using minimally invasive techniques. All 25 fingers in the eight cases resulted from machine-related damage with associated circumferentially injured areas in the finger soft tissues. The pathological conditions of the injury were complicated, with the primary trauma causing tissue injury, loss of blood supply, and necrosis. The original arterial network was damaged, leading to insufficient blood supply, and detrimentally affected the survival of the injured fingers. During the debridement process, the primary goal was maintaining the healthy tissues in the injured fingers and specifically avoiding damage to the fascial tissues that retained adequate blood supply. Fingers are cylindrical, and thus the external force received by each side of the finger was different during the trauma, causing different degrees of damage to the soft tissues. In the injured area, the damaged/necrotic tissues intermingled with healthy tissues with good blood circulation. Under microscope guidance, the tissues could be precisely distinguished, allowing removal of the contaminated, necrotic tissues while avoiding damage to the healthy tissues with good blood supply. Ruptured arteries were directly anastomosed in nine broken fingers in our study, and arteries were anastomosed in the presence of vascular grafts in three fingers. As the repaired blood vessels were networked with the remaining fascial tissue, the anastomosis improved the blood supply to the injured fingers. The blood supply from the original finger artery, as well as the reticular collateral branches in the fascial tissues, tissues surrounding the tendon, and tissues surrounding the bone, provided important additional blood supply. Under microscope guidance, we also observed that some damaged surfaces of the original arteries and distal fascial tissues provided continued blood supply to the bone and tendon. These fascial tissues with blood supply provided important markers for healthy bone and tendon. Distal bone marrow bleeding in the injured finger seen under the microscope indicated that the bone had blood supply, and when the blood supply was weak in the degloved fingers, lowering the hands showed active bleeding. Therefore, during the early phase of blood-supply reconstruction after the repair surgery, lowering the hands is beneficial to improve finger ischemia.
We modified the traditional surgical protocol that embeds the abdominal skin flap; instead, the injured fingers were embedded into the superficial layer of the abdominal subcutaneous fascia and stabilized by mattress suture placed between the bilateral skin of the fingers and the abdominal aponeurosis. This process enabled the skin flap and finger body to maintain proper tension without affecting the flap blood supply, creating good circumferential attachment between most of the finger surface and the finger body and leading to satisfactory healing after a single surgery. After the flaps were removed, the finger-flexor surface was repaired by the combined dorsalis pedis flap, dorsal toe flap, and toe-web flap via anastomosed blood vessels and nerves, allowing the flap, with arterial blood, to cover the fingertips. Dorsalis pedis tissues are similar to hand tissues, with similar flap thickness. The flap has a rich blood supply and gradually reestablishes skin sensation, leading to functional restoration of the injured area. The use of dorsalis pedis flaps to repair hand-skin damage has been reported previously.5,14 We combined the dorsalis pedis flap, dorsal toe flap, and toe-web flap, which increased the blood supply from the dorsalis pedis flap to the dorsal toe flap to some degree and increased the survival of the dorsal toe flap. In patients without a first dorsal metatarsal artery, we moderately increased the size of the dorsalis pedis flap so that the lateral tarsal arteries and the corresponding nutritional fascial vascular branches were obtained simultaneously to increase the survival of the distal flap.
The advantages and key points of our combined technique include: 1) the dorsalis pedis flap, dorsal toe flap, and toe-web flap covered most of the functional flexor area in the hand to maximize the restoration of hand function and appearance; 2) the degloved fingers were sufficiently covered by the abdominal superficial fascial flap; 3) multiple Z-plasty modifications were performed along the finger sides, and the two types of flaps integrated well, resulting in satisfactory firmness and softness; and 4) microscopic technique guiding debridement protected living tissue effectively, and anastomosis of digital artery improved the perfusion of injury fingers.
One drawback of our combined technique is that one main vessel on the foot is sacrificed. Therefore, this flap should be used with caution. However, this combined flap not only covered multiple degloving fingers, but provided ideal tissue that was most similar to hand tissue. All included cases had an accepted or ideal result in many aspects at the last follow-up. To avoid scar contracture, we followed certain procedures. First, when the flap was separated, we retained the fascia above the tendon to avoid adhesion of skin grafts and tendon. Second, split-thickness skin grafting was used to cover the flap donor area, toes were held in a separated position, and the joint was in functional position. In these cases, one 6-year-old child received this combined surgery strategy and had acceptable results. However, we also agreed to the reviewer’s comments. The indication of this combined surgery strategy for pediatric patients needs further discussion and investigation.
The limitations of the present study include the small sample size and the subjective nature of the patient-satisfaction survey results. Future studies require larger sample sizes in high-quality cohort studies and randomized controlled trials.
Conclusion
The approach of combined abdominal superficial fascial flap, dorsalis pedis flap, dorsal toe flap, and toe-web flap was effective and reliable to treat DIMF.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Krishnamoorthy R, Karthikeyan G. Degloving injuries of the hand. Indian J Plast Surg. 2011;44(2):227–236. | ||
Ulrich D, Pallua N. Treatment of avulsion injury of three fingers with a compound thoracodorsal artery perforator flap including serratus anterior fascia. Microsurgery. 2009;29(7):556–559. | ||
Kim KS, Kim ES, Kim DY, Lee SY, Cho BH. Resurfacing of a totally degloved hand using thin perforator-based cutaneous free flaps. Ann Plast Surg. 2003;50(1):77–81. | ||
Morrison WA, O’Brien BM, MacLeod AM. The foot as a donor site in reconstructive microsurgery. World J Surg. 1979;3(1):43–52. | ||
Ju J, Hou R. Reconstruction of penetrating injuries of the hand with dorsalis pedis composite free flaps: a series of 23 patients. J Plast Reconstr Aesthet Surg. 2012;65(10):1368–1376. | ||
Eo S, Kim Y, Kim JY, Oh S. The versatility of the dorsalis pedis compound free flap in hand reconstruction. Ann Plast Surg. 2008;61(2):157–163. | ||
Ju JH, Hou RX. Repair of a degloving injury of the thumb with a combined dorsal great toenail flap and dorsalis pedis flap: a case report. Arch Orthop Trauma Surg. 2013;133(10):1455–1458. | ||
Ju J, Zhao Q, Liu Y, et al. [Repair of whole-hand destructive injury and hand degloving injury with transplant of pedis compound free flap]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23(10):1153–1156. Chinese. | ||
Noaman HH. Salvage of complete degloved digits with reversed vascularized pedicled forearm flap: a new technique. J Hand Surg Am. 2012;37(4):832–836. | ||
Chung KC, Hamill JB, Walters MR, Hayward RA. The Michigan Hand Outcomes Questionnaire (MHQ): assessment of responsiveness to clinical change. Ann Plast Surg. 1999;42(6):619–622. | ||
Nazerani S, Motamedi MH, Nazerani T, Bidarmaghz B. Treatment of traumatic degloving injuries of the fingers and hand: introducing the “compartmented abdominal flap”. Tech Hand Up Extrem Surg. 2011;15(3):151–155. | ||
Yu G, Lei HY, Guo S, Yu H, Huang JH. Treatment of degloving injury of three fingers with an anterolateral thigh flap. Chin J Traumatol. 2011;14(2):126–128. | ||
Wang B, Zhang X, Jiang W, Ma T, Li H, Wang H. Reconstruction of distally degloved fingers with a cross-finger flap and a composite-free flap from the dorsum of the second toe. J Hand Surg Am. 2012;37(2):303–309. e1–e4. | ||
Wang L, Fu J, Li M, Han D, Yang L. Repair of hand defects by transfer of free tissue flaps from toes. Arch Orthop Trauma Surg. 2013;133(1):141–146. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.