Back to Journals » Infection and Drug Resistance » Volume 15
Treatment of Carbapenem-Resistant Multidrug-Resistant Gram-Negative Bacilli with Intracerebroventricular Injection of Polymyxin B: A Retrospective Study
Authors Liu D , Niu J, Chen G, Xu L
Received 14 October 2022
Accepted for publication 10 December 2022
Published 22 December 2022 Volume 2022:15 Pages 7653—7666
DOI https://doi.org/10.2147/IDR.S392818
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Dongsheng Liu,1 Jianxing Niu,1 Guoqiang Chen,1 Long Xu2
1Departments of Neurosurgery of Aviation General Hospital, Beijing, 100012, People’s Republic of China; 2Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, National Clinical Research Center for Neurological Diseases (NCRC-ND), Beijing, 100070, People’s Republic of China
Correspondence: Long Xu, Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, National Clinical Research Center for Neurological Diseases (NCRC-ND), No. 119 Nansihuanxilu Road, Fengtai District, Beijing, 100070, People’s Republic of China, Tel +86 13911129912, Fax +86 10-59976095, Email [email protected] Guoqiang Chen, Departments of Neurosurgery of Aviation General Hospital, No. 3, Anwai Beiyuan, Beiyuan Road, Chaoyang District, Beijing, 10001, People’s Republic of China, Tel +86 13311396583, Fax +86 10-59520156, Email [email protected]
Purpose: We evaluated the efficacy and administration time of intraventricular (IVT) polymyxin B in the treatment of carbapenem-resistant and multidrug-resistant/extensively drug-resistant (MDR/XDR) Gram-negative bacilli in central nervous system (CNS) infections and investigated prognostic factors.
Patients and Methods: This retrospective analysis comprised 41 post-surgical carbapenem-resistant CNS infections from October 2016 to October 2021. All patients were treated with effective intravenous antibiotics and IVT polymyxin B. Patient characteristics, therapeutic procedure, symptoms, cerebrospinal fluid (CSF) examination, laboratory tests, and complications were recorded. The effectiveness of IVT polymyxin B was evaluated using temperature, Glasgow Coma Scale, CSF contents, bacterial clearance rate, cure rate, and mortality. Mortality between early (7 days) and late administration of IVT polymyxin B was compared. Prognostic factors were evaluated using the pupillary light reflex and multiloculated hydrocephalus.
Results: The 41 patients acquired carbapenem-resistant MDR/XDR bacteria, including 24 Klebsiella pneumoniae, 15 Acinetobacter baumannii, 3 Pseudomonas aeruginosa, and 1 Enterobacter cloacae. The bacterial clearance rate was 32/41 (78.0%), and 9 patients (22.0%) with uncured bacterial infections died. Adverse events included 1 case of skin pigmentation. Among the 32 cured patients, 31 received a ventriculoperitoneal shunt, and 1 patient had an extraventricular drainage tube removed. Mortality in the late (> 7 days) group was higher (39.1% vs 0%, P < 0.05). The group without pupillary light reflex showed a higher death rate (41.2% vs 8.3%; P < 0.05). The multiloculated hydrocephalus group had a higher mortality rate than that of the normal group (34.8% vs 5.6%, P < 0.05). All 32 cured patients were followed up for 9 to 66 months, and all survived without recurrent infections.
Conclusion: Intraventricular polymyxin B is an effective treatment for carbapenem-resistant MDR/XDR Gram-negative bacilli, with a 78% cure rate and significant mortality reduction if administered within 7 days of bacterial identification. Multiloculated hydrocephalus and the pupillary light reflex may be used as prognostic indicators of mortality.
Keywords: polymyxin B, intracerebroventricular injection, central nervous system infections, cerebrospinal fluid, prognosis
Introduction
Treatment of infections of the central nervous system (CNS) is particularly challenging due to the limited permeability of the blood-brain barrier. Insufficient treatment may lead to increased morbidity and mortality as well as extended hospital stays. One study found that the risk of CNS infection following craniotomy was 1.5–5.5%, which increased to 9.6–15% if external ventricular drainage was inserted.1–5 Infections caused by multidrug-resistant/extensively drug-resistant (MDR/XDR) Gram-negative bacilli, such as Acinetobacter baumannii and Klebsiella pneumoniae, are difficult to treat because of the scarcity of effective drugs.6 Furthermore, carbapenem resistance is strongly correlated with death from meningitis.7 Fortunately, adjuvant intrathecal or intraventricular (ITH/IVT) colistin treatment may help patients with a CNS infection caused by MDR/XDR gram-negative bacilli isolates.8–10
Because the cerebrospinal fluid (CSF) lacks opsonins and has ineffective phagocytosis, bactericidal dosages of antibiotics are required to guarantee the removal of infection, unlike in other bodily compartments.11 Polymyxins are regarded as the most effective antibiotics and can eradicate Gram-negative bacteria. Polymyxins primarily target bacterial membranes, causing their disruption and cell death.12 Polymyxins also shuffle phospholipids, mediate hydroxyl radical death pathways, counterbalance endotoxins, and affect normal cell reproduction and respiration.13 According to a study that evaluated 40,625 isolates, resistance to polymyxin compounds was uncommon, and the highest polymyxin B resistance rates were observed for Klebsiella spp. (1.4%), followed by Acinetobacter spp. (0.8%) and Pseudomonas aeruginosa and Escherichia coli (both at 0.1%).14 It has even been suggested that polymyxin B is currently used as a “last-line” treatment for multidrug-resistant Gram-negative bacteria.15 However, as a result of their polycationic structure and high molecular weight, polymyxins have difficulty penetrating the blood-brain barrier when administered intravenously.16 Due to the blood-brain barrier, intravenous (IV) polymyxin B cannot reach the effective concentration in the CSF needed to treat an infection in the brain. IV injection results in a concentration of colistin in the CSF that is only 5–10% that of the blood level.17 Only intracerebroventricular injection resulted in CSF colistin concentrations above the minimum inhibitory concentration of 0.5 g/mL and penetration rates that were 11% greater than 7% compared to IV administration alone.18 IVT polymyxins may be the only way to quickly and effectively reach the bactericidal concentration.
The Standards and Practice Guidelines of the Infectious Diseases Society of America (IDSA) state that IVT injection in conjunction with IV polymyxin B or colistin is advised for the treatment of intracranial MDR gram-negative bacterial infections.19 IVT+IV colistin or polymyxin B is the best treatment for MDR/XDR Gram-negative CNS infections, according to multiple studies.20–22 Unfortunately, IVT polymyxin B is often used as a remedial treatment but is not recommended as an initial treatment for carbapenem-resistant MDR/XDR Gram-negative bacilli, and there have been few clinical studies on the correlation between the timing of IVT polymyxin administration and mortality.23 The IDSA guidelines also do not provide a possible effective start time window for IVT polymyxin B. This study aimed to determine the efficacy and administration time of IVT polymyxin B in the treatment of carbapenem-resistant and MDR/XDR Gram-negative bacilli in CNS infections and to investigate prognostic factors.
Materials and Methods
Selection and Exclusion Criteria
We retrospectively collected data from 41 patients with CNS infections from October 2016 to October 2021 in the Department of Neurosurgery of Beijing Aviation General Hospital. Selection criteria were: (1) clinical and radiographic evidence supported the diagnosis of CNS infections; (2) biochemical and routine examinations of the CSF suggested an intracranial infection; (3) two consecutive CSF bacterial cultures contained Gram-negative bacilli, and carbapenem-resistant strains were screened; (4) in addition to IV antibiotic, simultaneous IVT injection of polymyxin B was performed; (5) clinical data were complete. The exclusion criteria were: (1) patients developed CNS infection prior to neurosurgery; (2) patients with false-positive CSF cultures, which may have been caused by contamination from the trial process and sampling; (3) immunocompromised patients; (4) incomplete clinical data.
Microbial Culture Methods
CSF was collected and injected into Columbia blood AGAR culture flasks and then shaken at 35 °C for 24 h with a BACT/ALERT3D120 blood culture apparatus. After a few days, when the bacterial culture was positive, the bacterial suspension was standardized and placed in a VITEK2 for bacterial identification and to obtain drug sensitivity results. For polymyxin B drug sensitivity testing, bacterial suspensions were placed on MacConkey agar and test strips were used (E test method, BIO-KONT company, CHN). The polymyxin drug sensitivity results were evaluated according to the latest Clinical and Laboratory Standards Institute standards from 2015 to 2021 (https://clsi.org/meetings/susceptibility-testing-subcommittees).
Clinical Treatment
Among the 41 patients with CNS infection, 22 were transferred from other hospitals without identifying the pathogenic bacteria in the CSF, but the clinical manifestations, CSF examination, and imaging supported CNS infection. Empirical IV antibiotic therapy and CSF culture results were obtained. After the bacterial identification and drug susceptibility results were obtained, the original CSF drainage tube or ventricle shunt tube was immediately removed and a new CSF external drainage tube was inserted. Sensitive antibiotics were administered intravenously to fight infection, and 5 mg polymyxin B (XGEN PHARMACEUTICALS, US) was injected into the ventricle daily. The other 19 cases were transferred with bacterial identification, and the drug susceptibility results of CSF were obtained from another hospital. After admission, the original CSF drainage tube or ventricular shunt tube was immediately removed and a new ventricular shunt tube was placed. For the treatment of CNS infection, sensitive antibiotics were administered intravenously, and polymyxin B (5 mg/day) was administered intraventricularly. During anti-infective treatment, head CT was reviewed once a week to monitor ventricular adhesion and multiloculated hydrocephalus. When multiloculated hydrocephalus or isolated ventricle with peripheral brain tissue was compressed and clinical symptoms appeared, one or more ventricular drainages were performed.
Infection Cure Criteria and Management
The criteria for cure of infection were as follows. (1) The patient showed relief of symptoms and indicators of CNS illness, such as fever, neck stiffness, and changes in mental status, with meningeal irritation being the typical manifestation. (2) Biochemical and routine CSF levels returned to normal. Normal CSF was clear and colorless and levels of protein were 50–150 mg/L, glucose 2.5–4.5 mmol/L, leukocytes (0–5) ×106/L, and chloride 120–130 mmol/L. The bacteria in the CSF were continuously cultured three times without bacterial growth. (4) To evaluate the symptoms and imaging manifestations of patients with hydrocephalus, the ventricle drainage tube was replaced and drainage continued, and the duration of CSF purification was recorded. When the standard conditions for ventriculoperitoneal shunt were reached, it was performed. If the infection had been cured and the diagnosis of hydrocephalus was excluded, the drainage tube outside the ventricle was removed.
Clinical Outcomes
The data collected included clinical characteristics such as age, gender, primary disease, previous surgical procedures, and implants. The course of treatment included bacterial isolates, drug susceptibility, concomitant infections, empirical systemic antibiotics, IV antibiotics, and the duration of IV antibiotic administration. The pupil reflex and Glasgow Coma Scale (GCS) score before IVT polymyxin B, delay period (DP) from bacterial identification to IVT injection of polymyxin B, usage and dosage of IVT injection of polymyxin B, bacterial clearance time, body temperature, GCS score after bacterial clearance, presence of the multiloculated hydrocephalus, location of the isolated ventricles, number of drainage tubes placed in the ventricles, CSF purification time (time between bacterial identification in CSF and routine and biochemical normalization), CSF examination (bacterial culture, drug sensitivity, appearance, leukocytes, glucose, and protein), serum creatinine, and screening for adverse reactions including epilepsy, acute kidney injury, and skin pigmentation. All cured patients were followed up from 9 to 66 months. Prognostic factors were evaluated using the pupil light reflex and multiloculated hydrocephalus for mortality rates.
Statistics
The patients were grouped for statistical analysis by the DP of IVT polymyxin B, with DP ≤7 days for the early IVT injection group and DP >7 days for the late IVT injection group. The effectiveness of IVT polymyxin B was evaluated comparing the abovementioned indicators using paired samples t-tests and the independent samples Mann–Whitney U-Test. The chi-square test and Fisher’s exact test were used to compare mortality rates between the early and late groups. Statistical analysis was performed using SPSS (version 26.0). Numeric data with a normal distribution were represented as the mean ± standard deviation, and paired sample t-tests were used to compare pre-treatment and post-treatment variables. Non-normally distributed data were expressed as median (lower quartile, upper quartile). P<0.05 was considered statistically significant.
Results
A total of 41 patients with CNS infections between October 2016 and October 2021 were selected for this study. A total of 41 major pathogenic bacterial strains were isolated, including 24 cases of K. pneumoniae, 15 A. baumannii, 3 Pseudomonas aeruginosa, and 1 Enterobacter cloacae, all of which were MDR/XDR strains. In terms of drug resistance, the carbapenem resistance rate was 100%, polymyxin B resistance rate was 0%, and tigecycline resistance rate was 18.2%–33.3%. The results are presented in Table 1.
 |
Table 1 Drug Susceptibility Results of 41 MDR/XDR Gram-Negative Bacilli |
The age of the 41 patients was 37.7±19.1, and 70.7% were male. The primary diseases included intracerebral hemorrhage in 12 cases (29.3%), traumatic brain injury and CSF leakage in 9 cases (22.0%), traumatic brain injury in 8 cases (19.5%), brain aneurysm in 6 cases (14.6%), brain tumors in 4 cases (9.8%), chronic subdural hematoma in 1 case (2.4%), and hydrocephalus in 1 case (2.4%). The previous surgical procedures and conditions included decompressive craniectomy, intracerebral hemorrhage, external ventricular drainage, lumbar cisterna drainage, traumatic brain injury, evacuation of intracranial hematoma, excision of brain abscess, excision of intracranial tumor, intracranial aneurysm clipping, and Ommaya tube placement. The implants included extraventricular drainage tubes, lumbar cistern drainage tubes, subdural drainage tubes, and Ommaya tubes. These data are presented in Table 2.
 |
Table 2 Clinical Characteristics of Patients Enrolled in the Study |
Among the 41 major pathogenic bacteria, 28 were single infections and 13 were associated with concomitant infections, most of which were associated with Gram-positive cocci and some Gram-negative bacilli. Empirical systemic therapy or subsequent systemic therapy was performed prior to the identification of drug susceptibility results, including meropenem, vancomycin, cefoperazone sulbactam sodium, ceftriaxone, levofloxacin, ceftazidime, amikacin, piperacillin, tazobactam, and tigecycline. After identification of drug susceptibility results, 41 sensitive IV antibiotics were selected accordingly. The main antibiotics used in the 41 patients were tigecycline (n = 26), amikacin (n = 5), ceftazidime (n = 2), fosfomycin (n = 2), polymyxin (n = 2), levofloxacin (n = 1), ciprofloxacin (n = 1), cefoperazone (n = 1), and tobramycin (n = 1). In some severe cases, a second sensitive antibiotic was administered intravenously. The durations of IV antibiotic administration are listed in Table 3.
 |
Table 3 Cerebrospinal Fluid (CSF) Culture and Treatment Strategies for Each Patient |
The ventricle was injected daily with 5 mg polymyxin B. The median DP of IVT polymyxin B administration was 10.0 (5.0–26.0), the median time for continuous intraventricular polymyxin B injection was 19 (13, 27), the median time for bacterial clearance was 6.0 (5.0–10.0), and the median time for CSF purification was 83 (40–119). The bacterial clearance times of K. pneumonia and A. baumannii were 8.0 (6.0–12.0) days and 5.0 (4.0–7.0) days, respectively, and there was a significant difference between them (P < 0.05). Due to limited sample sizes, P. aeruginosa and E. cloacae were removed from the analysis. The comparison of the delay periods of the death group with those of the cured group showed that both were statistically significant (P < 0.05). These results are listed in Table 3.
The body temperature and GCS scores of all patients before and after bacterial clearance were significantly different (P < 0.05). Nonmetric tests before and after bacterial clearance, leukocytes, and glucose and protein levels were all significant (P < 0.05). These data are presented in Table 4.
 |
Table 4 Clinical Symptoms and Laboratory Data in Patients |
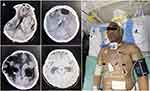
The bacterial clearance rate was 78.0%, and all 9 patients without bacterial clearance died. Thirty-two patients (78.0%) were cured, and 9 patients (22.0%) died, of which the 30-day mortality rate was 1/41 (2.4%), the 90-day mortality rate was 8/41 (19.5%), and the hospital mortality rate was 9/41 (22.0%). Adverse events included 0 cases of epilepsy, 0 cases of acute kidney injury, and 1 case of skin pigmentation (Figure 1). Among the 32 cases of infection cured, 31 cases received ventriculoperitoneal shunt, 1 case had the extraventricular drainage tube removed, and 18 cases developed multiloculated hydrocephalus. All 32 cured patients were followed up for 9 to 66 months, and all survived without recurrent infections (Table 5).
 |
Table 5 Clinical Outcomes and Complications |
Among the 41 patients, one ventricular drainage tube was indwelled in 24 cases (58.5%), two tubes in 13 cases (31.7%), three tubes in three cases (7.3%), and four tubes in one case (2.4%) (Figure 1).
Compared to the early (DP ≤7 days) IVT polymyxin B group, mortality in the late (DP >7 days) group was higher (39.1% vs 0%, P < 0.05). However, there was no statistical difference in the IVT polymyxin B delay period (days) between patients with multiloculated hydrocephalus and normal ventricles in the non-parametric test [13 (7, 26) vs 6 (3, 26); P < 0.05].
The pupil reflexes of 41 patients before IVT polymyxin B were evaluated; 24 (58.5%) were present and 17 (41.5%) vanished. Compared with the group with a pupillary light reaction before polymyxin B intraventricular injection, the group without a reflex had a higher death rate (41.2% vs 8.3%; P < 0.05). The multiloculated hydrocephalus group had a higher mortality rate than the normal ventricle groups (34.8 vs 5.6%, P < 0.05), as shown in Table 6.
 |
Table 6 Clinical Factors and Mortality |
Discussion
Based on how the 3163 strains of bacteria found in CSF samples by CHINET in 2021 were split, 11.3% were A. baumannii, 9.1% were K. pneumonia, 1.6% Pseudomonas aeruginosa, and 1.6% E. cloacae. In terms of drug resistance, the resistance rate of K. pneumoniae to meropenem increased from 2.9% in 2005 to 26% in 2022, and the resistance rate of A. baumannii to meropenem increased from 31% in 2005 to 76.6% in 2022 (see http://www.chinets.com). Carbapenem antibiotics are effective drugs for CNS infections because of their good blood-brain barrier permeability. Due to the lack of sensitive and high-permeability drugs, the rate of carbapenem-resistant MDR bacteria is increasing. Carbapenem-resistant Gram-negative bacteria are a leading cause of hospitalizations and deaths.24 In contrast to patients infected with carbapenem-susceptible K. pneumoniae, those with purpose-carbapenem-resistant K. pneumoniae have a higher mortality rate.25 Resistance to carbapenem may increase the mortality risk for patients with A. baumannii infection.26 Therefore, the detection rate of carbapenem-resistant Gram-negative bacteria is increasing each year and is closely related to high mortality. Intracranial infections caused by carbapenem-resistant K. pneumoniae and carbapenem-resistant A. baumannii have become difficult to solve in clinical practice.
Carbapenem-resistant strains should be treated with colistin sodium or polymyxin B according to the IDSA Standards and Practice Guidelines (strong, moderate).19 Patients with post-neurosurgical intracranial infection due to MDR/XDR Gram-negative bacteria have been shown in observational studies to benefit from the addition of ITH/IVT antimicrobial therapy, which is associated with a lower risk of mortality and a higher microbiological clearance rate and only mild adverse effects.23 The current standard of care for MDR/XDR A. baumannii ventriculitis/meningitis is IVT/ITH colistin, which provides a novel, relatively safe, and effective form of therapy.27 Therefore, IVT polymyxin B may be an effective treatment for CNS infections with MDR/XDR Gram-negative bacteria.
The Effectiveness of IVT Polymyxin B
The IDSA guidelines recommend 5 mg/day for 10–21 days of IVT polymyxin B.11 In the International Consensus Guidelines for the Optimal Use of Polymyxins, the dose for IVT polymyxin B is 5 mg/day for 18 days.28 Nevertheless, the quality of evidence is low, as stated in the guidelines. Despite the success of IVT + IV polymyxin B treatment, there are few clinical reports of other IV antibiotics combined with IVT polymyxin B. In our study, domestic polymyxin B was costly and not easily obtainable in China until 2018, so the majority of cases did not use the IV+IVT polymyxin B program recommended by the guidelines because of cost concerns. Therefore, IV antibiotics, such as tigecycline, amikacin, and fosfomycin, according to the drug susceptibility results, were used in combination with IVT polymyxin B (5 mg/day), with continuous IVT injection of polymyxin B for a median of 19 days (13, 27). According to published data, the bacterial clearance rate of IVT polymyxin B as an adjunct to standard IV treatment differed among studies: 65.2%,29 25%,8 66.7%,30 25%,31 78%,21 67%,32 and 67.6%.33 In our study, the bacterial clearance rate reached 78%, which is slightly higher than the bacterial clearance rate reported in the literature. The review and meta-analysis of Maria et al concluded that IVT antibiotics improved eradication rates for Gram-negative ventriculitis and meningitis, but they were not statistically significant.34 But, in carbapenem-resistant Gram-negative ventriculitis and meningitis, the effect was significant (10 times).34 However, the evidence was very poor. Further prospective large-scale studies are required to validate these findings.34
Some CSF management techniques may be helpful in improving bacterial clearance during treatment procedures. First, many reports believe that extracellular ventricular drainage and lumbar cisternectomy are high risk factors for intracranial infection,35–37 and the infection rate can reach 23.2%.36 Moreover, it has been reported that after the bacterial clearance of CSF, even if there are no bacteria in the bacterial culture of CSF, the fixed value bacteria in the extracellular ventricular drainage tube can still be detected by electron microscopy and PCR.38 In our treatment procedure, the temporary external ventricular drainage tube was removed immediately after bacterial clearance of IV antibiotics + IVT polymyxin B and a new ventricular drainage tube was inserted, which could prevent recurrence of infection caused by bacteria fixed in the drainage tube and improve the cure rate. Second, continuous ventricular drainage of CSF gradually cleared the CSF after placing a new extracellular ventricular drainage tube.39,40 In our study, CSF biochemistry and routine results also indicated gradual improvement to normal. Third, IVT polymyxin B can bypass the blood-brain barrier and higher concentration accumulates in the CSF than with IV antibiotics alone, reaching a minimum inhibitory concentration of 0.5 g/mL.18 IVT polymyxin B combined with continuous extraventricular drainage can drain inflammatory CSF while clearing bacteria to reduce the bacterial load of intracranial infection. Adequate long-term IV sensitive antibiotics have an effective therapeutic effect after an infection-induced increase in blood-brain barrier permeability.41
In this study, the median number of days of continuous intracerebroventricular injection of polymyxin B was 19 (13, 27), which was consistent with the guideline-recommended duration of continuous intracerebroventricular injection (10–21 or 18 days).19,28 The median bacterial clearance time in days was 6.0 (5.0–10.0). The nonparametric test in this study compared the bacterial clearance times of K. pneumoniae and A. baumannii and found that the median bacterial clearance time for K. pneumoniae was 8.0 (6.0–12.0), and that for A. baumannii was 5.0 (4.0–7.0), and the difference was statistically significant (P < 0.05). We believe that compared with the intracerebroventricular injection time for carbapenem-resistant K. pneumoniae, that for carbapenem-resistant A. baumannii should be appropriately extended by at least more than 8 days, which can reduce bacterial recurrence and improve the bacterial clearance rate.
Based on the clinical symptoms and laboratory results, we believe that the combination of IV-sensitive antibiotics and IVT polymyxin B can effectively control ventricular inflammation, improve GCS score, and have no effect on renal function.
Mortality
According to the literature, the mortality rates reported by studies using antibiotic IVT+IV treatment regimens varied and were 36.4%, 75%,27 34.9%,28 55.5%,13 20%,29 and 32.4%.30 The mortality rate of intracranial infections caused by MDR/XDR Gram-negative bacteria using only IV antibiotics in different studies were 56.2%,12 77.8%,13 72.7%,30 37.9%,8 91.6%,7 55.3%,14 and 33.3%.29 In our study, 32 patients (78.0%) were cured, and 9 patients (22.0%) died, of which the 30-day mortality rate was 1/41 (2.4%), the 90-day mortality rate was 8/41 (19.5%), and the hospital mortality rate was 9/41 (22.0%). Our findings are consistent with other literature reports on cure and mortality, with slightly better results than those of other studies.
We found that mortality was associated with the pupil response to light, administration time of IVT polymyxin B, and multiloculated hydrocephalus. First, patients with lost pupillary reflex before treatment had a higher mortality rate, indicating that the degree of brain injury is related to mortality and that the loss of optical reflex is a prognostic indicator. Second, we found that patients with delayed injection of IVT polymyxin B had a higher mortality rate. Instead of being utilized as an initial treatment, IVT injection is typically employed as a corrective treatment after systemic medication fails.31 However, when to initiate IVT treatment is not clear in the guidelines and depends more on clinical experience. It is widely accepted that IVT therapy is added sequentially to the treatment regimen for patients who exhibit persistent bacterial growth in CSF cultures or are clinically failing. IVT antibiotic use in ventriculitis treatment may be able to shorten the period of CSF bacterial clearance, especially in patients in a treatment-refractory state. Conversely, delayed bacterial clearance from the CSF is associated with adverse neurologic outcomes.31,42 Furthermore, delayed ITH/IVT colistin therapy contributed to mortality.43 This is consistent with our results. For carbapenem-resistant CNS infections, IVT polymyxin B should be initiated as soon as the bacteria are isolated. An effective time window for initiating therapy is generally within 7 days of bacterial identification and IVT polymyxin B therapy should be administered as soon as possible.
Lastly, multiloculated hydrocephalus is also called compartmentalized hydrocephalus or ventricular septations. Inflammation of the ependyma supports subependymal glial tissue development, where exudates and debris form fibroglial webs. Once the ependymal lining is disturbed, glial tufts create septations.44 It has been reported that VP patients with intracranial infection with Gram-negative bacilli have a higher probability of multiloculated hydrocephalus, with an incidence of approximately 32%.44 In our study, carbapenem-resistant MDR Gram-negative bacilli caused a higher mortality rate in ventricular compartmentalization patients with central nervous system infection. A possible reason is that compartmentalization may affect the distribution of CSF in the subarachnoid space and the ventricle, leading to the failure to kill bacteria completely, the infection does not heal, and even an asymmetrically dilated ventricle, causing acute cerebral hernia.
In our study, repeat CT scans following IVT polymyxin B + IV sensitive antibiotic therapy found multiloculated hydrocephalus. Regarding the cause, it has been hypothesized that the IVT administration of antibiotics may be a factor.45 In addition, it has been claimed that the delayed beginning of IVT irrigation and the administration of antibiotics contributed to an increased prevalence of multiloculated hydrocephalus.46 However, our study found no statistical difference in IVT polymyxin B DP between the patients with multiloculated hydrocephalus and those with normal ventricles. In addition, there was no indication of chemical ventriculitis after IVT polymyxin B administration. Therefore, more studies are required to determine the connection between multiloculated hydrocephalus and IVT polymyxin B.
Adverse Events
There were no instances of epilepsy or acute kidney injury. The only side effect in our study was skin hyperpigmentation in one patient who received 50 mg IV polymyxin B every 12 h for 31 days. The patient developed head and neck symptoms after 3 days of systemic polymyxin B treatment, and the color of the head and neck became dark brown after 10 days. (Figure 1). At the patient’s cervicothoracic junction, an interface of dark and light colors was visible. We call this phenomenon the “neck-thorax junction sign”, when other parts of the body’s skin are normal. After 31 days of continuous systemic administration of polymyxin B, the patient recovered and survived. Head and neck skin pigmentation partially recovered 3 months after drug discontinuation. Unfortunately, dermoscopy and dermatopathology were not performed in this patient.
The incidence of skin hyperpigmentation after IV polymyxin B treatment is approximately 8%, and it is assumed that this is related to an inflammatory process and melanocyte activation.47 Melanocytes are more abundant in the head and neck.48 High adnexal and nerve density is also characteristic of the head skin. The number of sebaceous glands on the head is 400–900/cm2.49,50 Polymyxin B usage may activate them, causing a non-uniform pigmentation distribution.
Limitations
This study had several limitations. (1) This was a single-center retrospective study, and prospective or retrospective multicenter randomized investigations are needed. (2) Our study had a small sample size (n = 41). (3) All carbapenem-resistant MDR/XDR Gram-negative bacilli isolated in this study were from critically ill patients who were treated with IVT polymyxin B + IV sensitive antibiotics. A strictly IV drug group was not used as the control group. (4) Polymyxin B was expensive and not easily obtainable in China until 2018; therefore, instead of using the IVT+IV polymyxin B regimen recommended by the guidelines, a systemic IV regimen based on susceptibility results was selected. An imbalance in a comparison study may have led to different results.
Conclusion
Intraventricular polymyxin B is an effective treatment for carbapenem-resistant multidrug-resistant Gram-negative bacilli, with a 78% cure rate and capacity to significantly reduce mortality if administered within 7 days after bacterial identification. Multiloculated hydrocephalus and the pupillary light reflex may be used as prognostic indicators of mortality.
Abbreviations
CNS, central nervous system; CSF, cerebrospinal fluid; DP, delay period; GCS, Glasgow Coma Scale; IDSA, Infectious Diseases Society of America; ITH, intrathecal; IV, intravenous; IVT, intraventricular; MDR/XDR, multidrug-resistant/extensively drug-resistant.
Ethical Statement
The study protocol was approved by Aviation General Hospital Medical Ethics Committee. (Protocol Number: KY2022-073-02). The study was conducted in accordance with the revised Helsinki Declaration in 2013. Written informed consent was obtained from patients or guardians of patients with coma, a parent or legal guardian of patients under 18 years of age. Written informed consent was obtained from the patient or guardian to have the case details and any accompanying images published.
Acknowledgments
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Mayhall CG, Archer NH, Lamb VA, et al. Ventriculostomy-related infections. A prospective epidemiologic study. N Engl J Med. 1984;310(9):553–559. doi:10.1056/NEJM198403013100903
2. Schade RP, Schinkel J, Visser LG, Van Dijk JMC, Voormolen JHC, Kuijper EJ. Bacterial meningitis caused by the use of ventricular or lumbar cerebrospinal fluid catheters. J Neurosurg. 2005;102(2):229–234. doi:10.3171/jns.2005.102.2.0229
3. Schade R, Schinkel J, Roelandse F, et al. Lack of value of routine analysis of cerebrospinal fluid for prediction and diagnosis of external drainage-related bacterial meningitis. J Neurosurg. 2006;104:101–108. doi:10.3171/JNS.2006.104.1.101
4. Korinek AM, Baugnon T, Golmard JL, van Effenterre R, Coriat P, Puybasset L. Risk factors for adult nosocomial meningitis after craniotomy: role of antibiotic prophylaxis. Neurosurgery. 2006;59(1):126–133;discussion 126–133. doi:10.1227/01.NEU.0000220477.47323.92
5. Kourbeti IS, Jacobs AV, Koslow M, Karabetsos D, Holzman RS. Risk factors associated with postcraniotomy meningitis. Neurosurgery. 2007;60(2):317–325;discussion 325–326. doi:10.1227/01.NEU.0000249266.26322.25
6. Metan G, Alp E, Aygen B, Sumerkan B. Acinetobacter baumannii meningitis in post-neurosurgical patients: clinical outcome and impact of carbapenem resistance. J Antimicrob Chemother. 2007;60(1):197–199. doi:10.1093/jac/dkm181
7. Moon C, Kwak YG, Kim ES, Kim ES, Lee CS, Lee C-S. Implications of postneurosurgical meningitis caused by carbapenem-resistant Acinetobacter baumannii. J Infect Chemother. 2013;19(5):916–919. doi:10.1007/s10156-013-0608-7
8. Rodríguez Guardado A, Blanco A, Asensi V, et al. Multidrug-resistant Acinetobacter meningitis in neurosurgical patients with intraventricular catheters: assessment of different treatments. J Antimicrob Chemother. 2008;61(4):908–913. doi:10.1093/jac/dkn018
9. Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46(8):1254–1263. doi:10.1086/529198
10. Murray CK, Hospenthal DR. Treatment of multidrug resistant Acinetobacter. Curr Opin Infect Dis. 2005;18(6):502–506. doi:10.1097/01.qco.0000185985.64759.41
11. Wen DY, Bottini AG, Hall WA, Haines SJ. Infections in neurologic surgery. The intraventricular use of antibiotics. Neurosurg Clin N Am. 1992;3(2):343–354. doi:10.1016/S1042-3680(18)30666-1
12. Mohapatra SS, Dwibedy SK, Padhy I. Polymyxins, the last-resort antibiotics: mode of action, resistance emergence, and potential solutions. J Biosci. 2021;46(3):85. doi:10.1007/s12038-021-00209-8
13. Ayoub Moubareck C et al. Polymyxins and bacterial membranes: a review of antibacterial activity and mechanisms of resistance. Membranes (Basel); 2020 10 8 181. doi:10.3390/membranes10080181.
14. Gales AC, Jones RN, Sader HS. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY Antimicrobial Surveillance Program (2006–09). J Antimicrob Chemother. 2011;66(9):2070–2074. doi:10.1093/jac/dkr239
15. Roberts KD, Azad MAK, Wang J, et al. Antimicrobial activity and toxicity of the major lipopeptide components of polymyxin B and colistin: last-line antibiotics against multidrug-resistant gram-negative bacteria. ACS Infect Dis. 2015;1(11):568–575. doi:10.1021/acsinfecdis.5b00085
16. López-Alvarez B, Martín-Láez R, Fariñas MC, Paternina-Vidal B, García-Palomo JD, Vázquez-Barquero A. Multidrug-resistant Acinetobacter baumannii ventriculitis: successful treatment with intraventricular colistin. Acta Neurochir. 2009;151(11):1465–1472. doi:10.1007/s00701-009-0382-6
17. Markantonis S, Markou N, Fousteri M, et al. Penetration of colistin into cerebrospinal fluid. Antimicrob Agents Chemother. 2009;53(11):4907–4910. doi:10.1128/AAC.00345-09
18. Ziaka M, Markantonis SL, Fousteri M. Combined intravenous and intraventricular administration of colistin methanesulfonate in critically ill patients with central nervous system infection. Antimicrob Agents Chemother. 2013;57(4):1938–1940. doi:10.1128/AAC.01461-12
19. Tunkel A, Hasbún R, Bhimraj A, et al. 2017 Infectious Diseases Society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017;64:701–706. doi:10.1093/cid/ciw861
20. Chusri S, Sakarunchai I, Kositpantawong N, et al. Outcomes of adjunctive therapy with intrathecal or intraventricular administration of colistin for post-neurosurgical meningitis and ventriculitis due to carbapenem-resistant Acinetobacter baumannii. Int J Antimicrob Agents. 2018;51(4):646–650. doi:10.1016/j.ijantimicag.2017.12.002
21. De Bonis P, Lofrese G, Scoppettuolo G, et al. Intraventricular versus intravenous colistin for the treatment of extensively drug resistant Acinetobacter baumannii meningitis. Eur J Neurol. 2016;23(1):68–75. doi:10.1111/ene.12789
22. Pan S, Huang X, Wang Y, et al. Efficacy of intravenous plus intrathecal/intracerebral ventricle injection of polymyxin B for post-neurosurgical intracranial infections due to MDR/XDR Acinetobacter baumannii: a retrospective cohort study. Antimicrob Resist Infect Control. 2018;7(1):8. doi:10.1186/s13756-018-0305-5
23. Hu Y, He W, Yao D, Dai H. Intrathecal or intraventricular antimicrobial therapy for post-neurosurgical intracranial infection due to multidrug-resistant and extensively drug-resistant Gram-negative bacteria: a systematic review and meta-analysis. Int J Antimicrob Agents. 2019;54(5):556–561. doi:10.1016/j.ijantimicag.2019.08.002
24. Babiker A, Clarke LG, Saul M, et al. Changing epidemiology and decreased mortality associated with carbapenem-resistant gram-negative bacteria, 2000–2017. Clin Infect Dis. 2021;73(11):e4521–e4530. doi:10.1093/cid/ciaa1464
25. Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16(1):18. doi:10.1186/s12941-017-0191-3
26. Lemos EV, de la Hoz FP, Einarson TR, et al. Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin Microbiol Infect. 2014;20(5):416–423. doi:10.1111/1469-0691.12363
27. Karaiskos I, Galani L, Baziaka F, Giamarellou H. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: a literature review. Int J Antimicrob Agents. 2013;41(6):499–508. doi:10.1016/j.ijantimicag.2013.02.006
28. Tsuji BT, Pogue JM, Zavascki AP, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39(1):10–39. doi:10.1002/phar.2209
29. Fernandez-Viladrich P, Corbella X, Corral L, Tubau F, Mateu A. Successful treatment of ventriculitis due to carbapenem-resistant Acinetobacter baumannii with intraventricular colistin sulfomethate sodium. Clin Infect Dis. 1999;28(4):916–917. doi:10.1086/517243
30. Tuon FF, Rocha JL, Arend LN, Wallbach K, Zanin HA, Pilonetto M. Treatment and outcome of nine cases of KPC-producing Klebsiella pneumoniae meningitis. J Infect. 2013;67(2):161–164. doi:10.1016/j.jinf.2013.04.003
31. Wang JH, Lin PC, Chou CH, et al. Intraventricular antimicrobial therapy in postneurosurgical Gram-negative bacillary meningitis or ventriculitis: a hospital-based retrospective study. J Microbiol Immunol Infect. 2014;47(3):204–210. doi:10.1016/j.jmii.2012.08.028
32. Shofty B, Neuberger A, Naffaa ME, et al. Intrathecal or intraventricular therapy for post-neurosurgical Gram-negative meningitis: matched cohort study. Clin Microbiol Infect. 2016;22(1):66–70. doi:10.1016/j.cmi.2015.09.023
33. Fotakopoulos G, Makris D, Chatzi M, Tsimitrea E, Zakynthinos E, Fountas K. Outcomes in meningitis/ventriculitis treated with intravenous or intraventricular plus intravenous colistin. Acta Neurochir. 2016;158(3):603–610;discussion 610. doi:10.1007/s00701-016-2702-y
34. Karvouniaris M, Brotis AG, Tsiamalou P, Fountas KN. The role of intraventricular antibiotics in the treatment of nosocomial ventriculitis/meningitis from gram-negative pathogens: a systematic review and meta-analysis. World Neurosurg. 2018;120:e637–e650. doi:10.1016/j.wneu.2018.08.138
35. Hoefnagel D, Dammers R, Laak-Poort MT, Avezaat C. Risk factors for infections related to external ventricular drainage. Acta Neurochirurgica. 2007. doi:10.1007/s00701-007-1458-9
36. Camacho E, Boszczowski Í, Basso M, et al. Infection rate and risk factors associated with infections related to external ventricular drain. Infection. 2010. doi:10.1007/s15010-010-0073-5
37. Xi-ling W Analysis of High Risk Factors of Intracranial Infection after Craniotomy in Neurosurgery Department. Journal of Beihua University. 2013.
38. Stoodley P, Jr EEB, Nistico L, et al. Direct demonstration of Staphylococcus biofilm in an external ventricular drain in a patient with a history of recurrent ventriculoperitoneal shunt failure. Pediatr Neurosurg. 2010;46:127–132. doi:10.1159/000319396
39. Zhang Y, Zhao R, Shi W, Zheng J, Li H, Li Z. Predictor of a permanent shunt after treatment of external ventricular draining in pediatric postinfective hydrocephalus—a retrospective cohort study. Childs Nervous Syst. 2021;37:1877–1882. doi:10.1007/s00381-021-05054-6
40. Sun C, Du H, Yin L, He M, Tian Y, Li H. Choice for the removal of bloody cerebrospinal fluid in postcoiling aneurysmal subarachnoid hemorrhage: external ventricular drainage or lumbar drainage? Turk Neurosurg. 2014;24(5):737–744. doi:10.5137/1019-5149.JTN.9837-13.2
41. Galea I. The blood-brain barrier in systemic infection and inflammation. Cell Mol Immunol. 2021;18(11):2489–2501. doi:10.1038/s41423-021-00757-x
42. Lebel MH, McCracken GH. Delayed cerebrospinal fluid sterilization and adverse outcome of bacterial meningitis in infants and children. Pediatrics. 1989;83(2):161–167. doi:10.1542/peds.83.2.161
43. Khawcharoenporn T, Apisarnthanarak A, Mundy LM. Intrathecal colistin for drug-resistant Acinetobacter baumannii central nervous system infection: a case series and systematic review. Clin Microbiol Infect. 2010;16(7):888–894. doi:10.1111/j.1469-0691.2009.03019.x
44. Jamjoom AB, Mohammed AA, Al-Boukai A, Jamjoom ZA, Rahman N, Jamjoom HT. Multiloculated hydrocephalus related to cerebrospinal fluid shunt infection. Acta neurochir. 1996;138(6):714–719. doi:10.1007/BF01411477
45. Schultz P, Leeds NE. Intraventricular septations complicating neonatal meningitis. J Neurosurg. 1973;38(5):620–626. doi:10.3171/jns.1973.38.5.0620
46. Pandey S, Yao PW, Qian Z, Ji T, Wang K, Gao L. Clinical characteristics of hydrocephalus following the treatment of pyogenic ventriculitis caused by multi/extensive drug-resistant gram-negative bacilli, Acinetobacter baumannii, and Klebsiella pneumoniae. Front Surg. 2022;9:854627. doi:10.3389/fsurg.2022.854627
47. Mattos KPH, Cintra ML, Gouvêa IR, Ferreira LÁ, Velho PE, Moriel P. Skin hyperpigmentation following intravenous polymyxin B treatment associated with melanocyte activation and inflammatory process. J Clin Pharm Ther. 2017;42(5):573–578. doi:10.1111/jcpt.12543
48. Tuthill RJ. Histologic diagnosis of inflammatory diseases of the skin. an algorithmic method based on pattern analysis, 3rd edition. Am J Surg Pathol. 2005;29(9):1264–1265. doi:10.1097/01.pas.0000174010.91697.1d
49. Smith KR, Thiboutot DM. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. 2008;49(2):271–281. doi:10.1194/jlr.R700015-JLR200
50. Nolano M, Provitera V, Caporaso G, et al. Cutaneous innervation of the human face as assessed by skin biopsy. J Anat. 2013;222(2):161–169. doi:10.1111/joa.12001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.