Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Treatment of Androgenetic Alopecia: Current Guidance and Unmet Needs
Authors Kaiser M, Abdin R, Gaumond SI, Issa NT, Jimenez JJ
Received 7 March 2023
Accepted for publication 23 May 2023
Published 31 May 2023 Volume 2023:16 Pages 1387—1406
DOI https://doi.org/10.2147/CCID.S385861
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Michael Kaiser,1 Rama Abdin,2 Simonetta I Gaumond,1 Naiem T Issa,3,4 Joaquin J Jimenez1
1Dr. Phillip Frost Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine, Miami, FL, USA; 2Charles E. Schmidt College of Medicine, Florida Atlantic University, Boca Raton, FL, USA; 3Forefront Dermatology, Vienna, VA, USA; 4Issa Research and Consulting, LLC, Springfield, VA, USA
Correspondence: Joaquin J Jimenez, Phillip Frost Department of Dermatology and Cutaneous Surgery, University of Miami Miller School of Medicine, Miami, FL, USA, Tel +1 305-243-6586, Email [email protected]
Abstract: Androgenetic alopecia (AGA) is the most common cause of hair loss in men and women. Traditionally, topical minoxidil and oral finasteride have been the standard of care yielding mixed results. New treatments such as Low-Level Laser Therapy (LLLT), microneedling, platelet-rich plasma (PRP), and others have been extensively studied in the literature, and the purpose of this review is to provide a comprehensive discussion of the latest treatment methods and their efficacy in treating AGA. Novel therapies such as oral minoxidil, topical finasteride, topical spironolactone, botulinum toxin, and stem cell therapy offer interesting alternatives to standard of care therapies for patients. In this review, we present data from recent studies on the clinical efficacy of these treatments. Furthermore, as new treatments have emerged, clinicians have tested combination therapies to assess whether there may be a synergistic relationship between multiple modalities. While there has been a great increase in the treatments available for AGA, the quality of evidence varies greatly and there is still a great need for randomized double blinded clinical trials to adequately assess the clinical efficacy of some treatments. While PRP and LLLT have demonstrated encouraging results, standardized treatment protocols are needed to adequately inform clinicians on how to use such therapies. Given the abundance of new therapeutic options, clinicians and patients must weigh the benefits and risks of each treatment option for AGA.
Keywords: androgenetic alopecia, minoxidil, finasteride, platelet rich plasma, microneedling
Introduction
Male Pattern Hair Loss (MPHL) and Female Pattern Hair Loss (FPHL), also known as androgenetic alopecia (AGA), is the most common cause of hair loss.1 An estimated 30% of Caucasian men will suffer from AGA by the age of 30, and approximately 80% of patients aged 70 and older are affected by AGA as prevalence increases with age.1 Roughly 50% of females are affected by AGA.2 The principal cause of AGA is thought to involve changes in androgen metabolism with increased age leading to progressive shortening of the anagen phase of hair growth and a decline in hair follicle size.3 Additionally, there is an increased telogen period leading to involution of the hair follicle.4 The increased activation of androgen receptors in AGA progressively shortens hair follicles until they can no longer penetrate through the epidermis.5 Patients with AGA have been found to have higher levels of dihydrotestosterone (DHT), and regions of scalp affected by AGA have increased androgen receptors.5 AGA involves the loss of terminal hairs and replacement with small vellus hairs usually in the temporal, vertex, and mid-frontal areas of the scalp.6 The typical pattern of hair loss in AGA begins with a bitemporal recession of the hairline followed by vertex hair thinning.7 In women, AGA typically affects the crown and bitemporal scalp areas without affecting the frontal hairline.4 Minoxidil and finasteride remain the only medical treatments approved by the Food and Drug administration (FDA), and the Lasermax Haircomb is the only device approved by the FDA for treatment by the AGA.8 Given the inconsistent results and dissatisfactory adverse effects associated with each, alternative treatments have been evaluated for the treatment of AGA. In this review, we will examine and present data on the clinical efficacy of the multitude of treatments available for AGA and give recommendations on quality of evidence supporting these treatment claims.
Topical Minoxidil Therapy
Mechanism of Action
The initial research and development of topical minoxidil for the treatment of AGA began in 1960.9 Minoxidil was originally developed as an oral treatment for hypertension.10 The main side effect was found to be hypertrichosis, which gave rise to the first topical solutions aimed at combatting hair loss.10 Minoxidil is a prodrug that must be converted to its bioactive metabolite, minoxidil sulfate, via SULT1A sulfotransferase residing within the hair follicle.11,12 It is hypothesized that topical minoxidil works through several mechanisms including arteriolar vasodilation, anti-inflammatory effects, and Wingless and Int-1 gene (Wnt)/β-catenin signaling. Minoxidil acts on ATP gated smooth muscle K+ channels leading to vasodilation. This is thought to increase blood supply and nutrients to hair follicles, facilitating increased growth.13 Minoxidil has also been shown to reduce pro-inflammatory cytokines such as interleukin-2 (IL-2) and prostacyclins, which may play a role in the pathogenesis of AGA.13–15 Lastly, minoxidil is hypothesized to upregulate the Wnt/β-catenin signaling pathway by increasing vascular endothelial growth factor (VEGF) levels.16 Minoxidil formulations approved for men include 2% and 5% solutions as well as 5% foam, whereas a 2% solution and 5% foam are approved for women.17
Efficacy
The efficacy of topical minoxidil for AGA varies widely (Table 1). For men, the 5% topical solution has been shown to be more effective than the 2% solution when applied twice daily.18 In females, studies show that 5% solution is superior or equivalent to the 2% solution.19,20 Goren et al reported that after 16 weeks of continuous topical application of minoxidil, less than 40% of patients responded.21 The earliest effects of minoxidil on hair growth begin at 6–8 weeks and reached maximal effect by 12–16 weeks without significant further improvement with additional time.22 Olsen et al evaluated men using daily topical minoxidil for 5 years and found that hair growth peaked at 1 year followed by a slow, steady decline afterward.23 It has been estimated that 60% of male patients may not respond to topical minoxidil therapy due to decreased baseline levels of the sulfotransferase required to activate minoxidil to its active metabolite.24 Furthermore, analyzing sulfotransferase activity in plucked hair follicles was found to be an accurate predictor of female response to topical minoxidil treatment.25 In summary, daily application of topical 5% minoxidil monotherapy appears to be more effective than 2% minoxidil; its effectiveness is dependent on the concentration of sulfotransferase available within the hair follicle, and results take at least 6–8 weeks to appear.
 |
Table 1 Summary of Major Studies for AGA Treatments |
Adverse Effects
Although minoxidil has beneficial results for hair growth, long-term compliance is often challenging in patients due to adverse effects and the need for daily application. While topical minoxidil is considered safe, patients often experience an irritant contact dermatitis causing pruritis and scaling.12 It may also cause a transient increase in hair shedding at the beginning of the treatment, which can temporarily worsen physical appearance and dissuade patients from continuing the treatment regimen.10,20 This shedding period arises from alterations to the hair cycle such that the hair follicle enters the anagen phase prematurely, which requires it to pass through a shorter telogen phase and thus a period of hair shedding occurs before clinical improvement can be observed.33,34 The most common adverse effect of topical minoxidil is hypertrichosis.20 The second most common adverse effect of topical minoxidil is headache, which affected 2–4% of the 153 patients in a large scale study.19
Oral Finasteride
Mechanism of Action
Oral finasteride (5mg/day) was approved by the FDA in 1992 for the treatment of benign prostatic hyperplasia.35 Due to its anti-androgen properties, finasteride was examined as a potential treatment for AGA in men. In 1997 it was approved for the treatment of male AGA at a dosage of 1mg/day.36 Finasteride is a selective 5α-reductase type II inhibitor that is also FDA approved for the treatment of AGA in men. In the scalp, testosterone is converted to DHT by the enzyme 5α-reductase.37 The pathogenesis of AGA is suggested to be due to DHT mediated miniaturization of the hair follicles, ultimately rendering them unable to fully penetrate the epidermis.38 This subsequently causes progressive hair loss, especially in men. This mechanism is corroborated by findings suggesting that men who are deficient in the type II 5α-reductase are not affected by AGA.38
Efficacy
Finasteride at a dosage of 1 mg/day has been shown to reduce the progression of AGA.38 A systematic review by Mella et al found oral finasteride to significantly improve hair regrowth and mean hair count compared to placebo across 10 studies.39 A 10-year follow-up study by Rossi et al further found that daily finasteride use in men with AGA beyond 5 years resulted in continued improved hair count in 21% of patients. Best results in hair regrowth were in the vertex region of the scalp, while the frontal region showed minimal clinical improvement.37 A Japanese study examining 801 men with AGA treated with finasteride 1 mg/day found an improvement in modified global photographic assessment (MGPA) over a 5-year follow-up period.40 However, patients with worse MGPA scores at baseline exhibited much less improvement. The authors also noted that the degree of improvement peaked after 1 year of sustained treatment and there was minimal improvement after 4 years of use. A retrospective study examining 166 Korean men with AGA being treated with 1 mg of finasteride daily found that there was sustained improvement of hair growth at 5 years. However, patients with frontal and vertex type AGA, approximately 10% and 16% of patients, respectively, experienced relapse of AGA after peak response.41
Off-label use of finasteride has demonstrated clinical efficacy in female patients with AGA.42 The dosage of finasteride in females varied widely, ranging from 1 to 5 mg daily and postmenopausal women were the predominant population examined. Overall, 2.5 to 5 mg daily improved hair loss in females with AGA, while lower doses of 1 mg daily provided very little improvement.43 In summary, finasteride is effective for AGA given that it directly targets the main pathogenic mechanism of DHT formation via inhibition of 5α-reductase type II. While some studies show that peak effect of hair regrowth is achieved after 1 year, many patients will continue to have further regrowth. Notably, maintenance therapy is also required to sustain the achieved regrowth effect.
Adverse Effects
The adverse effects of finasteride are well documented in men and women and include sexual dysfunction, birth defects, and depression.44 While current guidelines recommend taking finasteride for an indefinite amount of time, there are limited data on the safety of long-term use. Sexual side effects remain the most common adverse effects of finasteride use, and the rate of occurrence ranges between 1% to nearly 40%.44 Adverse effects of finasteride include gynecomastia, muscle atrophy, decreased libido, and impotence.45 The adverse effects of finasteride remain a major barrier to long-term compliance. Clinicians should warn patients of the risks and together weigh them against the benefits of potentially improving hair loss. Long-term studies have analyzed the relationship between finasteride and prostate cancer. The Prostate Cancer Prevention Trial showed that finasteride may have a protective effect on the development of prostate cancer but could also increase the risk of developing high-grade prostate cancer.46 Due to conflicting data in the association between prostate cancer and finasteride, Wang et al performed a comprehensive review and concluded that overall finasteride does reduce the risk of prostate cancer. However, they also found that there was an increase in the degree of malignancy of prostate cancer in patients using finasteride.46 Patients should be informed of the risk versus benefit to using finasteride and should be monitored for development of prostate cancer.
Off-Label Drugs and Drug Formulations Used in AGA
Oral Minoxidil
Oral minoxidil was originally used to treat resistant cases of hypertension in the 1970s.47 Minoxidil is a potent vasodilator that was used to reduce systolic and diastolic pressure in select patients.47 As previously discussed, topical minoxidil is one of the main treatments for AGA, but more recently systemic therapy with low dose oral minoxidil has been proposed as an effective alternative therapy. Long-term compliance with topical minoxidil is difficult to achieve due to the twice daily application requirement. It is thought that low dose oral minoxidil is far easier to use given the requirement to take one pill per day. The dosage of oral minoxidil for the treatment of AGA is relatively low, ranging from 0.25 mg to 5 mg daily, much lower than the dosage originally used to treat hypertension (10 to 40 mg daily).10,48 Oral minoxidil is converted to its active metabolite in the liver as opposed to the scalp. Given that patients have varying sulfotransferase expression in the scalp, metabolism by the liver increases bioavailability and can lead to greater improvement in patients who have failed topical minoxidil treatment.49
Data on Efficacy
The advantages of oral minoxidil over topical minoxidil include its convenience, cosmesis, cost, and compliance. It is more convenient to take oral minoxidil than to apply it daily; it is more cosmetically effective as it does not alter gray hair color or generate product residue as topical minoxidil does; and due to its convenience, there is good reported compliance with therapy.50
Ramos et al compared the use of 1 mg oral minoxidil to 5% topical minoxidil in 52 women.51 They found a non-significant difference in total hair density increase between the two treatment groups. This study proposes that oral minoxidil should be considered as an alternative option for patients with poor compliance or adverse effects with topical minoxidil. Vastarella et al assessed the use of oral minoxidil in 12 women who had previously failed conventional treatments.52 Patients were started with oral minoxidil 0.50 mg daily and then increased to 1.5–2 mg daily at 3 months. Overall, there was a statistically significant increase in hair density per unit area and total number of hairs in the frontal scalp area at 24 weeks.
Low dose oral minoxidil has also been shown to be efficacious in the treatment of AGA in men.48 Jimenez-Cauhe et al suggests that oral minoxidil 5 mg/day yields a faster clinical response than other AGA therapies, including topical minoxidil and 5-alpha-reductase inhibitors.53 Males who underwent treatment with 5 mg daily of oral minoxidil therapy for 6 months demonstrated a significant increase in total hair counts at 12 weeks and 24 weeks.34 The results of this study suggest that low dose oral minoxidil is an effective treatment of androgenetic alopecia. However, given the lack of randomization and control within this study, double-blind, placebo-controlled studies are required to better understand the efficacy of oral minoxidil.
Randolph et al reviewed existing studies of oral minoxidil for the treatment of AGA and found that 0.25 mg to 1.25 mg daily was effective for treating AGA in men and women.10 Jimenez-cauhe et al evaluated oral minoxidil 5 mg daily in a subgroup of 16 male patients for 6 months and found that 90% exhibited clinical improvement, with ~30% demonstrating great improvement.48 Adverse effects were more common in the 5 mg dosage group, with ~25% of patients reporting hypertrichosis and 5% reporting lower extremity edema. Overall there appears to be a dose-dependent increase in efficacy and adverse effects, but otherwise oral minoxidil is well tolerated.10
Adverse Effects
Sanabria et al summarized the adverse effects of low-dose oral minoxidil in the treatment of androgenetic alopecia.54 Hypertrichosis was the most common adverse effect occurring in 55.4% of patients. In men, hypertrichosis was associated with younger age and was found to be dose dependent. Hair shedding at treatment onset was reported in 32% of patients and was also associated with younger age in men. Pedal edema was reported in 6% of patients. Of note, 35 patients (8%) discontinued treatment due to lack of efficacy, hypertrichosis, fear of interaction with other drugs, intention to get pregnant, and hair shedding.
Topical Finasteride
Mechanism of Action
The adverse effects of systemic therapy with oral finasteride combined with the uncertain safety of long-term therapy gave rise to the potential for a topical finasteride formulation. Given that finasteride blocks the conversion of testosterone to DHT, systemic use may lead to numerous adverse effects, as previously noted. Topical finasteride locally inhibits DHT, reduces DHT production in the hair follicle, and has fewer systemic effects. Furthermore, finasteride is a teratogen but could be a safer alternative treatment in a topical formulation.55 Topical administration would locally inhibit DHT and reduce DHT production in the hair follicle while minimizing systemic effects.
Data on Efficacy
Casserini et al found that topical 0.25% finasteride solution applied twice daily lowered scalp DHT levels similarly to oral finasteride therapy.56 Piraccini et al performed a Phase III randomized controlled clinical trial evaluating the efficacy of daily topical finasteride 0.25% spray in 323 patients with AGA.57 After 24 weeks, there was a statistically significant increase in target area hair count compared to placebo. No significant difference in mean hair counts was found between the topical and oral groups. Notably, the plasma finasteride concentration was over 100-fold less than the oral variant. Another randomized double blinded clinical trial by Hajheydari et al compared topical 1% finasteride gel to 1 mg oral finasteride.58,59 Both treatment groups had significant increase in terminal hair count compared to baseline, but there was no significant difference between the two. Overall, the findings of these studies suggest that topical finasteride promotes hair growth comparable to oral finasteride while having minimal effects on plasma DHT levels, thereby diminishing adverse effects.
Adverse Effects
The most common adverse effects due to topical finasteride are skin irritation and pruritis at the application site with no patients experiencing sexual dysfunction.57 Current evidence obtained from placebo-controlled and head-to-head studies comparing topical to oral finasteride suggest that twice daily application of topical finasteride is equivalent in efficacy, with favorable pharmacokinetics minimizing systemic absorption. However, further studies are required to assess the optimal formulation and dosing.
Combination Topical Minoxidil and Topical Finasteride
With the emergence of topical finasteride as a viable treatment option for AGA, clinicians have attempted to combine topical minoxidil with topical finasteride to boost the efficacy of both treatments. Given their orthogonal mechanisms of action, as previously discussed, investigation into the potential synergy of both topical treatments has become of great interest. Suchonwanit et al examined topical 0.25% finasteride mixed with topical 3% minoxidil compared to topical 3% minoxidil monotherapy in 40 male patients with AGA.60 Both treatment groups achieved significant increase in hair density and hair diameter compared to baseline. Combined therapy had a greater increase in hair density than monotherapy at both 16 and 24 weeks. Additionally, hair diameter increase was greatest in the combination treatment group at 24 weeks. Importantly, the authors found that plasma DHT decreased only by 5% and no patients had significant adverse effects from either therapy. Topical 5% minoxidil mixed with topical 0.1% finasteride was also shown to maintain hair density and prevent further hair loss.61,62 Several studies reported no adverse effects from topical finasteride and minoxidil treatment.60,61 Patients were apprehensive about using oral finasteride and reported more side effects compared to topical finasteride.
Oral Dutasteride
Mechanism of Action
As previously discussed, the conversion of testosterone to the more potent DHT by 5α-reductase is an important mechanism of AGA. Humans express two known isozymes: type I and type II 5α-reductase. Type I is present in the dermal papillae and follicle epithelial cells, while type II is predominantly expressed in male genitalia as well as the anterior and parietal scalp dermal papilla.63 Oral dutasteride inhibits both type I and type II 5α-reductase and was first approved as a treatment for benign prostatic hyperplasia (BPH).63
Data on Efficacy
Oral dutasteride was compared at three dosages (0.02 mg/d, 0.1 mg/d, or 0.5 mg/d) to finasteride (1 mg/d) or placebo in a randomized double blinded control trial in 917 males with AGA.64 Dutasteride resulted in a dose-dependent increase in hair count (primary endpoint) and hair width after 24 weeks with a minimum dose of 0.1 mg/d required for efficacy. Dutasteride 0.5 mg/d was also found to be superior to finasteride at 12 and 24 weeks. Jung et al investigated dutasteride 0.5 mg/d in 31 male patients who failed oral finasteride.65 Approximately 80% of patients demonstrated clinical improvement after 6 months of dutasteride therapy. Hair density increased ~10% with statistical significance, while hair thickness increased ~20%. Thus, dutasteride may be an effective therapeutic option in patients who fail oral finasteride.
Adverse Effects
Oral dutasteride has been associated with various sexual dysfunction side effects including decreased libido, impotence, ejaculatory dysfunction, and gynecomastia. One study found that 17% of patients experienced alterations in libido in response to oral finasteride therapy.65 Tsunemi et al assessed the long-term safety of oral dutasteride in 120 patients taking 0.5mg daily for 52 weeks.63 The incidence of any adverse effect from dutasteride treatment was 53%. The most common drug-related adverse effect was erectile dysfunction seen in roughly 10% of patients. As such, the side effect profile associated with dutasteride poses a major obstacle.
Low Level Laser Therapy Treatment
Mechanism of Action
Low-level laser therapy (LLLT) represents a relatively new and efficacious treatment for AGA. LLLT has been used in dermatology to reduce inflammation and promote wound healing.66 LLLT has also been used in dermatology to improve wrinkles, scarring from acne, improve blood flow, and improve hypertrophic scars and burns.67 LLLT was first shown to potentiate hair growth in mice in the late 1960s. Mice that had been shaved regrew hair after receiving treatment with LLLT.68 Furthermore, clinicians using laser for permanent hair removal noticed that in peripheral areas where laser therapy had been applied, there was a paradoxical increase in hair growth.68 Further investigation demonstrated efficacy in humans. Due to its cost-effectiveness and noninvasive nature, LLLT is becoming a popular first-line treatment for AGA. LLLT uses light with wavelengths between 600 and 1100 nm to stimulate hair regrowth. While this mechanism is not completely understood, LLLT is believed to foster hair growth by promoting anagen-phase reentry of telogen hair follicles, increasing the duration of anagen phase, and preventing premature conversion of anagen hairs to catagen-phase hairs (similar to minoxidil).69–72 In 2018, the FDA approved LLLT as a therapy for AGA.73 Currently, the only FDA approved LLLT device for the treatment of AGA is the Lasermax Haircomb.8
Data on Efficacy
LLLT has largely demonstrated positive effects in AGA (Figure 1). A retrospective observational study examining LLLT 655 nm for 3 sessions weekly (8 to 15 min per session) in 32 patients with AGA found 28 patients to have moderate to significant improvement. Improvement was recognized as early as 3 months and lasting up to 24 months.26 A systematic review by Afifi et al found that nine out of 11 studies had statistically significant improvements in hair count and hair density in patients with AGA undergoing treatment with LLLT. In 2 out of 4 studies, improvements in hair strength and hair thickness were observed.74 A more recent meta-analysis by Liu et al found that in 11 randomized double blinded controlled trials patients treated with LLLT demonstrated a statistically significant improvement in hair density compared to placebo device.73 Additionally, increased hair growth was observed in males and females as well as in both short- and long-term follow-up. Another notable finding was that low treatment frequency was more effective than high treatment frequency LLLT therapy. One of the largest studies to date by Qiu et al examined LLLT in 1383 patients with AGA. LLLT was delivered at a wavelength of 650nm, and treatment was received for 20 min every other day. LLLT was found to be effective in 52.0% of patients with mild AGA and 58.0% with moderate-to-severe AGA.27 Additionally, it was significantly more effective for 28.0% of patients with mild AGA and 20.0% with moderate-to-severe AGA.
 |
Figure 1 Efficacy of Low-level laser therapy for the treatment of androgenetic alopecia. Reproduced from Suchonwanit P, Chalermroj N, Khunkhet S. Low-level laser therapy for the treatment of androgenetic alopecia in Thai men and women: a 24-week, randomized, double-blind, sham device-controlled trial. Lasers Med Sci. 2019;34(6):1107–1114. Creative Commons Attribution 4.0 International License. (https://creativecommons.org/licenses/by/4.0/).75 |
Adverse Effects
Adverse effects due to LLLT were minimal. Nonetheless, adverse effects included temporary hair shedding, pruritis, tenderness, and acne.70,75 Overall, the minimal adverse effects and low cost of treatment make LLLT a favorable adjunct therapy to minoxidil and finasteride or an alternative option for patients who cannot tolerate their side effects.
Combination Topical Minoxidil with LLLT
Esmat et al found that patients receiving LLLT with topical 5% minoxidil had the highest patient satisfaction compared to monotherapy, showed a statistically significant increase in the number of follicles at 4 months and the greatest overall improvement at 4 months.76 Importantly, this study showed the potential for LLLT treatment to confer an early therapeutic advantage, while patients waited for the delayed efficacy of minoxidil. A systematic review of the combination of LLLT and minoxidil therapy was performed by Kaiser et al and found that there were mixed results in the literature concerning the efficacy of combined therapy.69 Of the five available studies analyzed in their review, three studies found that combination therapy was superior to monotherapy, while two found that there was no statistically significant difference between therapies. Studies were limited by small sample sizes and lack of long-term follow-up. A meta-analysis by Zhou et al attempted to compare minoxidil monotherapy to minoxidil and LLLT combination therapy.77 The authors noted that there was considerable heterogeneity in the studies, but using their parameters, they found that combination of LLLT and minoxidil demonstrated superior efficacy to monotherapy. While combination therapy may confer a therapeutic advantage, further evaluation with larger sample sizes and a more uniform LLLT treatment regimen is necessary. There was also no increase in adverse effects with combination therapy.
Platelet Rich Plasma Treatment
Mechanism of Action
Platelet Rich Plasma (PRP) is a technique that has been employed in a variety of medical fields including orthopedic surgery, sports medicine, and cosmetic procedures. The regenerative properties of PRP have been used extensively to improve tendinopathy, tears in tendons, and disorders of bone structure.78 PRP has become an increasingly popular therapeutic modality in dermatology for skin rejuvenation, acne scarring, and vitiligo.79 PRP is the fraction of plasma and platelets isolated from autologous venous blood of patients. PRP contains chemokines, growth factors, cell signaling molecules, and cytokines with important implications in the control and regulation of many cellular processes. Among the many molecules contained within PRP, the most important components for treating AGA are alpha granules produced and stored in platelets. These alpha granules contain platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), transforming growth factor beta (TGFβ), and insulin-like growth factors (IGF).80,81 These factors are especially important in the induction of stem cell differentiation as well as promoting angiogenesis. The main role of PRP in treating AGA is thought to be from the large release of PDGF, which plays an important role in reducing follicular apoptosis and promoting follicular cell growth.29 DHT plays a prominent role in the miniaturization of hair follicles while also reducing local IGF-1 production. PRP contains large amounts of IGF-1 and can counteract the inhibitory effect of DHT on the hair follicle.82
Data on Efficacy
Recent studies have shown that PRP can be a viable treatment for AGA. Gkini et al examined PRP treatment in 20 patients with AGA.28 PRP was prepared using the single spin method with activation. Hair regrowth was statistically significant compared to baseline at both 6 months and 1 year. Hair growth peaked at 3 months and was sustained for the entire one-year follow-up period. Singhal et al investigated PRP therapy for 10 patients with AGA.29 PRP was prepared using the two-spin method, and an activator was used prior to injection. All 10 patients reported satisfactory hair regrowth at 3 months and the average hair removed by hair pull test decreased by 65%. A meta-analysis by Gupta et al evaluated studies using PRP as a treatment for AGA.83 Nearly every study demonstrated a statistically significant increase in hair growth and hair density in the PRP treatment group compared to the placebo. The authors recommended that PRP therapy should be conducted at 1-month intervals for 3 months followed by maintenance therapy. Another meta-analysis performed by Giordano et al examined six randomized controlled trials using PRP injection for the treatment of AGA. Similarly, the authors found that PRP injection increased hair number and cross-sectional hair thickness compared to control groups.84 While the initial data seems promising for the therapeutic efficacy of PRP, both previously mentioned meta-analyses noted that the existing studies had small sample sizes and inadequate follow-up times to adequately measure the treatment response in patients.
Verma et al compared PRP monotherapy to minoxidil therapy.30 PRP was prepared using the double spin method and activated with calcium gluconate. Injections of 0.1–0.2 mL of PRP were administered at monthly intervals for 4 months. In the minoxidil monotherapy group, 1 mL of minoxidil was applied topically twice daily for 6 months. The PRP therapy group was found to have improved hair pull test and improved patient satisfaction compared to the minoxidil group. The PRP therapy group was also found to have a greater improvement in global hair photography compared to minoxidil. Although there appear to be promising clinical results from the use of PRP in patients with AGA, lack of standardization of PRP protocols remains a barrier in adequately characterizing the therapeutic response in patients. In 2021, the National Indian Association of Dermatologists, Venereologists, and Leprologists (IADVL) PRP Taskforce published their recommendations for the preparation of PRP for AGA. The authors recommended using a wide bore needle for drawing blood to prepare for PRP, using a double spin method, and recommended against using an activator of PRP prior to injection.85
Adverse Effects
PRP has emerged as a viable, minimally invasive alternative treatment for patients with AGA. The overall risk of adverse effects remains small, however there is a risk of infection. Additionally scalp sensitivity and mild scalp scaling are the most common adverse effects.30 Not all patients are candidates for PRP. PRP therapy should be avoided in patients with a history of malignancy, platelet disorders, anemia, bleeding disorders, pregnant woman, or immunocompromised patients.29
Microneedling
Mechanism of Action
The development of microneedling as a therapeutic technique can be dated back to the 1900s when it was used for scar treatment in plastic surgery. Since its advent, it has been implicated in collagen induction therapy, acne treatment, hyperpigmentation, and androgenetic alopecia.86–88 Hollow or solid microneedling devices are available and can be used for directly creating channels in the skin or delivering small molecules or nanoparticles for local or systemic therapy.89 Microneedling has also been discussed as a potential breakthrough topical or transdermal drug delivery system.90 The device consists of micron-sized needles ranging from 100 to 2000 μm fixed to a base plate.90 Its mechanism of action is based on controlled, repetitive, tissue microinjury, which creates transdermal microchannels within the stratum corneum. This is thought to promote growth factors, activate bulge stem cells, and increase expression of genes associated with hair growth.91 Microneedling is also thought to stimulate hair growth via upregulation of Wnt/ß-catenin signaling and induction of vascular endothelial growth factor.87
Many microneedling devices are present on the market with variations in needle length, drum size, and automation depending on the target of therapy and desired outcome.92,93 The wide variety in microneedling therapy parameters can make establishing a standard protocol for the treatment of AGA difficult.
Efficacy
A pilot study by Dhurat et al sought to evaluate the effectiveness of microneedling treatment in men with AGA.31 In this study, 44 men were treated with 5% topical minoxidil monotherapy and 50 men were treated with 5% topical minoxidil therapy in conjunction with weekly microneedling. The average change in hair count from baseline to week 12 was significantly greater for the combination therapy group compared to the minoxidil monotherapy. Microneedling was also investigated over 6 months with 3 sessions spaced 4 weeks apart in a 24-week open-label, non-randomized study of 29 females and 14 males with AGA.32 Participants were advised to continue with topical minoxidil therapy 24 hr after treatment. All patients reported partial or complete perceived reduction in hair loss with negative pull tests. On trichoscopy, males had a 25.16% and 17.45% increase in frontal and vertex hair density, respectively, and females experienced a 32.35% and 35.88% increase in frontal and vellus hair density, respectively, by the end of the study. The authors recommend microneedling for those who seek quick improvement as well as in cases of hair loss refractory to standard of care treatments.
Microneedling can serve as a generally tolerable and valuable augmentation tool for standard of care therapies, even in treatment refractory androgenetic alopecia. Side effects include pain during treatment, transient pinpoint bleeding, erythema, and even enlargement of lateral cervical lymph nodes.32,94 Other potential adverse effects include pruritis, seborrheic dermatitis, and headache.95 However, some studies reported no adverse events.31,96,97 Given the microtrauma created during therapy, there is a risk of infection as well. However, microneedling overall exhibits minimal side effects with low risk for serious adverse events.
Combination Treatment with PRP, Minoxidil, LLLT, and Microneedling
Given the different mechanisms of action of PRP, minoxidil, LLLT, and microneedling, clinicians have experimented with combination therapy. Together, these different modalities act on different pathways to treat AGA, potentially allowing for synergistic results when used together in patients with AGA.
Investigation of PRP with minoxidil has yielded encouraging results in existing studies. Alves et al compared 5% topical minoxidil solution twice daily with PRP injection in a split scalp study of 13 patients.98 Half of the scalp was treated with topical minoxidil and PRP combination and the other half of the scalp was treated only with topical minoxidil. Combination therapy resulted in a significant increase in hair density and mean hair count compared to minoxidil monotherapy. Although the sample size was small and follow-up time was limited to 6 months, the results were encouraging. Pakhomova et al performed a three-arm study examining PRP with 5% topical minoxidil compared to topical minoxidil monotherapy or PRP monotherapy in 69 male patients with AGA.99 Combination therapy outperformed PRP monotherapy as overall hair density increased nearly threefold compared to PRP monotherapy. Other studies found similar results with PRP and minoxidil combination therapy outperforming minoxidil or PRP monotherapy, suggesting that the combination of these two treatments could have synergistic benefits.100–102
There is growing evidence that combination therapy with PRP and microneedling can improve hair growth (Figure 2). Gentile et al compared LLLT with PRP and microneedling in 23 patients with AGA.103 Combined treatment demonstrated an increase in mean hair density at 58 weeks in all patients compared to baseline. Although there was no control group, this study demonstrated that combination therapy is both efficacious and safe. Shah et al examined PRP with microneedling and minoxidil in 50 patients with AGA.95 In this study, 25 patients received only topical 5% minoxidil twice daily and 25 patients received topical 5% minoxidil with monthly PRP injections and microneedling for 6 months. Both patient satisfaction and clinician graded improvements were highest in the combination therapy group. Yepuri et al trialed PRP and microneedling combination therapy every 4 weeks for up to 6 months in 60 patients who previously did not respond to minoxidil or finasteride monotherapy.104 Over 80% saw at least a 40% improvement in hair growth. Adequate treatment response was observed after four total sessions of PRP and microneedling, which can aid in future studies seeking to establish a standardized treatment regimen. A retrospective study by Jha et al performed a comprehensive evaluation of minoxidil, PRP, and microneedling treatment modalities.105 In this study, 93 patients were divided into three treatment groups composed of 31 patients treated with topical 5% minoxidil twice daily, 31 patients treated with PRP and topical 5% minoxidil, and 31 patients treated with topical minoxidil, PRP, and microneedling. PRP was administered in 3-week intervals for a total of 4 sessions. The authors found that triple combination therapy yielded the highest number of negative hair pull tests, the highest terminal to vellus hair ratio, and the greatest improvement in hair growth than the other treatment groups.
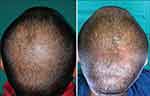 |
Figure 2 Before and after of a patient with AGA treated with PRP and microneedling. Reproduced from Aggarwal K, Gupta S, Jangra RS, Mahendra A, Yadav A, Sharma A. Dermoscopic assessment of microneedling alone versus microneedling with platelet-rich plasma in cases of male pattern alopecia: a split-head comparative study. Int J Trichol. 2020;12(4): 156, under the Creative Commons Attribution-Non Commercial-ShareAlike 4.0 License.106 |
Overall, there appears to be increasing evidence that combination therapies outperform monotherapy options. Given the favorable side effect profiles of LLLT, topical minoxidil, PRP, and microneedling, patients are not at an increased risk of adverse outcomes with combined therapy. Patients must also be candidates for PRP, as previously discussed, and should be informed of the potential costs associated with all modalities.
Oral Spironolactone
Mechanism of Action
Spironolactone is an aldosterone antagonist that is metabolized by the liver to its primary metabolite canrenone.107 It is used primarily as an anti-hypertensive agent acting as a potassium sparing diuretic. Spironolactone can bind and inhibit androgen receptors, which can treat hirsutism and acne in female patients, particularly those with polycystic ovarian syndrome (PCOS).108 Spironolactone has also been used as a treatment for acne vulgaris in women due to its capacity to reduce sebum production.109 Due to its anti-androgen properties, it is used off label to treat female pattern hair loss (FPHL) only. It is believed that oral spironolactone can halt the progression of FPHL through decreasing the levels of circulating androgens by inhibiting adrenal androgen production and inhibiting the effect of androgens in the hair follicle.110–112
Data on Efficacy
Burns et al found that patients with a Sinclair hair loss severity score greater than 2.5 saw one integer of improvement in hair loss regardless of concomitant therapy.111,113 James et al found in a systematic review that nearly 50% of women taking oral spironolactone as monotherapy for FPHL saw an improvement in hair loss.111 Furthermore, a dosage below 100 mg of spironolactone was ineffective in altering the disease course. Long term use greater than 12 months at a dosage between 100 mg and 200 mg was required to see clinical improvement in FPHL.111 A retrospective study by Famenini et al found that 74% of females receiving long-term spironolactone therapy for FPHL reported clinical improvement and a halt in progression of FPHL.114
Adverse Effects
The adverse effects commonly reported by patients on oral spironolactone were commonly hypotension, hyperkalemia, and occasionally urticaria.111 The recommended treatment regimen was in non-pregnant females with FPHL beginning at 50 mg daily with an optimal dosage between 100 mg and 200 mg daily for at least 6 months in order to see clinical improvement.111
Topical Spironolactone
Topically applied spironolactone offers the same local inhibition of androgens on the hair follicle without the systemic side effect profile of oral spironolactone. Hussein et al examined the treatment response in 26 patients (16 males and 10 females) with AGA to topical 1% spironolactone.112 All patients applied 1% topical spironolactone daily for 6 months. Nearly 77% of patients experienced clinical improvement measured by an increase in hair count in response to topical spironolactone treatment. A study compared topical 5% spironolactone in 16 patients to 16 patients treated with topical 0.1% finasteride for 6 months and found that topical spironolactone improved hair density and diameter in both males and females greater than topical finasteride.115 Combination therapy of topical 5% spironolactone and topical 5% minoxidil was evaluated by Ammal et al in 120 patients with AGA.116 This three-armed study was divided as such: 40 patients received 5% topical spironolactone treatment, 40 patients received 5% topical minoxidil, and 40 patients were treated with a combination of both therapies. Clinical improvement was evaluated by dermoscopy, which showed an increase in upright regrowing hairs in all groups. Combination therapy showed the greatest improvement in dermoscopic features, which suggests that minoxidil and spironolactone combination therapy could be beneficial to patients.
Botulinum Toxin
Mechanism of Action
Botulinum toxin is produced by the bacteria Clostridium botulinum and has been employed for a variety of uses including skin rejuvenation, facial palsy, and facial synkinesis.117 The predominant form, botulinum toxin A, inhibits the release of acetylcholine into the neuromuscular junction.118 The proposed mechanism of action for botulinum toxin in the treatment of AGA is a reduction in scalp muscle tone that results in vasodilation and enhancement of local microcirculation, thus increasing hair follicle oxygen perfusion and simultaneous removal of accumulated DHT. Another hypothesis is that the toxin could inhibit neuromodulators such as substance P or calcitonin gene-related peptide (CGRP) that may contribute to the pathogenesis of AGA.119 Zhang et al theorized that in the vertex area of the scalp there is lower blood perfusion due to muscle contraction and botulinum toxin may alleviate this lack of perfusion by relaxing the frontalis or occipitalis muscles.119,120 Local hypoxia is also thought to increase levels of DHT, potentiating the progressive hair loss associated with AGA.119,121 DHT has been shown to induce TGF-β1 in dermal papilla cells, which suppresses growth of epithelial cells within the follicle.122 Botulinum toxin has been shown to inhibit TGF-β1 secretion in fibroblasts and may have a similar effect in dermal papilla cells.122
Data on Efficacy
An open-label study examined the effect of intramuscular botulinum toxin injection in 40 patients with AGA.121 Patients were injected with 150 units of botulinum toxin in the scalp for 2 sessions, with 24 weeks in between each session, over the course of 48 weeks. Patients experienced a 75% treatment response rate, with an 18% increase in mean hair count being statistically significant compared to baseline. Zhou et al compared botulinum toxin injection to a combination of botulinum toxin and oral finasteride.118 Injections were done every 3 months for a total of 4 sessions. 100 units of botulinum toxin were injected into the scalp during sessions. The increase in hair growth compared to baseline was statistically significant, and there was no statistically significant difference between the botulinum toxin and combined groups. Wang et al performed a systematic review of botulinum toxin administered as a treatment for AGA and found mixed results.117 The authors found that there were considerable differences in the dosing of botulinum toxin ranging from 50 units to nearly 500 units across 11 published studies. A study by Cho et al investigated seven patients and found only one patient demonstrated hair growth, while one patient had worsening of hair loss and the other five patients had no response.117,123 Another systematic review by English et al reviewed five clinical studies and found that intramuscularly injected botulinum toxin response rates ranged from 70% to 79%.124 Intradermally injected botulinum toxin demonstrated only a 5% change in percent hair count. Most studies lacked control groups and had small sample sizes. Before botulinum toxin can be recommended as a treatment for AGA, more rigorous clinical trials including double blinded evaluation, control groups, and larger sample sizes are required.
Adverse Effects
The adverse effects associated with botulinum toxin were generally mild and included headache, injection site pain, and erythema.118 Kowing et al reported adverse effects of madarosis and facial alopecia following 100 unit injections of botulinum toxin every 3 months.125 Other studies have reported potentially worsening hair-line regression.117,126 Overall, botulinum toxin appears to be a safe treatment for AGA but currently lacks the evidence required to become a standard recommended treatment.
Stem Cell Therapy
The regenerative potential of stem cell therapy is thought to be useful in the treatment of AGA. Stem cells are believed to modulate AGA by reversing its effects and forming new mature hair follicles.127 Hair follicle stem cells located in the hair bulge interact with stem cells in the dermal papilla. Activation of Wnt signaling pathway by hair follicle stem cells can modulate hair follicle growth and differentiation.128 It is believed that androgens downregulate important signaling factors from cells in the dermal papilla responsible for normal hair follicle stem cell differentiation.129 Elmaadawi et al found that intradermal injection of autologous stem cells in 20 patients with AGA demonstrated significant improvement in hair growth, which was visualized by dermoscopy and immunostaining.130 Other studies have shown that topical application of adipocyte-derived stem cells can improve hair growth up to 20% in as little as 8 weeks.131 Although recent studies have not shown any toxicities or adverse effects associated with stem cell application, the long-term safety has not been studied. The treatment of AGA with stem cell therapy remains an experimental treatment. Not only is it costly but intradermal application of stem cells is also invasive, and its long-term safety is still unknown. Additionally, the cost of transporting live stem cell culture is costly and can rarely cause graft-versus-host disease (GVHD).
Another treatment using autologous stem cells for AGA has been developed using autonomous cellular micrografts (ACM). In this method, autologous stem cells are obtained from skin biopsies of the patient’s scalp and then processed and reinjected as a solution into the area of hair loss on the patient’s scalp.132 This novel technique has produced some promising data. In a 6 month follow-up study of 140 patients treated with ACM, hair density, hair thickness, and the number of follicular units increased in every region injected.132 While this therapy is early in development, it could provide substantial benefit to patients.
Supplements and Dietary Options
The use of nutritional supplements in the treatment of AGA has also been under investigation, particularly those that are hypothesized to inhibit 5-alpha reductase, stimulate IGF-1 production, decrease oxidative stress, and provide valuable nutrients or blood flow to hair follicles.133 Saw palmetto, pumpkin seed oil, and Forti5 combination supplement are all thought to inhibit 5-alpha reductase; and studies have shown that these supplements may be used to stabilize hair loss or even stimulate hair growth in men with AGA. Antioxidants such as vitamin E and vitamin C have also shown efficacy in treating female AGA by increasing hair density and thickness, and although side effects are limited, in large doses these effects can be paradoxical. Protein-based supplements and probiotics, such as kimchi and cheonggukjang, also appear to be clinically effective in treating AGA by providing essential nutrients and increasing blood flow to hair follicles. Despite the favorable side effect profile of most of these supplements, it is important to note that these products may not undergo FDA premarket approval, and current studies investigating their efficacy are generally limited by small sample size, lack of control group, and/or lack of head-to-head comparison with standard therapies.
Drugs in the Pipeline
Clascoterone is a unique therapeutic option currently being evaluated for treatment of AGA. It is a novel androgen receptor antagonist that competes with DHT binding sites on cytoplasmic androgen receptors.134 Clascoterone is FDA approved to treat acne vulgaris and more recently has been explored as a topical treatment for AGA.135 It is currently undergoing Phase II clinical trials for the treatment of AGA.136
Discussion
Androgenetic alopecia is a complex disorder that can be managed through a myriad of therapies. Management of AGA should be a patient-centered process requiring physician consultation to assess the patient’s expectations, goals of care, and adverse effects associated with potential treatment options. It is also important to inform patients of the cost of treatment options and consider the patient’s comorbidities when selecting a treatment plan. As always, it is important to offer minimally invasive treatments first and escalate treatment accordingly until patient satisfaction is achieved.
In 2018, the S3 guidelines for the treatment of AGA in men and women were updated to include the latest available data for each treatment of AGA.137 According to these guidelines, treatments with the greatest body of evidence for men included oral finasteride, oral dutasteride, and topical minoxidil. LLLT received a lower ranking for “B level evidence”, while PRP was the lowest included therapeutic option and was ranked lowest due to “C evidence” from poorly designed studies. In females topical minoxidil received the highest marks, followed by LLLT and PRP. The purpose of this review is to summarize existing therapies for AGA, their adverse effects, clinical efficacy, and potential synergy with other modalities. Since the 2018 version of the S3 guidelines was published, studies with better experimental design supporting LLLT and PRP have been published.
Currently, topical minoxidil remains the safest starting point to assess therapeutic response. Patients with more financial resources may also potentially be recommended combination therapy with LLLT. Both modalities offer a minimally invasive therapeutic option for treating AGA. PRP injection, while more costly and more invasive than LLLT or topical minoxidil, can be used orthogonally in the management of AGA. As previously discussed, patients must be candidates to receive PRP and must understand the cost of the procedure. PRP can be employed in conjunction with minoxidil and/or LLLT. Microneedling offers a safe and minimally invasive augmentative option, which can improve the efficacy of topical minoxidil, LLLT, and PRP. Together, these techniques offer patients valuable options for managing AGA. While oral finasteride is typically offered for long-term maintenance therapy for AGA, the adverse effects remain major barriers to long-term compliance. Hair transplant remains an important tool in reversing the cosmetic results of AGA but is often very costly and may not be an option for patients. Additionally, hair transplant does not alter the progression of AGA and requires long-term anti-AGA therapy.
There is an abundance of alternative experimental therapies for AGA that have demonstrated encouraging results but require far more clinical testing before they can be incorporated as mainstream treatments for AGA. Topical finasteride represents an exciting alternative to oral finasteride and has been shown to offer similar clinical efficacy while minimizing the off-target effects of lowering plasma DHT concentrations. While stem cell therapy is novel, there are major logistic and financial barriers to overcome before it can be used to treat AGA.
Conclusion
While AGA remains difficult to manage, there are novel and exciting therapeutic options that have become widely available to patients. Current evidence on combination therapy has demonstrated enough beneficial results for it to be considered in patients, and it may eventually become the standard of care. Clinicians currently have incredible flexibility to offer cost-effective therapeutic options with favorable side-effect profiles for patients. Topical minoxidil has been a first-line treatment for AGA for decades and has demonstrated some efficacy. Twice daily application and delayed onset of action are difficult obstacles for patients to achieve long-term compliance. Given the synergistic relationship between LLLT and topical minoxidil, the use of both therapies concurrently could improve compliance especially early on in the use of topical minoxidil. Oral minoxidil is an alternative to topical minoxidil and is well tolerated at low doses ranging from 0.5 to 2mg daily and is far easier to achieve long-term compliance with a once daily oral pill therapy. Early data on efficacy appear promising, but more rigorous studies are required to adequately assess the efficacy.
Oral finasteride has also been a mainstay therapy for male AGA. While there does appear to be data supporting the ongoing use of oral finasteride, adverse effects including decreased libido, erectile dysfunction, and limited data on the long-term safety of oral finasteride may impair patient adherence. Topical finasteride is an interesting alternative therapy that is still being evaluated for efficacy. Viable alternatives to finasteride are available should patients not tolerate the adverse effects of sustained treatment. LLLT is one of the most recent FDA approved therapies for AGA and has the most favorable side effect profile. Daily use with LLLT is efficacious, noninvasive, and relatively inexpensive. Platelet Rich Plasma is a technique that has become more popular for AGA, but the increased cost and invasiveness can be off-putting for patients. PRP could instead be considered in patients who have failed multiple other first-line modalities. Techniques such as microneedling can be used as an augmentation tool for all therapies, especially topical minoxidil and LLLT. Microneedling is both cost-effective and minimally invasive. Overall, patients and clinicians have a variety of new therapeutic options for managing AGA that can be considered.
Disclosure
Dr Naiem T Issa reports a patent WO2021178871A1 issued to University of Miami. The authors report no other conflicts of interest in this work.
References
1. Lolli F, Pallotti F, Rossi A, et al. Androgenetic alopecia: a review. Endocrine. 2017;57(1):9–17. doi:10.1007/s12020-017-1280-y
2. Price VH. Androgenetic alopecia in women. J Investig Dermatol Symp Proc. 2003;8(1):24–27. doi:10.1046/j.1523-1747.2003.12168.x
3. Trüeb RM. Molecular mechanisms of androgenetic alopecia. Exp Gerontol. 2002;37(8–9):981–990. doi:10.1016/S0531-5565(02)00093-1
4. Kaliyadan F, Nambiar A, Vijayaraghavan S. Androgenetic alopecia: an update. Indian J Dermatol Venereol Leprol. 2013;79(5):613. doi:10.4103/0378-6323.116730
5. Ho CH, Sood T, Zito PM. Androgenetic alopecia. In: StatPearls. StatPearls Publishing; 2021.
6. Rathnayake D, Sinclair R. Male androgenetic alopecia. Expert Opin Pharmacother. 2010;11(8):1295–1304. doi:10.1517/14656561003752730
7. Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med. 2002;4(22):1–11. doi:10.1017/S1462399402005112
8. Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(1):136–141. e5. doi:10.1016/j.jaad.2017.02.054
9. Zins GR. The history of the development of minoxidil. Clin Dermatol. 1988;6(4):132–147. doi:10.1016/0738-081X(88)90078-8
10. Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84(3):737–746. doi:10.1016/j.jaad.2020.06.1009
11. Barnett AC, Tsvetanov S, Gamage N, et al. Active site mutations and substrate inhibition in human sulfotransferase 1A1 and 1A3. J Biol Chem. 2004;279(18):18799–18805. doi:10.1074/jbc.M312253200
12. Suchonwanit P, Thammarucha S, Leerunyakul K. Minoxidil and its use in hair disorders: a review. Drug Des Devel Ther. 2019;13:2777. doi:10.2147/DDDT.S214907
13. Gupta A, Talukder M, Venkataraman M, et al. Minoxidil: a comprehensive review. J DermatolTreat. 2022;33(4):1896–1906. doi:10.1080/09546634.2021.1945527
14. Pekmezci E, Turkoğlu M, Gökalp H, et al. Minoxidil downregulates interleukin-1 alpha gene expression in HaCaT cells. Int J Trichology. 2018;10(3):108. doi:10.4103/ijt.ijt_18_17
15. Kvedar JC, Baden HP, Levine L. Selective inhibition by minoxidil of prostacyclin production by cells in culture. Biochem Pharmacol. 1988;37(5):867–874. doi:10.1016/0006-2952(88)90174-8
16. Kwack MH, Kang BM, Kim MK, et al. Minoxidil activates β-catenin pathway in human dermal papilla cells: a possible explanation for its anagen prolongation effect. J Dermatol Sci. 2011;62(3):154–159. doi:10.1016/j.jdermsci.2011.01.013
17. Kelly Y, Blanco A, Tosti A. Androgenetic alopecia: an update of treatment options. Drugs. 2016;76(14):1349–1364. doi:10.1007/s40265-016-0629-5
18. Olsen EA, Dunlap FE, Funicella T, et al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002;47(3):377–385. doi:10.1067/mjd.2002.124088
19. Lucky AW, Piacquadio DJ, Ditre CM, et al. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol. 2004;50(4):541–553. doi:10.1016/j.jaad.2003.06.014
20. Blumeyer A, Tosti A, Messenger A, et al. Evidence‐based (S3) guideline for the treatment of androgenetic alopecia in women and in men. JDDG. 2011;9:S1–S57. doi:10.1111/j.1610-0379.2011.07802.x
21. Goren A, Shapiro J, Roberts J, et al. Clinical utility and validity of minoxidil response testing in androgenetic alopecia. Dermatol Ther. 2015;28(1):13–16. doi:10.1111/dth.12164
22. Messenger A, Rundegren J. Minoxidil: mechanisms of action on hair growth. BrJ Dermatol. 2004;150(2):186–194. doi:10.1111/j.1365-2133.2004.05785.x
23. Olsen EA, Weiner MS, Amara IA, et al. Five-year follow-up of men with androgenetic alopecia treated with topical minoxidil. J Am Acad Dermatol. 1990;22(4):643–646. doi:10.1016/0190-9622(90)70089-Z
24. Goren A, Castano JA, McCoy J, et al. Novel enzymatic assay predicts minoxidil response in the treatment of androgenetic alopecia. Dermatol Ther. 2013;27(3):171–173. doi:10.1111/dth.12111
25. Roberts J, Desai N, McCoy J, et al. Sulfotransferase activity in plucked hair follicles predicts response to topical minoxidil in the treatment of female androgenetic alopecia. Dermatol Ther. 2014;27(4):252–254. doi:10.1111/dth.12130
26. Munck A, Gavazzoni MF, Trüeb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6(2):45. doi:10.4103/0974-7753.138584
27. Qiu J, Yi Y, Jiang L, et al. Efficacy assessment for low-level laser therapy in the treatment of androgenetic alopecia: a real-world study on 1383 patients. Lasers Med Sci. 2022;37:1–6.
28. Gkini M-A, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg. 2014;7(4):213. doi:10.4103/0974-2077.150743
29. Singhal P, Agarwal S, Dhot P, et al. Efficacy of platelet-rich plasma in treatment of androgenic alopecia. Asian J Transfus Sci. 2015;9(2):159. doi:10.4103/0973-6247.162713
30. Verma K, Tegta G, Verma G, et al. A study to compare the efficacy of platelet-rich plasma and minoxidil therapy for the treatment of androgenetic alopecia. Int J Trichology. 2019;11(2):68. doi:10.4103/ijt.ijt_64_18
31. Dhurat R, Sukesh MS, Avhad G, et al. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichology. 2013;5(1):6. doi:10.4103/0974-7753.114700
32. Starace M, Alessandrini A, Brandi N, et al. Preliminary results of the use of scalp microneedling in different types of alopecia. J Cosmet Dermatol. 2020;19(3):646–650. doi:10.1111/jocd.13061
33. Mori O, Uno H. The effect of topical minoxidil on hair follicular cycles of rats. J Dermatol. 1990;17(5):276–281. doi:10.1111/j.1346-8138.1990.tb01641.x
34. Panchaprateep R, Lueangarun S. Efficacy and safety of oral minoxidil 5 mg once daily in the treatment of male patients with androgenetic alopecia: an open-label and global photographic assessment. Dermatol Ther. 2020;10:1345–1357. doi:10.1007/s13555-020-00448-x
35. Andy G, John M, Mirna S, et al. Controversies in the treatment of androgenetic alopecia: the history of finasteride. Dermatol Ther. 2019;32(2):e12647. doi:10.1111/dth.12647
36. Libecco JF, Bergfeld WF. Finasteride in the treatment of alopecia. Expert Opin Pharmacother. 2004;5(4):933–940. doi:10.1517/14656566.5.4.933
37. Rossi A, Cantisani C, Scarnò M, et al. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10‐year follow‐up. Dermatol Ther. 2011;24(4):455–461. doi:10.1111/j.1529-8019.2011.01441.x
38. Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. J Am Acad Dermatol. 1998;39(4):578–589. doi:10.1016/S0190-9622(98)70007-6
39. Mella JM, Perret MC, Manzotti M, et al. Efficacy and safety of finasteride therapy for androgenetic alopecia: a systematic review. Arch Dermatol. 2010;146(10):1141–1150. doi:10.1001/archdermatol.2010.256
40. Yoshitake T, Takeda A, Ohki K, et al. Five‐year efficacy of finasteride in 801 Japanese men with androgenetic alopecia. J Dermatol. 2015;42(7):735–738. doi:10.1111/1346-8138.12890
41. Shin JW, Chung E-H, Kim M-B, et al. Evaluation of long‐term efficacy of finasteride in Korean men with androgenetic alopecia using the basic and specific classification system. J Dermatol. 2019;46(2):139–143. doi:10.1111/1346-8138.14719
42. Thai KE, Sinclair R. Finasteride for female androgenetic alopecia. Br J Dermatol. 2002;147(4):812–813. doi:10.1046/j.1365-2133.2002.49084.x
43. Hu AC, Chapman LW, Mesinkovska NA. The efficacy and use of finasteride in women: a systematic review. Int J Dermatol. 2019;58(7):759–776. doi:10.1111/ijd.14370
44. Hirshburg JM, Kelsey PA, Therrien CA, et al. Adverse effects and safety of 5-alpha reductase inhibitors (finasteride, dutasteride): a systematic review. J Clin Aesthet Dermatol. 2016;9(7):56.
45. Ganzer CA, Jacobs AR, Iqbal F. Persistent sexual, emotional, and cognitive impairment post-finasteride: a survey of men reporting symptoms. Am J Men Health. 2015;9(3):222–228. doi:10.1177/1557988314538445
46. Wang L, Lei Y, Gao Y, et al. Association of finasteride with prostate cancer: a systematic review and meta-analysis. Medicine. 2020;99:15.
47. Villani A, Fabbrocini G, Ocampo‐Candiani J, et al. Review of oral minoxidil as treatment of hair disorders: in search of the perfect dose. J Eur Acad Dermatol Venereol. 2021;35(7):1485–1492. doi:10.1111/jdv.17216
48. Jimenez-Cauhe J, Saceda-Corralo D, Rodrigues-Barata R, et al. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. J Am Acad Dermatol. 2019;81(2):648–649. doi:10.1016/j.jaad.2019.04.054
49. Ramos P, Goren A, Sinclair R, et al. Oral minoxidil bio‐activation by hair follicle outer root sheath cell sulfotransferase enzymes predicts clinical efficacy in female pattern hair loss. J Eur Acad Dermatol Venereol. 2020;34(1):e40–e41. doi:10.1111/jdv.15891
50. Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31(6):e12707. doi:10.1111/dth.12707
51. Ramos PM, Sinclair RD, Kasprzak M, et al. Minoxidil 1 mg oral versus minoxidil 5% topical solution for the treatment of female-pattern hair loss: a randomized clinical trial. J Am Acad Dermatol. 2020;82(1):252–253. doi:10.1016/j.jaad.2019.08.060
52. Vastarella M, Cantelli M, Patrì A, et al. Efficacy and safety of oral minoxidil in female androgenetic alopecia. Dermatol Ther. 2020;33(6). doi:10.1111/dth.14234
53. Jimenez-Cauhe J, Saceda-Corralo D, Moreno-Arrones OM, et al. Reply to: “Very-low-dose oral minoxidil in male androgenetic alopecia: a study with quantitative trichoscopic documentation”. J Am Acad Dermatol. 2020;82(1):e23–e24. doi:10.1016/j.jaad.2019.08.085
54. Sanabria B, Vanzela TDN, Miot HA, et al. Adverse effects of low-dose oral minoxidil for androgenetic alopecia in 435 patients. J Am Acad Dermatol. 2021;84(4):1175–1178. doi:10.1016/j.jaad.2020.11.035
55. Suchonwanit P, Iamsumang W, Leerunyakul K. Topical finasteride for the treatment of male androgenetic alopecia and female pattern hair loss: a review of the current literature. J DermatolTreat. 2022;33(2):643–648. doi:10.1080/09546634.2020.1782324
56. Caserini M, Radicioni M, Leuratti C, et al. A novel finasteride 0.25% topical solution for androgenetic alopecia: pharmacokinetics and effects on plasma androgen levels in healthy male volunteers. Int J Clin Pharmacol Ther. 2014;52(10):842–849. doi:10.5414/CP202119
57. Piraccini B, Blume‐Peytavi U, Scarci F, et al. Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a Phase III, randomized, controlled clinical trial. J Eur Acad Dermatol Venereol. 2022;36(2):286–294. doi:10.1111/jdv.17738
58. Hajheydari Z, Akbari J, Saeedi M, et al. Comparing the therapeutic effects of finasteride gel and tablet in treatment of the androgenetic alopecia. Indian J Dermatol Venereol Leprol. 2009;75(1):47. doi:10.4103/0378-6323.45220
59. Gupta AK, Talukder M. Topical finasteride for male and female pattern hair loss: is it a safe and effective alternative? J Cosmet Dermatol. 2022;21(5):1841–1848. doi:10.1111/jocd.14895
60. Suchonwanit P, Srisuwanwattana P, Chalermroj N, et al. A randomized, double‐blind controlled study of the efficacy and safety of topical solution of 0.25% finasteride admixed with 3% minoxidil vs. 3% minoxidil solution in the treatment of male androgenetic alopecia. J Eur Acad Dermatol Venereol. 2018;32(12):2257–2263. doi:10.1111/jdv.15171
61. Chandrashekar B, Nandhini T, Vasanth V, et al. Topical minoxidil fortified with finasteride: an account of maintenance of hair density after replacing oral finasteride. Indian Dermatol Online J. 2015;6(1):17. doi:10.4103/2229-5178.148925
62. Rai PB, Khushwaha P, Jain N, et al. Comparing the therapeutic efficacy of topical minoxidil and finasteride with topical minoxidil and oral finasteride in androgenetic alopecia: a randomized trial. Int J Res Dermatol. 2018;4(3):386–390. doi:10.18203/issn.2455-4529.IntJResDermatol20183163
63. Tsunemi Y, Irisawa R, Yoshiie H, et al. Long‐term safety and efficacy of dutasteride in the treatment of male patients with androgenetic alopecia. J Dermatol. 2016;43(9):1051–1058. doi:10.1111/1346-8138.13310
64. Harcha WG, Barboza Martínez J, Tsai T-F, et al. A randomized, active-and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol. 2014;70(3):489–498. e3. doi:10.1016/j.jaad.2013.10.049
65. Jung JY, Yeon JH, Choi JW, et al. Effect of dutasteride 0.5 mg/d in men with androgenetic alopecia recalcitrant to finasteride. Int J Dermatol. 2014;53(11):1351–1357. doi:10.1111/ijd.12060
66. Gupta AK, Foley KA. A critical assessment of the evidence for low-level laser therapy in the treatment of hair loss. Dermatol Surg. 2017;43(2):188–197. doi:10.1097/DSS.0000000000000904
67. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. In: Seminars in Cutaneous Medicine and Surgery. NIH Public Access; 2013.
68. Friedman S, Schnoor P. Novel approach to treating androgenetic alopecia in females with photobiomodulation (low-level laser therapy). Dermatol Surg. 2017;43(6):856–867. doi:10.1097/DSS.0000000000001114
69. Kaiser MA, Almeida SM, Rodriguez M, et al. Low-level light therapy and minoxidil combination treatment in androgenetic alopecia: a review of the literature. Skin Appendage Disord. 2023;9(2):1–7. doi:10.1159/000527782
70. Egger A, Resnik SR, Aickara D, et al. Examining the safety and efficacy of low-level laser therapy for male and female pattern hair loss: a review of the literature. Skin Appendage Dis. 2020;6(5):259–267. doi:10.1159/000509001
71. Avci P, Gupta GK, Clark J, et al. Low‐level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2014;46(2):144–151. doi:10.1002/lsm.22170
72. Wikramanayake TC, Rodriguez R, Choudhary S, et al. Effects of the Lexington LaserComb on hair regrowth in the C3H/HeJ mouse model of alopecia areata. Lasers Med Sci. 2012;27(2):431–436. doi:10.1007/s10103-011-0953-7
73. Liu K-H, Liu D, Chen Y-T, et al. Comparative effectiveness of low-level laser therapy for adult androgenic alopecia: a system review and meta-analysis of randomized controlled trials. Lasers Med Sci. 2019;34(6):1063–1069. doi:10.1007/s10103-019-02723-6
74. Afifi L, Maranda EL, Zarei M, et al. Low‐level laser therapy as a treatment for androgenetic alopecia. Lasers Surg Med. 2017;49(1):27–39. doi:10.1002/lsm.22512
75. Suchonwanit P, Chalermroj N, Khunkhet S. Low-level laser therapy for the treatment of androgenetic alopecia in Thai men and women: a 24-week, randomized, double-blind, sham device-controlled trial. Lasers Med Sci. 2019;34(6):1107–1114. doi:10.1007/s10103-018-02699-9
76. Esmat SM, Hegazy RA, Gawdat HI, et al. Low level light‐minoxidil 5% combination versus either therapeutic modality alone in management of female patterned hair loss: a randomized controlled study. Lasers Surg Med. 2017;49(9):835–843. doi:10.1002/lsm.22684
77. Zhou Y, Chen C, Qu Q, et al. The effectiveness of combination therapies for androgenetic alopecia: a systematic review and meta‐analysis. Dermatol Ther. 2020;33(4):e13741. doi:10.1111/dth.13741
78. Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259–2272. doi:10.1177/0363546509349921
79. Hesseler MJ, Shyam N. Platelet-rich plasma and its utility in medical dermatology: a systematic review. J Am Acad Dermatol. 2019;81(3):834–846. doi:10.1016/j.jaad.2019.04.037
80. Emer J. Platelet-rich plasma (PRP): current applications in dermatology. Skin Therapy Lett. 2019;24(5):1–6.
81. Leo MS, Kumar AS, Kirit R, et al. Systematic review of the use of platelet‐rich plasma in aesthetic dermatology. J Cosmet Dermatol. 2015;14(4):315–323. doi:10.1111/jocd.12167
82. Gupta AK, Carviel J. A mechanistic model of platelet-rich plasma treatment for androgenetic alopecia. Dermatol Surg. 2016;42(12):1335–1339. doi:10.1097/DSS.0000000000000901
83. Gupta AK, Cole J, Deutsch DP, et al. Platelet-rich plasma as a treatment for androgenetic alopecia. Dermatol Surg. 2019;45(10):1262–1273. doi:10.1097/DSS.0000000000001894
84. Giordano S, Romeo M, Lankinen P. Platelet‐rich plasma for androgenetic alopecia: does it work? Evidence from meta analysis. J Cosmet Dermatol. 2017;16(3):374–381. doi:10.1111/jocd.12331
85. Dashore S, Chouhan K, Nanda S, et al. Preparation of platelet-rich plasma: national IADVL PRP taskforce recommendations. Indian Dermatol Online J. 2021;12(Suppl 1):S12. doi:10.4103/idoj.idoj_269_21
86. Singh A, Yadav S. Microneedling: advances and widening horizons. Indian Dermatol Online J. 2016;7:4.
87. Kim YS, Jeong KH, Kim JE, et al. Repeated microneedle stimulation induces enhanced hair growth in a murine model. Ann Dermatol. 2016;28(5):586. doi:10.5021/ad.2016.28.5.586
88. Liebl H, Kloth LC. Skin cell proliferation stimulated by microneedles. J Am Coll Clin Wound Spec. 2012;4(1):2–6. doi:10.1016/j.jccw.2012.11.001
89. Gowda BJ, Ahmed MG, Sanjana A. Can microneedles replace hypodermic needles? Painless drug delivery. Resonance. 2022;27(1):63–85. doi:10.1007/s12045-022-1294-5
90. Gowda BJ, Ahmed MG, Hani U, et al. Microneedles as a momentous platform for psoriasis therapy and diagnosis: a state-of-The-art review. Int J Pharm. 2023;632:122591. doi:10.1016/j.ijpharm.2023.122591
91. Katzer T, Leite Junior A, Beck R, et al. Physiopathology and current treatments of androgenetic alopecia: going beyond androgens and anti-androgens. Dermatol Ther. 2019;32(5). doi:10.1111/dth.13059
92. Doddaballapur S. Microneedling with dermaroller. J Cutan Aesthet Surg. 2009;2(2):110. doi:10.4103/0974-2077.58529
93. Jha AK, Vinay K. Androgenetic alopecia and microneedling: every needling is not microneedling. J Am Acad Dermatol. 2019;81(2):e43–e44. doi:10.1016/j.jaad.2019.02.070
94. Jha AK, Udayan UK, Roy PK, et al. Original article: platelet-rich plasma with microneedling in androgenetic alopecia along with dermoscopic pre- and post-treatment evaluation. J Cosmet Dermatol. 2018;17(3):313–318. doi:10.1111/jocd.12394
95. Shah KB, Shah AN, Solanki RB, et al. A comparative study of microneedling with platelet-rich plasma plus topical minoxidil (5%) and topical minoxidil (5%) alone in androgenetic alopecia. Int J Trichology. 2017;9(1):14. doi:10.4103/ijt.ijt_75_16
96. Dhurat R, Sukesh M. Response to microneedling treatment in men with androgenetic alopecia who failed to respond to conventional therapy. Indian J Dermatol. 2015;60(3):260. doi:10.4103/0019-5154.156361
97. Lee YB, Eun YS, Lee JH, et al. Effects of topical application of growth factors followed by microneedle therapy in women with female pattern hair loss: a pilot study. J Dermatol. 2013;40(1):81–83. doi:10.1111/j.1346-8138.2012.01680.x
98. Alves R, Grimalt R. Platelet-rich plasma in combination with 5% minoxidil topical solution and 1 mg oral finasteride for the treatment of androgenetic alopecia: a randomized placebo-controlled, double-blind, half-head study. Dermatol Surg. 2018;44(1):126–130. doi:10.1097/DSS.0000000000001198
99. Pakhomova EE, Smirnova IO. Comparative evaluation of the clinical efficacy of prp-therapy, minoxidil, and their combination with immunohistochemical study of the dynamics of cell proliferation in the treatment of men with androgenetic alopecia. Int J Mol Sci. 2020;21(18):6516. doi:10.3390/ijms21186516
100. Singh S, Kumar V, Rai T. Comparison of efficacy of platelet-rich plasma therapy with or without topical 5% minoxidil in male-type baldness: a randomized, double-blind placebo control trial. Indian J Dermatol Venereol Leprol. 2020;86(2):150–157. doi:10.4103/ijdvl.IJDVL_589_18
101. Ramadan WM, Hassan AM, Ismail MA, et al. Evaluation of adding platelet‐rich plasma to combined medical therapy in androgenetic alopecia. J Cosmet Dermatol. 2021;20(5):1427–1434. doi:10.1111/jocd.13935
102. Ray R, Sharma A. Comparison of 5% minoxidil lotion monotherapy versus its combination with autologous platelet rich plasma in androgenetic alopecia in hundred males. Med J Armed Forces India. 2021;77(3):355–362. doi:10.1016/j.mjafi.2020.11.010
103. Gentile P, Dionisi L, Pizzicannella J, et al. A randomized blinded retrospective study: the combined use of micro-needling technique, low-level laser therapy and autologous non-activated platelet-rich plasma improves hair re-growth in patients with androgenic alopecia. Expert Opin Biol Ther. 2020;20(9):1099–1109. doi:10.1080/14712598.2020.1797676
104. Yepuri V, Venkataram M. Platelet-rich plasma with microneedling in androgenetic alopecia: study of efficacy of the treatment and the number of sessions required. J Cutan Aesthet Surg. 2021;14(2):184. doi:10.4103/JCAS.JCAS_33_20
105. Jha AK, Vinay K, Zeeshan M, et al. Platelet‐rich plasma and microneedling improves hair growth in patients of androgenetic alopecia when used as an adjuvant to minoxidil. J Cosmet Dermatol. 2019;18(5):1330–1335. doi:10.1111/jocd.12864
106. Aggarwal K, Gupta S, Jangra R, et al. Dermoscopic assessment of microneedling alone versus microneedling with platelet-rich plasma in cases of male pattern alopecia: a split-head comparative study. Int J Trichology. 2020;12(4):156. doi:10.4103/ijt.ijt_64_20
107. Sinclair RD. Female pattern hair loss: a pilot study investigating combination therapy with low‐dose oral minoxidil and spironolactone. Int J Dermatol. 2018;57(1):104–109. doi:10.1111/ijd.13838
108. Carone L, Oxberry SG, Twycross R, et al. Spironolactone. J Pain Symptom Manage. 2017;53(2):288–292. doi:10.1016/j.jpainsymman.2016.12.320
109. Goodfellow A, ALAGHBAND-ZADEH J, Carter G, et al. Oral spironolactone improves acne vulgaris and reduces sebum excretion. Br J Dermatol. 1984;111(2):209–214. doi:10.1111/j.1365-2133.1984.tb04045.x
110. Rathnayake D, Sinclair R. Innovative use of spironolactone as an antiandrogen in the treatment of female pattern hair loss. Dermatol Clin. 2010;28(3):611–618. doi:10.1016/j.det.2010.03.011
111. James JF, Jamerson TA, Aguh C. Efficacy and safety profile of oral spironolactone use for androgenic alopecia—a systematic review. J Am Acad Dermatol. 2021;2021:1.
112. Hussein SM, Soliman AM, Abd El-Alim SH, et al. Assessment of the efficacy of topical antiandrogen; spironolactone in patients with androgenetic alopecia by dermoscopy. J Pak Assoc Dermatol. 2022;32(3):493–501.
113. Burns LJ, De Souza B, Flynn E, et al. Spironolactone for treatment of female pattern hair loss. J Am Acad Dermatol. 2020;83(1):276–278. doi:10.1016/j.jaad.2020.03.087
114. Famenini S, Slaught C, Duan L, et al. Demographics of women with female pattern hair loss and the effectiveness of spironolactone therapy. J Am Acad Dermatol. 2015;73(4):705–706. doi:10.1016/j.jaad.2015.06.063
115. Ahmed SA, Ayman EY, Mousa A. Topical finasteride versus topical spironolactone in the treatment of androgenetic alopecia. Med J Cairo Univ. 2020;88:1017–1022. doi:10.21608/mjcu.2020.110836
116. Ammar AM, Elshahid AR, Abdel‐Dayem HA, et al. Dermoscopic evaluation of the efficacy of combination of topical spironolactone 5% and minoxidil 5% solutions in the treatment of androgenetic alopecia: a cross sectional‐comparative study. J Cosmet Dermatol. 2022;21(11):5790–5799. doi:10.1111/jocd.15328
117. Wang Y, Zhang H, Zheng Q, et al. Botulinum toxin as a double‐edged sword in alopecia: a systematic review. J Cosmet Dermatol. 2020;19(10):2560–2565. doi:10.1111/jocd.13647
118. Zhou Y, Yu S, Zhao J, et al. Effectiveness and safety of botulinum toxin type A in the treatment of androgenetic alopecia. Biomed Res Int. 2020;2020:1–7. doi:10.1155/2020/1501893
119. Carloni R, Pechevy L, Postel F, et al. Is there a therapeutic effect of botulinum toxin on scalp alopecia? Physiopathology and reported cases: a systematic review of the literature. J Plast Reconstr Aesthet Surg. 2020;73(12):2210–2216. doi:10.1016/j.bjps.2020.05.035
120. Zhang L, Yu Q, Wang Y, et al. A small dose of botulinum toxin A is effective for treating androgenetic alopecia in Chinese patients. Dermatol Ther. 2019;32(4):e12785. doi:10.1111/dth.12785
121. Freund BJ, Schwartz M. Treatment of male pattern baldness with botulinum toxin: a pilot study. Plast Reconstr Surg. 2010;126(5):246e–248e. doi:10.1097/PRS.0b013e3181ef816d
122. Shon U, Kim MH, Lee DY, et al. The effect of intradermal botulinum toxin on androgenetic alopecia and its possible mechanism. J Am Acad Dermatol. 2020;83(6):1838–1839. doi:10.1016/j.jaad.2020.04.082
123. Cho HR, Lew B-L, Lew H, et al. Treatment effects of intradermal botulinum toxin type A injection on alopecia areata. Dermatol Surg. 2010;36:2175–2181. doi:10.1111/j.1524-4725.2010.01709.x
124. English RS, Ruiz S. Use of botulinum toxin for androgenic alopecia: a systematic review. Skin Appendage Dis. 2022;2022:1–8.
125. Kowing D. Madarosis and facial alopecia presumed secondary to botulinum a toxin injections. Optom Vision Sci. 2005;82(7):579–582. doi:10.1097/01.opx.0000171332.53664.df
126. Di Pietro A, Piraccini BM. Frontal alopecia after repeated botulinum toxin type A injections for forehead wrinkles: an underestimated entity? Skin Appendage Disord. 2016;2(1–2):67–69. doi:10.1159/000448380
127. Gentile P, Garcovich S. Advances in regenerative stem cell therapy in androgenic alopecia and hair loss: wnt pathway, growth-factor, and mesenchymal stem cell signaling impact analysis on cell growth and hair follicle development. Cells. 2019;8(5):466. doi:10.3390/cells8050466
128. Talavera-Adame D, Newman D, Newman N. Conventional and novel stem cell based therapies for androgenic alopecia. Stem Cells Cloning. 2017;10:11. doi:10.2147/SCCAA.S138150
129. Leiros GJ, Attorresi AI, Balaña ME. Hair follicle stem cell differentiation is inhibited through cross‐talk between Wnt/β‐catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012;166(5):1035–1042. doi:10.1111/j.1365-2133.2012.10856.x
130. Elmaadawi IH, Mohamed BM, Ibrahim ZAS, et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia. J DermatolTreat. 2018;29(5):431–440. doi:10.1080/09546634.2016.1227419
131. Tak YJ, Lee SY, Cho AR, et al. A randomized, double-blind, vehicle-controlled clinical study of hair regeneration using adipose-derived stem cell constituent extract in androgenetic alopecia. Stem Cells Transl Med. 2020;9(8):839–849. doi:10.1002/sctm.19-0410
132. Zari S. Short-term efficacy of autologous cellular micrografts in male and female androgenetic alopecia: a retrospective cohort study. Clin Cosmet Investig Dermatol. 2021;Volume 14:1725–1736. doi:10.2147/CCID.S334807
133. Drake L, Drake L, Han JJ, et al. Evaluation of the safety and effectiveness of nutritional supplements for treating hair loss: a systematic review. JAMA Dermatol. 2022;158(10):1187–1191. doi:10.1001/jamadermatol.2022.3025
134. Sun H, Sebaratnam D. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45(7):913–914. doi:10.1111/ced.14292
135. Nestor MS, Ablon G, Gade A, et al. Treatment options for androgenetic alopecia: efficacy, side effects, compliance, financial considerations, and ethics. J Cosmet Dermatol. 2021;20(12):3759–3781. doi:10.1111/jocd.14537
136. Rosette C, Rosette N, Mazzetti A, et al. Cortexolone 17α-propionate (clascoterone) is an androgen receptor antagonist in dermal papilla cells in vitro. J Drugs Dermatol. 2019;18(2):197–201.
137. Kanti V, Messenger A, Dobos G, et al. Evidence‐based (S3) guideline for the treatment of androgenetic alopecia in women and in men–short version. J Eur Acad Dermatol Venereol. 2018;32(1):11–22. doi:10.1111/jdv.14624
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
